Abstract
The tolerance of the dune grass Leymus mollis (Triticeae; Poaceae) to various biotic and abiotic stresses makes it a very useful genetic resource for wheat breeding. Wide hybridization between L. mollis and wheat allows the introduction of Leymus chromosomes into the wheat genetic background and facilitates the integration of useful traits into wheat. However, the genetic basis controlling the physiological tolerance of L. mollis to multiple environmental stresses remains largely unexplored. Using suppression subtractive hybridization, we identified 112 osmotic-stress-responsive genes from L. mollis and confirmed their differential expression under osmotic stress. These genes were categorized into 13 functional categories, including cell defense and stress response, transcriptional regulation, signal transduction, biosynthesis of compatible solutes and cell wall metabolism. Representative genes were validated by northern blot and RT-PCR analyses of expression patterns in response to osmotic stress and abscisic acid treatment. The genes identified here represent a useful source of expressed sequence tags (ESTs) for the analysis and identification of Leymus chromosomes introduced into wheat. Furthermore, being highly conserved, genetically associated with osmotic stress tolerance and transferable to wheat, these ESTs provide significant tools for the development of EST-derived molecular markers for introgression of osmotic stress tolerance genes into wheat.
Keywords: Leymus, wheat, osmotic stress, ESTs
Introduction
Abiotic stresses such as drought and salt are considered prime constraints on the growth and productivity of crop plants worldwide. Desertification and salinization are rapidly increasing on a global scale, reducing average yields for most major crop plants by more than 50% (Bartels and Sunkar 2005). With climate change, significant drying in some regions (Allen et al. 2010) and an increase in the frequency and severity of extreme droughts are expected (IPCC 2007). Exposure to drought stress leads to cellular dehydration, which causes osmotic stress and removal of water from the cytoplasm into the extracellular space. Drought also promotes the overproduction of reactive oxygen species (ROS), which harm cellular structures, functions and metabolism (Eltayeb et al. 2007). Physiological drought stress responses include stomatal closure, repression of cell growth and photosynthesis, and activation of respiration.
Drought tolerance is regulated by the induction of multiple genes that may either directly protect plant cells through their products (LEA proteins, chaperones, enzymes for osmolyte biosynthesis and detoxification) or regulate the expression of other genes such as those for transcription factors, secondary messengers, phosphatases and kinases (Reddy et al. 2008).
Wheat (Triticum aestivum L.), which is one of the most important staple food crops worldwide, is adversely affected by periodic drought in more than half of its area of production (Rajaram 2001). The productivity of wheat is often limited by a shortage of water (Aprile et al. 2009). Micro-array transcriptional profiling study using durum wheat (T. durum) and two bread wheat genotypes (Cultivar Chinese spring and its 5A chromosome deletion line) revealed several drought responses that can be associated to the different genomic structures (Aprile et al. 2009). Tran-scriptomic analyses used to study drought responses in wild emmer wheat (T. turgidum) revealed the enrichment of drought induced transcripts involved in multilevel regulatory and signaling processes such as transcriptional regulation, kinase activity and hormone related genes (Krugman et al. 2010, 2011). These studies have also indicated the differential expression of genes known to be involved in drought adaptation mechanisms such as cell wall adjustment, osmo-regulation and dehydration protection.
Improving wheat drought tolerance through selection and breeding requires a high level of heritable genetic variation among various genotypes or wild relatives (Ashraf 2010). A large amount of genetic diversity for adaptation to drought stress is present in wild relatives of plant species that may have specific adaptive mechanisms and express novel stress-responsive genes (Reddy et al. 2008).
The dune grass Leymus mollis (Triticeae; Poaceae) is a wild relative of wheat that grows mainly along sea coasts and in inland dry areas (Fan et al. 2009). It is evolutionarily distant from wheat, and has exceptionally large spikes, strong rhizomes and vigorous growth. It is considered to be very useful as a genetic resource for wheat breeding (Kishii et al. 2003), being tolerant to salt and drought (McGuire and Dvorak 1981), resistant to various diseases, including scab (Mujeeb-Kazi et al. 1983) and powdery mildew (Faith 1983) and highly adaptable to nutrient deprivation and harsh conditions. Yet few studies have investigated its stress tolerance. Gagné and Houle (2002) reported that L. mollis seedlings were more tolerant to sand burial, salt spray, soil salinity and drought stress than Honckenya peploides (Caryophyllaceae). Aptekar and Rejmánek (2000) reported that L. mollis maintained high bud viability even after 13 days’ complete submergence. Recently, Wang et al. (2010) reported the creation of wheat–Leymus chromosome addition lines for breeding wheat with high phosphorus efficiency. Despite the high tolerance to multiple environmental stresses in L. mollis, the genetic basis for its biochemical and physiological responses to abiotic stresses remains largely unexplored. The identification of stress-responsive genes from stress-tolerant species and understanding of their role in stress adaptation will help to improve the tolerance of stress-susceptible crops (Reddy et al. 2008).
Marker-assisted breeding can identify and select lines containing expressed sequences of stress-tolerance genes within a breeding population. Suppression subtractive hybridization (SSH) is an efficient technique to compare two populations of mRNA (Diatchenko et al. 1996) and identify novel genes and species specific expressed sequence tags (ESTs) that are expressed in one population but not in the other. ESTs are a potential valuable source of molecular markers and allow a simple strategy to study the transcribed parts of complex and highly redundant genomes like that of wheat (Yu et al. 2004). EST-derived markers are more advantageous than anonymous sequence-derived markers in that they are genetically associated with a trait of interest, could be all or part of the gene directly affecting the trait (Yu et al. 2004), are often conserved (Thiel et al. 2003), and are more transferable between species (Cordeiro et al. 2001, Taylor et al. 2001). Moreover, EST-derived markers can provide opportunities for gene discovery and enhance the role of genetic markers in assays for variation in transcribed and known-function genes (Yu et al. 2004).
We used SSH to identify important genes and ESTs for osmotic stress tolerance from L. mollis. We isolated and functionally categorized 112 non-redundant sequences that were differentially expressed. We investigated the expression patterns of some representative genes in response to osmotic stress and ABA treatments. Furthermore, we investigated the gene copy number of some genes in L. mollis and the wheat cultivars ‘Norin 61’ and ‘Chinese Spring’ (CS) in relation to osmotic stress tolerance. Some of these genes represent valuable resources for the development of EST-derived molecular markers for osmotic stress.
