Abstract
To reveal varietally differing glucosinolate (GSL) contents in radish (Raphanus sativus L.) cultivated in Japan, the total and individual GSLs of 28 cultivars were analyzed using high-performance liquid chromatography. In these cultivars, GSL types including three aliphatic GSLs (glucoraphenin, glucoerucin, and 4-methylthio-3-butenyl GSL (4MTB-GSL)) and three indolyl GSLs (4-hydroxyglucobrassicin, glucobrassicin, and 4-methoxy-glucobrassicin) were detected. No cultivar-specific type of GSL was identified. The dominant GSL was 4MTB-GSL, but its contents differed remarkably: 8.6 μmol/g in ‘Koushin’ to 135.7 μmol/g in ‘Karami 199’. Over about 90% of all GSLs in Japanese radish type are 4MTB-GSL, a higher percentage than in Chinese or European garden radish cultivars. A simple, rapid method for estimating total GSL contents in crude extracts was established because of the small variation of glucosinolate composition in Japanese cultivars. The total GSL content can be estimated using an equation for prediction with absorbance at 425 nm in a mixture of GSL crude extract and palladium (II) chloride solution: Total GSL (μmol/g) = 305.47 × A425 − 29.66. Its coefficient of determination (R2) and standard error of prediction (SEP) are 0.968 and 8.052. This method enables total GSL content estimation from more than 200 samples per person per day.
Keywords: colorimetric quantification, pungency, GSL component, varietal difference
Introduction
Plant tissues of Brassicaceae, including radish, contain glucosinolates (GSLs), which are thioglucosides in secondary metabolites. More than 120 types of GSLs having different substituents (R) have been identified. By disruption of cells, GSLs are hydrolyzed with inherent myrosinase (EC3.2.1.147), resulting in production of isothiocyanates (ITCs), which are pungent components that are characteristic of Brassicaceae plants (Fahey et al. 2001, Mithen et al. 2000).
The major GSL in radish, which is an important vegetable in Japan, is 4-methylthio-3-butenyl glucosinolate (4MTB-GSL). Radishes also contain small amounts of indolyl GSLs and other aliphatic GSLs (Montaut et al. 2010). 4MTB-GSL and glucoraphenin are hydrolyzed respectively by myrosinase into 4-methylthio-3-butenyl isothiocyanate (4MTB-ITC) and 4-methylsulfinyl-3-butenyl isothiocyanate (4MSB-ITC). 4MTB-ITC, a pungent ingredient that is specific for radish (Friis and Kiaer 1966), strongly influences the taste of grated fresh radish and radish salad. The breakdown of 4MTB-ITC by reaction with water produces a yellow pigment and a flavor component that is responsible for the color and flavor of traditional Japanese pickles known as “Takuan” (Ozawa et al. 1990a, 1990b). Reportedly, 4MTB-ITC has antimutagenicity (Nakamura et al. 2001, Uda et al. 2000) and anticarcinogenicity (Yamasaki et al. 2009). Its anticarcinogenicity reportedly results from selective cytotoxicity and apoptotic activity (Barillari et al. 2007, Papi et al. 2008). ITCs derived from other GSLs might share this functionality. Therefore, it is important to elucidate the variation of GSLs, relative contents of each GSL, composition ratios and the total amounts of GSLs in radish roots.
As a method for analysis of GSLs in radish roots, the desulfation method is commonly used: it is high-performance liquid chromatography (HPLC) analysis of desulfo-GSL obtained by desulfation of extracted GSLs with sulfatase (Bjerg and Sørensen 1987, Bjorkqvist and Hase 1988). The ion-pair method has also been developed, by which extracted GSLs are analyzed directly using HPLC with a reverse-phase column using an ion-pair reagent without desulfation (Mellon et al. 2002, Rangkadilok et al. 2002). These methods provide reliable quantitative data and information related to the GSL variation, but they require much time and labor for analysis and special equipment. Therefore, a simple method for quantitative analysis of GSLs is necessary for the fields of plant breeding and food processing.
Varietal differences of GSL contents in radish roots produced in Japan have been reported by Carlson et al. (1985) and Ishii et al. (1989). However, the cultivars they used are landraces propagated by open pollination, which are regarded as having high variation in many genetic traits within a cultivar and which are not cultivated at present. No information related to current cultivars is available. In this study, we performed profiling of GSL compositions and GSL contents in radish cultivars grown in Japan. Furthermore, we developed a simple and rapid method for quantitative analysis of the total GSLs using the palladium colorimetric method.
Materials and Methods
Plant materials and cultivation methods
The 28 cultivars of radish (Raphanus sativus L.) presented in Table 1 were used for comparison of GSL contents using HPLC. All are commercial varieties. Based on geneology, cultivar names and breeder information, these cultivars were classified into eight cultivar groups of four types. They included 14 cultivars of the Japanese common radish type, which include five cultivars of the Green neck group like Miyashige, one cultivar of the Miyashige group, one cultivar of the Shougoin group and seven cultivars of the Nerima group. Four cultivars of the Japanese pungent radish type, four cultivars of the Chinese radish type, which are two cultivars of the Northern China group and two cultivars of the Southern China group and six cultivars of the European garden radish type were also used.
Table 1.
Glucosinolate contents in roots of 28 radish cultivars
| Cultivar | Seed propagation systema | Year | Glucosinolate (μmol/g) | % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Aliphatic GSL | Total indolyl GSL | Total GSL | 4MTB-GSL/Total GSL | Aliphatic GSL/Total GSL | Indolyl GSL/total GSL | |||||
|
| ||||||||||
| Glucoraphenin | Glucoerucin | 4MTB-GSL | ||||||||
| Japanese common radish type | ||||||||||
| Green neck group | ||||||||||
| Taibyou-sobutori | F1 | 2005 | 1.8 ± 0.5 | 0.2 ± 0.2 | 44.1 ± 0.7 | 0.6 ± 0.2 | 46.8 