Materials and Methods
Plant materials and growth conditions
Young L. mollis plants were collected from Hamamura Beach, Tottori city (35°30′N), Japan.
Seedlings were washed thoroughly and maintained at 25°C, 60–70% relative humidity (RH), with their roots in distilled water for 3 days. They were then transferred to 10-L plastic containers for hydroponic culture in a growth chamber under natural light at 22–25°C and 60–70% RH. The hydroponic culture consisted of continuously aerated 1/4 strength Hoagland solution (HS), renewed every 2 days and with the pH adjusted to 5.6 with 1 M KOH or NaOH.
Drought and ABA treatments
Polyethylene glycol (PEG) 6000 which influences osmotic potential was used to induce osmotic stress in this study. Three biological replications of plants uniform in shape and size were subjected to 15% (w/v) PEG in 1/4 strength HS for 3 days until stress symptoms such as leaf rolling were visible. ABA treatment used 10−5 M ABA in 1/4 strength HS (pH 5.6). Plants were held in a growth chamber maintained at 25°C, 50–60% RH, with 200 μmol m−2 s−1 photosynthetically active radiation. Control plants were maintained in 1/4 strength HS. Leaves were sampled according to days-time course for osmotic stress or hours-time course for ABA treatment. Samples were immediately frozen in liquid nitrogen and stored at −80°C.
Poly(A)+ RNA isolation and construction of SSH library
Total RNA was isolated from leaf tissues with TriPure Isolation Reagent (Roche, Mannheim, Germany) and treated with RNase-free DNase I (Takara, Ohtsu, Japan) to remove any genomic DNA. Poly(A)+ RNA was purified with an Oligotex-dT30 <Super> mRNA purification kit (Takara, Ohtsu, Japan) according to the manufacturer’s instructions. Suppressive subtraction hybridization (SSH) was performed according to Diatchenko et al. (1996) using a PCR-Select cDNA Subtraction Kit (Clontech, Palo Alto, CA, USA) to identify genes that were differentially expressed in response to osmotic stress. cDNAs were synthesized from 2 μg poly(A)+ RNA isolated from osmotic stressed and control plants and used in forward- and reverse-subtraction hybridizations according to the manufacturer’s instructions. The forward-subtracted secondary PCR products were cloned into the pCR 2.1-TOPO TA cloning vector (Invitrogen, Carlsbad, CA, USA) and then transformed into E. coli DH5a competent cells according to the manufacturer’s instructions. A total of 196 positive colonies were randomly picked, and the cDNA inserts were confirmed by PCR using GoTaq Green Master Mix (Promega, Madison, WI, USA).
Confirmation of differential expression
The differential expression of the subtracted clones was tested by using a PCR-Select Differential Screening Kit (Clontech) according to the manufacturer’s instructions with some modifications. Forward-subtracted cDNA inserts were used to prepare two identical copies of Hybond-N+ nylon membranes (GE Healthcare, Little Chalfont, UK). Membranes were saturated in denaturing solution (0.5 M NaOH, 1.5 M NaCl), neutralized in neutralization solution (0.5 M Tris-HCl, pH 7.4, 1.5 M NaCl) and then air-dried for 30 min. Forward- and reverse-subtracted probes were prepared with a PCR DIG Probe Synthesis Kit (Roche). Hybridization followed standard procedures (Sambrook et al. 1989). Hybridized DIG-labeled probes were detected with a DIG Luminescent Detection Kit (Roche) and hybridization signals were visualized with an LAS-4000 Mini CCD camera (Fujifilm, Tokyo, Japan).
ESTs sequence analysis
Total of 144 ESTs were sequenced with a BigDye Terminator v. 3.1 Cycle Sequencing Kit (AB Applied Biosystems, Foster City, CA, USA) in an ABI 3130 Genetic Analyzer (Applied Biosystems). Sequences were analyzed using GENETYX version 9 software (GENETYX Corporation, Tokyo, Japan). Vector and adaptor sequences were removed, and the resulting high-quality and non-redundant sequences were used in sequence similarity searches against the NCBI non-redundant database (http://www.ncbi.nlm.nih.gov; National Center for Biotechnology Information, Bethesda, MD, USA) and the TAIR database (http://www.arabidopsis.org) with BLAST default parameters. ESTs were categorized by function against the MIPS (Munich Information for Protein Sequences) functional catalog (http://mips.gsf.de/projects/function).
Southern and northern blot analyses and RT-PCR
For northern blot analysis, total RNA (20 μg) from osmotic stressed or ABA-treated plants was denatured in formaldehyde gel and transferred to Hybond-N+ nylon membranes. For Southern analysis, genomic DNA was isolated with an Isoplant II kit (Nippon Gene, Tokyo, Japan). The DNA (10 μg) was digested with EcoRI and HindIII restriction enzymes, electrophoresed in 1.5% agarose gel, denatured, neutralized and then transferred to Hybond-N+ nylon membranes. Probes were labeled, hybridized and detected as described above in both northern and Southern analyses. For RT-PCR, 1 μg total RNA was used to synthesize first-strand cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions. Then 1 μL of the first-strand cDNA was used for PCR using gene-specific primers (Table 1), and with actin as an internal control as described previously (Eltayeb et al. 2010).
Table 1.
Sequences of the primers used for RT-PCR analysis
| Genea | Primer sequence |
|---|---|
| ARF-F | 5′-CATCATATTCCCAGGGGAGCGGCGCAGA-3′ |
| ARF-R | 5′-GCTTGGGTCTGATGGAAGGAAGGTTGCCAG-3′ |
| CIP-F | 5′-GTACGGTTGGCTCCGTCGCATTTGAGAGC-3′ |
| CIP-F | 5′-CAGCACCGAGGACAGGGATGCCATCTTC-3′ |
| PEAMT-F | 5′-GAAGCCACTCTGCTCCCGGATCATCGCGTC-3′ |
| PEAMT-R | 5′-GAAGACCGCACCGACCAGTTCCTGAGGGTC-3′ |
| actin-F | 5′-TGG ACT CTG GTG ATG GTG TC-3′ |
| actin-R | 5′-CCT CCA ATC CAA ACA CTG TA-3′ |
ARF, ETTIN-like auxin response factor; CIP, Chloroplast inositol phosphatase; PEAMT, phosphoethanolamine methyltransferase
Results
SSH cDNA library and confirmation of differential expression
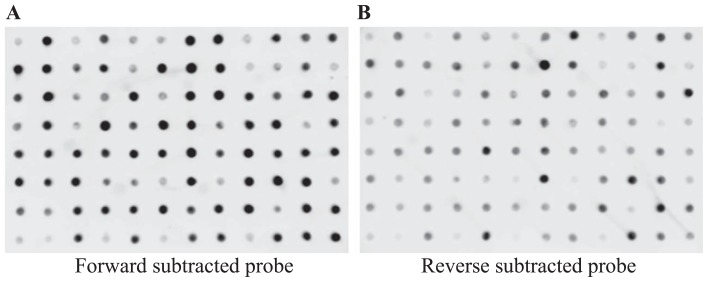
To identify important genes for osmotic stress tolerance, we performed SSH using L. mollis plants grown under normal conditions as the driver and plants grown under osmotic stress as the tester. Upon cloning of the forward-subtracted products, we obtained several hundreds of positive clones transformed with differentially expressed sequences. To confirm this differential expression, we differentially screened 96 randomly picked clones using forward-subtracted probes (drought-stressed SSH cDNAs) or reverse-subtracted probes (unstressed SSH cDNAs). Most of the clones showed strong hybridization signals when hybridized with the forward-subtracted probe (Fig. 1A) but faint or no signals with the reverse-subtracted probe (Fig. 1B). Fewer clones that hybridized with the reverse-subtracted probe may be considered background (Diatchenko et al. 1996). Clear variations observed in the intensities of the hybridization signals reflect the enrichment of the osmotic stress SSH cDNA library with various transcripts differentially up-regulated in response to osmotic stress.
Fig. 1.
Confirmation of differential expression of clones from the osmotic stress SSH cDNA library. cDNAs from the forward subtraction were randomly picked and used to prepare two identical Hybond-N+ nylon membranes. Clones were hybridized with (A) forward- or (B) reverse-subtracted probes.
Sequence analysis of the forward-subtracted ESTs
We cloned and sequenced cDNAs of the forward-subtracted library, which is enriched with differentially up-regulated sequences and potentially more related to osmotic stress tolerance. Inserts of the positive clones were verified by PCR, and clones showing a single band were sequenced. After removal of adaptor and primer sequences, the resulting high quality sequences were used for BLAST homology searches of the NCBI and TAIR databases. Total of 112 sequences with high homology to genes from other species were identified (Supplemental Table 1). All sequences of these ESTs have been submitted to the NCBI dbEST division of GenBank, with accession numbers JK317124 to JK317239.
Functional categories of the ESTs
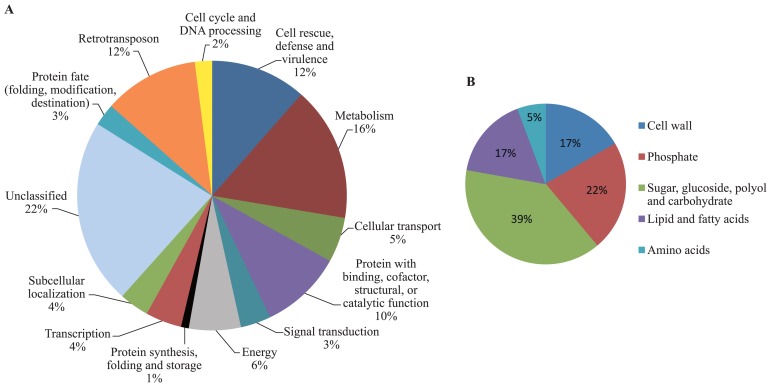
The functional categorization of ESTs was carried using the MIPS functional catalogue based on the pathway where a specific protein acts. The osmotic stress-responsive ESTs identified are involved in various cellular functions represented by 13 functional categories, including metabolism, cell rescue and defense, transcription and regulation of gene functions and transport facilitation (Fig. 2A). Of the 112 ESTs, genes involved in metabolism (16%), cell rescue and defense (stress response, detoxification and virulence, 12%), cellular transport (transport compounds, facilities and routes, 5%) and transcription (RNA synthesis, processing and modification, 4%) were highly represented classes of genes with known potential function. Twenty-two percent of the ESTs represented genes for unclassified proteins.
Fig. 2.
Functional categories of the osmotic stress-responsive ESTs. The putative function of each EST was based on the MIPS functional catalogue (http://mips.gsf.de/projects/function). (A) Main functional categories of all ESTs. (B) Metabolism functional sub-categories.
We further sub-categorized the metabolism ESTs by function (Fig. 2B). Interestingly, high representations of genes related directly to sugar, glucoside, polyol, or carbohydrate metabolism (39%), phosphate metabolism (22%) and cell wall metabolism (17%) were observed.
Diversity of drought-induced genes in L. mollis
Several genes have functions related to drought tolerance, including those encoding dehydrin (DHN; JK317151), annexin (JK317142) and late-embryogenesis-abundant (LEA) family protein (JK317130) (Supplemental Table 1). Stress-responsive genes involved in detoxification under stressful conditions include those for thioredoxin h (JK317145) and glutathione S-transferase (GST; JK317230).
In plants, several mitogen-activated protein kinases (MAPKs) are activated in response to hyperosmotic stress (Mahajan and Tuteja 2005). Genes for four L. mollis MAPKs responded to osmotic stress: histidine kinase (JK317179 and JK317233), protein kinase (JK317136) and serine/threonine protein kinase (JK317191). Osmotic stress up-regulated genes for transcription factors such as ETTIN-like auxin response factor (ARF; JK317176) and zinc finger proteins (JK317197).
Genes involved in the synthesis of JA, an important stress signaling compound, were identified, including those for lipoxygenase (LOX; JK317160 and JK317174) and allene oxide cyclase (AOC; JK317139). We also identified genes involved in the biosynthesis and metabolism of glycine betaine, a solute which protects cells from dehydration injury (Ashraf and Foolad 2007), such as those for phosphoethanol-amine methyltransferase (PEAMT; JK317166) and glycine decarboxylase (JK317169).
Osmotic stress up-regulated several genes related to the metabolism of cell walls, such as those for xyloglucan endotransglucosylase/hydrolase (XTH; JK317149) and beta-glucanase (JK317146).
Northern blot and RT-PCR analyses of some ESTs
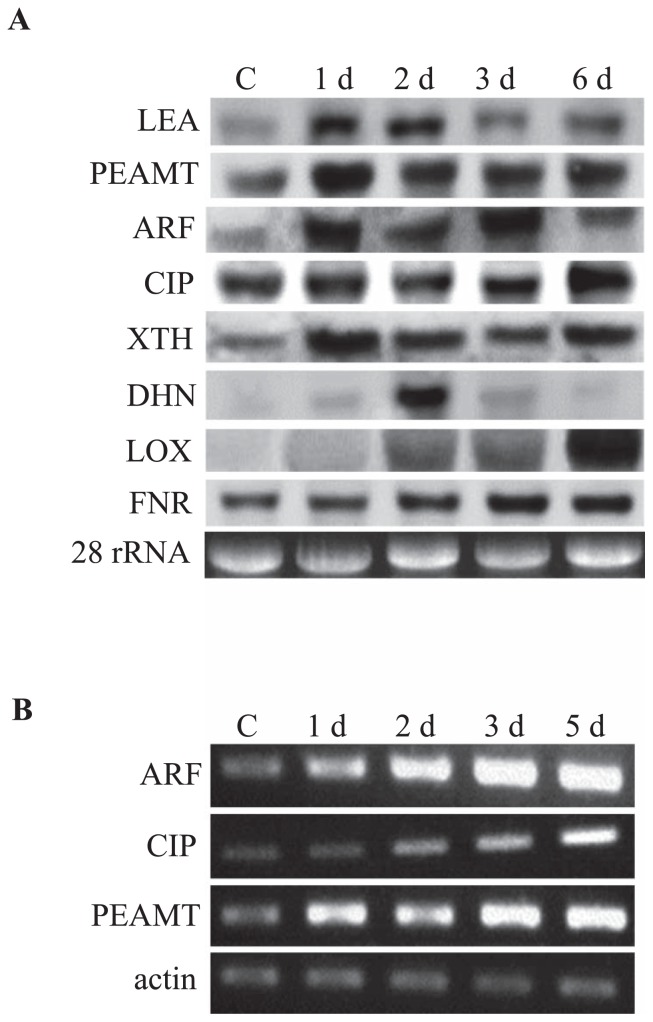
The expression patterns of ESTs representing different functional categories were verified by using northern blot and RT-PCR analyses. Northern blot analysis of eight genes confirmed their substantial induction by osmotic stress (Fig. 3A). Genes encoding LEA, PEAMT, ARF, chloroplast inositol phosphate (CIP; JK317156) and XTH were induced within 1 day of osmotic stress and those for DHN, LOX and ferredoxin-NADP(H) oxidoreductase (FNR; JK317158) were induced within 2 days (Fig. 3A). Although LEA, CIP and FNR genes showed slight expression under control conditions, their expression increased remarkably with osmotic stress. RT-PCR analysis of ARF, CIP and PEAMT confirmed their induction under osmotic stress (Fig. 3B).
Fig. 3.
Expression analyses of selected ESTs under osmotic stress. (A) Northern blot analysis. Total RNA was denatured, transferred to Hybond-N+ nylon membranes and hybridized with DIG-labeled probes. (B) Expression analysis by RT-PCR. LEA, late embryogenesis abundant family protein; PEAMT, phosphoethanolamine methyltransferase; ARF, ETTIN-like auxin response factor; CIP, chloroplast inositol phosphate; XTH, xyloglucan endotransglucosylase/hydrolase; DHN, dehydrin; LOX, lipoxygenase; FNR, ferredoxin-NADP(H) oxidoreductase. C, unstressed control; 1 d, 2 d, 3 d, 5 d and 6 d indicate days of osmotic stress.
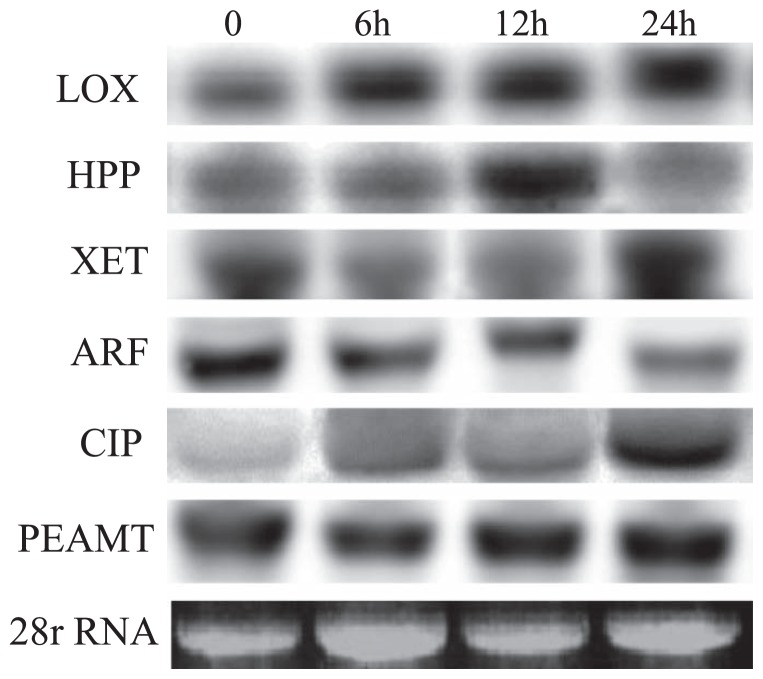
ABA is an important mediator in triggering many of the physiological and molecular adaptive responses of plants to adverse environmental conditions (Giraudat 1995). Northern blot analysis showed that ABA induced genes for LOX, CIP and PEAMT within 6 h. Although these genes were slightly expressed under control condition, their expression increased with ABA treatment. Hydroxyphenyl pyruvate (HPP) integral membrane protein (JK317159) was induced after 12 h (Fig. 4). In contrast, ABA down-regulated the gene for ARF (Fig. 4).
Fig. 4.
Northern blot analyses of selected ESTs under ABA treatment. Total RNA was denatured, transferred to Hybond-N+ nylon membranes, and hybridized with DIG-labeled probes. LOX, lipoxygenase; HPP, hydroxyphenyl pyruvate integral membrane protein; XTH, xyloglucan endotransglucosylase/hydrolase; ARF, ETTIN-like auxin response factor; CIP, chloroplast inositol phosphate; PEAMT, phosphoethanolamine methyltransferase; C, untreated control; 6 h, 12 h and 24 h indicate hours of ABA treatment.
Copy number of genes for ARF, CIP and PEAMT
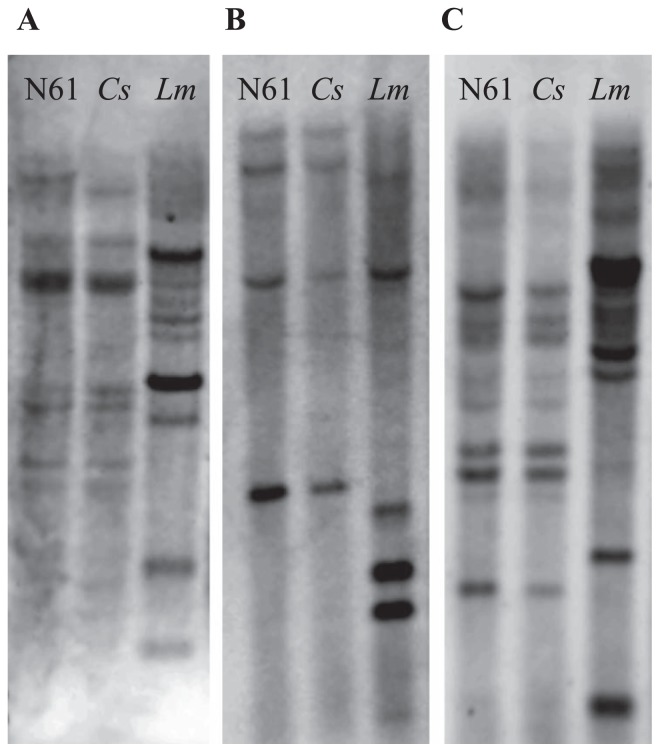
To investigate whether different copy numbers of osmotic stress-responsive genes are present in the genomes of cultivated wheat and its wild relative, we performed Southern hybridization analysis using genomic DNA isolated from Norin 61, CS and L. mollis. The genome of L. mollis had more copies of ESTs corresponding to ARF (Fig. 5A), CIP (Fig. 5B) and PEAMT (Fig. 5C) than Norin 61 and CS had.
Fig. 5.
Estimation of the copy number of genes for (A) ARF, (B) CIP and (C) PEAMT by Southern blot analysis. DNA was digested, electrophoresed, denatured, neutralized, transferred to Hybond-N+ nylon membrane and hybridized with DIG-labeled probes. ARF, ETTIN-like auxin response factor; CIP, chloroplast inositol phosphate; PEAMT, phosphoethanolamine methyltransferase.
Discussion
The tolerance of L. mollis to multiple environmental stresses has attracted the interest of many wheat breeders. The ability to cross-hybridize L. mollis and wheat allows the introduction of Leymus chromosomes into the wheat genetic background and facilitates the integration of useful traits into wheat (Forsström and Merker 2001, Wang et al. 2010). As a drought-tolerant wild relative of wheat, L. mollis represents an excellent resource for the identification of highly conserved and transferable ESTs for osmotic stress tolerance.
Under drought stress, plants operate various defense mechanisms by expressing several stress-responsive genes, through which the tolerance to drought and water deficit is enhanced (Bray 1997, Reddy et al. 2004). In this study, we used SSH technology to identify osmotic stress-responsive genes in L. mollis. The osmotic stress-responsive sequences were verified by differential expression screening (Fig. 1A, 1B), which confirmed the elimination of sequences that might be expressed equally under osmotic stress and control conditions. We identified 112 differentially expressed sequences (Supplemental Table 1) in 13 functional categories (Fig. 2A). Some of these genes may be regarded as potentially important for osmotic and drought stress tolerance and therefore could be useful to improve wheat through wide hybridization and marker assisted breeding. Moreover, up-regulation of L. mollis specific ESTs corresponding to unclassified proteins (22%) might be very useful as new genetic resources for characterization and integration into wheat.
Induction of genes involved in cell wall metabolism
Interestingly, 17% of the metabolism genes are related directly to the metabolism of cell walls (Fig. 2B), indicating their significant role in osmotic stress adaptation in L. mollis. In wild emmer wheat, genes involved in cell wall adjustment were also found to be differentially regulated in response to drought (Krugman et al. 2010). Water deficit controls tissue turgor pressure and affects the extensibility of cell walls. Plant cells respond to these changes by either loosening or tightening the wall, a phenomenon believed to be mediated at physiological, biochemical and genetic levels (Moore et al. 2008). XTH enzyme activity, which has been found to increase at the site of wall loosening (Moore et al. 2008), is believed to be part of the cell wall loosening phenomenon. This activity appears to be partly regulated by ABA (Wu et al. 1994). The gene for XTH was up-regulated within 1 day of both osmotic stress (Fig. 3A) and ABA (Fig. 4) treatment. These results agree with the induction of Capsicum annuum CaXTH3 by drought and high salinity (Cho et al. 2006). Moreover, transgenic Arabidopsis and tomato plants over-expressing CaXTH3 were much more tolerant to severe water deficit (Cho et al. 2006, Choi et al. 2011). Our results suggest the importance of XTH in enhancing osmotic stress tolerance in L. mollis.
Carbohydrate, phosphate and lipid metabolism
Thirty-nine percent of the metabolism ESTs is related to the metabolism of carbohydrates (Fig. 2B). In wild emmer wheat, 29% of the genes found to be regulated under drought were classified as involved in the metabolism of carbohydrate, lipids and catalytic activities (Krugman et al. 2011). The accumulation of water-soluble carbohydrates such as glucose, fructose and sucrose is widely regarded as an adaptive response of plants to drought stress (Xue et al. 2008). Some studies have reported changes in the expression of some genes involved in carbohydrate metabolism during drought stress (Bartels and Sunkar 2005, Bray 2002, Yamaguchi-Shinozaki and Shinozaki 2006). The high proportion of genes linked to metabolism identified here reflect the importance of maintaining proper cellular metabolism and carbohydrate pools in L. mollis to survive and adapt to osmotic stress.
Twenty-two percent of the metabolism ESTs is related to phosphate metabolism (Fig. 2B). CIP expression was induced under both osmotic stress (Fig. 3A, 3B) and ABA (Fig. 4). Inositol phosphatases (CIPs) and their turnover products have been suggested to play important roles in stress signaling in eukaryotic cells (Yang et al. 2008). Enhanced drought tolerance in transgenic plants overexpressing different types of CIPs has been reported (Perera et al. 2008, Yang et al. 2008). The induction of CIP here highlights its necessary role in providing L. mollis with tolerance to osmotic stress.
In the metabolism of lipids and fatty acids sub-category, PEAMT is involved in the synthesis of phosphatidylcholine, a precursor for the compatible osmolyte glycine betaine (GB). As major organic osmolyte, GB appears to be a critical determinant of stress tolerance and is synthesized by many plants in response to abiotic stresses (Chen and Murata 2002). PEAMT was greatly up-regulated within 1 day of osmotic stress (Fig. 3A, 3B) and ABA treatment (Fig. 4), indicating the importance of GB in enhancing osmotic stress tolerance in L. mollis.
We identified two ESTs for key genes involved in the biosynthesis of JA: those encoding LOX and AOC. JA is an important signaling molecule in plants, responding to biotic and abiotic stresses, including drought. LOX is involved in the first step of the biosynthesis of JA by the conversion of a-linolenic acid to 12-oxo-phytodienoic acid, with the sequential action of the enzymes allene oxide synthase and AOC. The EST for LOX was induced within 2 days of osmotic stress (Fig. 3A) and 6 h after ABA treatment (Fig. 4), reflecting the importance of the JA signaling pathway in alleviating osmotic stress in L. mollis.
Up-regulation of genes involved in cell rescue and signal transduction
ESTs related to cell rescue and defense functions represented 12% of all ESTs. Drought stress leads to several changes in plant cells, including the expression of LEA/dehydrin-type genes and the activation of enzymes involved in the production and removal of ROS. We identified ESTs corresponding to DHN, LEA protein, GST and thioredoxin h (Supplemental Table 1). Osmotic stress induced DHN EST expression (Fig. 3A). LEA proteins operate in whole-plant tolerance to drought, cold and other environmental stresses (Hong-Bo et al. 2005). Overexpression of a barley group 3 LEA gene, HVA1, in wheat improved the tolerance to osmotic stress and enhanced recovery after drought and salinity stresses (Sivamani et al. 2000). GST is involved in the detoxification of ROS and thioredoxin h in cell redox regulation under drought stress.
In the signal transduction category, we identified four MAPKs: histidine kinase, protein kinase and serine/threo-nine protein kinase. MAPKs respond to extracellular stimuli such as osmotic stress, regulate various cellular activities and gene expression and coordinate a variety of response patterns (Pearson et al. 2001). The identification of MAPKs correlates with the up-regulation of the LEA/dehydrin-type genes, GST and thioredoxin. Increased transcription of genes for MAPKs, particularly histidine kinase and MAPK kinase, results ultimately in the accumulation of osmolytes that help to re-establish the osmotic balance and protect against stress damage, or in the induction of repair mechanisms by the induction of LEA/dehydrin-type stress genes (Mahajan and Tuteja 2005, Torres and Forman 2003).
Induction of cellular transport genes
EST for ABC transport protein (JK317155) was up-regulated. ABC transporters participate in chlorophyll biosynthesis, stomatal movement, ion influx and detoxification processes (Martinoia et al. 2002). We also identified an EST for ferredoxin-NADP(H) oxidoreductase (FNR; JK317158), which catalyzes the final step in the photosynthetic linear electron transfer chain in chloroplasts (Grzyb et al. 2008). FNR transports electrons from reduced ferredoxin (Fd) to NADP+ to form NADPH. The expression of the FNR EST increased with increasing osmotic stress (Fig. 3A), possibly reflecting its importance in regulating the NADP+/NADPH ratio and the redox state of ferredoxin under osmotic stress conditions in L. mollis.
Transcriptional regulation in response to drought
Transcription factors (TFs) are critical regulators of gene expression and environmental stress responses. They play an essential role in the signal transduction network that leads from the perception of stress signals to the expression of stress-responsive genes (Hussain et al. 2011). Four percent of the ESTs were classified as transcription factors, and include members of the auxin response and zinc finger. Transcriptional factors were also found to be up-regulated in response to drought in wild emmer wheat (Ergen et al. 2009). ARF was up-regulated under osmotic stress (Fig. 3A, 3B) but down regulated by ABA (Fig. 4). High auxin activity is correlated with a high growth rate; therefore, specific down regulation of shoot growth as an adaptive mechanism to stress involves a decrease in leaf auxin concentration, or in auxin signaling, to minimize water loss (Popko et al. 2010). These results indicate that an interaction between auxin and ABA is necessary to achieve the optimal physiological status for osmotic stress adaptation in L. mollis.
Variation in the copy number of ARF, CIP and PEAMT
One way to increase the expression of a gene is to simply increase its copy number (Knox et al. 2010). Southern blot analysis revealed a variation in the copy number of ARF, CIP and PEAMT between L. mollis and the wheat genotypes Norin 62 and CS (Fig. 5). The L. mollis genome showed up to 9 bands corresponding to ARF (Fig. 5A), 6 to CIP (Fig. 5B) and 10 to PEAMT (Fig. 5C), compared to 7, 4 and 8 bands, respectively, in the genomes of both N62 and CS. These variations suggest fewer copy numbers in Norin 61 and CS, which might affect their osmotic stress tolerance. Similarly, Knox et al. (2010) reported that the C-repeat binding factor (CBF), which regulates freezing tolerance, occurs in greater copy numbers in winter wheat than in spring wheat. A significant correlation between the mean tolerance to salt stress and the DREB1A copy number was found in transgenic potato plants (Behnam et al. 2006).
Importance of Leymus ESTs in wheat breeding
The genetic diversity that could be utilized in improving cultivated wheat could be expanded and captured through several approaches, including genetic transformation, mutation breeding, the use of synthetic polyploids and wide hybridization. In particular, the introduction of alien chromosomes through wide hybridization offers great opportunities for enriching the wheat gene pool. Although several wheat–Leymus chromosome addition lines have been produced, genetic research in this area has been encumbered by a lack of functional and conserved gene marker sequences (Larson et al. 2011). Leymus ESTs identified here could serve as useful markers for the identification of Leymus chromosomes introduced into wheat. They provide a valuable resource for the development of EST-derived molecular markers that correspond to genes associated with osmotic stress tolerance, and will be valuable for facilitating efficient marker-assisted breeding and map-based cloning. Furthermore, being conserved and transferable to wheat, these ESTs could be very useful to increase the copy number of important genes linked with osmotic stress tolerance.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Global Center of Excellence for Dryland Science of the Arid Land Research Center, Tottori University.
Literature Cited
- Allen C.D., Macalady A.K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., Kitzberger T., Rigling A., Breshears D.D., Hogg E.H., et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259: 660–684 [Google Scholar]
- Aprile A., Mastrangelo A.M., De Leonardis A.M., Galiba G., Roncaglia E., Ferrari F., De Bellis L., Turchi L., Giuliano G., Cattivelli L. (2009) Transcriptional profiling in response to terminal drought stress reveals differential responses along the wheat genome. BMC Genomics 10: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptekar R., Rejmánek M. (2000) The effect of sea-water submergence on rhizome bud viability of the introduced Ammophila arenaria and the native Leymus mollis in California. J. Coastal Conserv. 6: 107–111 [Google Scholar]
- Ashraf M. (2010) Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 28: 169–183 [DOI] [PubMed] [Google Scholar]
- Ashraf M., Foolad M.R. (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exper. Bot. 206–216 [Google Scholar]
- Bartels D., Sunkar R. (2005) Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 24: 23–58 [Google Scholar]
- Behnam B., Kikuchi A., Celebi-Toprak F., Yamanaka S., Kasuga M., Yamaguchi-Shinozaki K., Watanabe K.N. (2006) The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasomic tetraploid potato (Solanum tuberosum). Plant Biotechnol. 23: 169–177 [Google Scholar]
- Bray E.A. (1997) Plant responses to water deficit. Trends Plant Sci. 2: 48–54 [Google Scholar]
- Bray E.A. (2002) Classification of genes differentially expressed during water-deficit stress in Arabidopsis thaliana: an analysis using microarray and differential expression data. Ann. Bot. 89: 803– 811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.H.H., Murata N. (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 5: 250–257 [DOI] [PubMed] [Google Scholar]
- Cho S.K., Kim J.E., Park J.A., Eom T.J., Kim W.T. (2006) Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 580: 3136–3144 [DOI] [PubMed] [Google Scholar]
- Choi J.Y., Seo Y.S., Kim S.J., Kim W.T., Shin J.S. (2011) Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/ hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 30: 867–877 [DOI] [PubMed] [Google Scholar]
- Cordeiro G.M., Casu R., McIntyre C.L., Manners J.M., Henry R.J. (2001) Microsatellite markers from sugarcane (Saccharum spp.) ESTs cross transferable to erianthus and sorghum. Plant Sci. 160: 1115–1123 [DOI] [PubMed] [Google Scholar]
- Diatchenko L., Lau Y.F.C., Campbell A.P., Chenchik A., Moqadam F., Huang B., Lukyanov K., Gurskaya N., Sverdlov E.D., Siebert P.D. (1996) Suppression subtractive hybridization: a method of generating differentially regulated or tissue-specific cDNA probes and libraries. Proc. Natl. Acad. Sci. USA 93: 6025–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltayeb A.E., Kawano N., Badawi G.H., Kaminaka H., Sanekata T., Shibahara T., Inanaga S., Tanaka K. (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225: 1255–1264 [DOI] [PubMed] [Google Scholar]
- Eltayeb A.E., Yamamoto S., Habora M.E.E., Matsukubo Y., Aono M., Tsujimoto H., Tanaka K. (2010) Greater protection against oxidative damages imposed by various environmental stresses in transgenic potato with higher level of reduced glutathione. Breed. Sci. 60: 101–109 [Google Scholar]
- Ergen N.Z., Thimmapuram J., Bohnert H.J., Budak H. (2009) Transcriptome pathways unique to dehydration tolerant relatives of modern wheat. Funct. Integr. Genomics 9: 377–396 [DOI] [PubMed] [Google Scholar]
- Faith A.M.B. (1983) Analysis of the breeding potential of wheat-Agropyron and wheat-Elymus derivatives. I. Agronomic and quality characteristics. Hereditas 98: 287–295 [DOI] [PubMed] [Google Scholar]
- Fan X., Sha L.N., Yang R.W., Zhang H.Q., Kang H.Y., Ding C.B., Zhang L., Zheng Y.L., Zhou Y.H. (2009) Phylogeny and evolutionary history of Leymus (Triticeae; Poaceae) based on a single-copy nuclear gene encoding plastid acetyl-CoA carboxylase. BMC Evol. Biol. 9: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsström P.O., Merker A. (2001) Sources of wheat powdery mildew resistance from wheat-rye and wheat-Leymus hybrids. Hereditas 134: 115–119 [DOI] [PubMed] [Google Scholar]
- Gagné J.M., Houle G. (2002) Factors responsible for Honckenya peploides (Caryophyllaceae) and Leymus mollis (Poaceae). Am. J. Bot. 3: 479–485 [DOI] [PubMed] [Google Scholar]
- Giraudat J. (1995) Abscisic acid signaling. Curr. Opin. Cell Biol. 7: 232–238 [DOI] [PubMed] [Google Scholar]
- Grzyb J., Malec P., Rumak I., Garstka M., Strzalka K. (2008) Two isoforms of ferredoxin:NADP+ oxidoreductase from wheat leaves: purification and initial biochemical characterization. Photosynth. Res. 96: 99–112 [DOI] [PubMed] [Google Scholar]
- Hong-Bo S., Zong-Suo L., Ming-An S. (2005) LEA proteins in higher plants: Structure, function, gene expression and regulation. Colloids Surf. B. 45: 131–135 [DOI] [PubMed] [Google Scholar]
- Hussain S.S., Kayano M.A., Amjad M. (2011) Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol. Progr. 27: 297–306 [DOI] [PubMed] [Google Scholar]
- IPCC(2007) Climate change 2007. The physical science basis. In: Solomon, S., Qin D., Manning M., Chen Z., Marquis M., Averyt K.B., Tignor M., Miller H.L. (eds.) Contribution of Working Group I to the Fourth Assessment. Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom/New York, NY, USA, p. 996 [Google Scholar]
- Kishii M., Wang R.R.C., Tsujimoto H. (2003) Characteristics and behaviour of the chromosomes of Leymus mollis and L. racemosus (Triticeae, Poaceae) during mitosis and meiosis. Chromo. Res. 11: 741–748 [DOI] [PubMed] [Google Scholar]
- Knox A.K., Dhillon T., Cheng H., Tondelli A., Pecchioni N., Stockinger E.J. (2010) CBF gene copy number variation at Frost Resistance-2 is associated with levels of freezing tolerance in temperate-climate cereals. Theor. Appl. Genet. 121: 21–35 [DOI] [PubMed] [Google Scholar]
- Krugman T., Chagué V., Peleg Z., Balzergue S., Just J., Korol A.B., Nevo E., Saranga Y., Chalhoub B., Fahima T. (2010) Multilevel regulation and signalling processes associated with adaptation to terminal drought in wild emmer wheat. Funct. Integr. Genomics 10: 167–186 [DOI] [PubMed] [Google Scholar]
- Krugman T., Peleg Z., Quansah L., Chagué V., Korol A.B., Nevo E., Saranga Y., Fait A., Chalhoub B., Fahima T. (2011) Alteration in expression of hormone-related genes in wild emmer wheat roots associated with drought adaptation mechanisms. Funct. Integr. Genomics 11: 565–583 [DOI] [PubMed] [Google Scholar]
- Larson S.R., Kishii M., Tsujimoto H., Qi L., Chen P., Lazo G.R., Jensen K.B., Wang R.R. (2011) Leymus EST linkage maps identify 4NsL–5NsL reciprocal translocation, wheat-Leymus chromosome introgressions, and functionally important gene loci. Theor. Appl. Genet. 124: 189–206 [DOI] [PubMed] [Google Scholar]
- Mahajan S., Tuteja N. (2005) Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444: 139–158 [DOI] [PubMed] [Google Scholar]
- Martinoia E., Klein M., Geisler M., Bovet L., Forestier C., Kolukisaoglu U., Muller-Rober B., Schulz B. (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214: 345–355 [DOI] [PubMed] [Google Scholar]
- McGuire P.E., Dvorak J. (1981) High salt-tolerance potential in wheatgrasses. Crop Sci. 21: 702–705 [Google Scholar]
- Moore J.B., Vicre-Gibouin M., Farrant J.M., Driouich A. (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol. Plant. 134: 237–245 [DOI] [PubMed] [Google Scholar]
- Mujeeb-Kazi A., Bernard M., Bekele G.T., Mirand J.L. (1983) In-corporation of alien genetic information from Elymus giganteus into Triticum aestivum. In: Sakamoto, S. (ed.) Proceedings of the 6th International Wheat Genetic Symposium, Maruzen, Kyoto, pp. 223–231 [Google Scholar]
- Pearson G., Robinson F., Gibson T.B., Xu B.E., Karandikar M., Berman K., Cobb M.H. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Perera Y.I., Hung C.-Y., Moore C.D., Stevenson-Paulik J., Bossa W.F. (2008) Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell 20: 2876–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko J., Hansch R., Mendel R.R., Polle A., Teichmann T. (2010) The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 12: 242–258 [DOI] [PubMed] [Google Scholar]
- Rajaram S. (2001) Prospects and promise of wheat breeding in the 21st century. Euphytica 119: 3–15 [Google Scholar]
- Reddy A.R., Chaitanya K.V., Vivekanandan M. (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 161: 1189–1202 [DOI] [PubMed] [Google Scholar]
- Reddy P.C., Sairanganayakulu G., Thippeswamy M., Reddy P.S., Reddy K.M., Sudhakar C. (2008) Identification of stress-induced genes from the drought tolerant semi-arid legume crop horsegram (Macrotyloma uniflorum (Lam.) Verdc.) through analysis of subtracted expressed sequence tags. Plant Sci. 175: 372–384 [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, p7.52 [Google Scholar]
- Sivamani E., Bahieldin A., Wraith J.M., Al-Niemi T., Dyer W.E., Ho T.H.D., Qu R. (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 155: 1–9 [DOI] [PubMed] [Google Scholar]
- Taylor C., Madsen K., Borg S., Moller M.G., Boelt B., Holm P.B. (2001) The development of sequence-tagged sites (STSs) in Lolium perenne L.: the application of primer sets derived from other genera. Theor. Appl. Genet. 103: 648–658 [Google Scholar]
- Thiel T., Michalek W., Varshney R.K., Graner A. (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 106: 411–422 [DOI] [PubMed] [Google Scholar]
- Torres M., Forman H.J. (2003) Redox signaling and the MAP kinase pathways. Biofactors 17: 287–296 [DOI] [PubMed] [Google Scholar]
- Wang S., Yin L., Tanaka H., Tanaka K., Tsujimoto H. (2010) Identification of wheat alien chromosome addition lines for breeding wheat with high phosphorus efficiency. Breed. Sci. 60: 371–379 [Google Scholar]
- Wu Y., Spollen W.G., Sharp R.E., Hetherington P.R., Fry S.C. (1994) Root growth maintenance at low water potentials. Increased activity of xyloglucan endotransglycolase and its possible regulation by abscisic acid. Plant Physiol. 106: 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.-P., McIntyre C.L., Glassop D., Shorter R. (2008) Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol. Biol. 67: 197– 214 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57: 781–803 [DOI] [PubMed] [Google Scholar]
- Yang L., Tang R., Zhu J., Liu H., Mueller-Roeber B., Xia H., Zhang H. (2008) Enhancement of stress tolerance in transgenic tobacco plants constitutively expressing AtIpk2β, an inositol poly-phosphate 6-/3-kinase from Arabidopsis thaliana. Plant Mol. Biol. 66: 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.K., Dake T.M., Singh S., Benscher D., Li W., Gill B., Sorrells M.E. (2004) Development and mapping of EST-derived simple sequence repeat markers for hexaploid wheat. Genome 47: 805–818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.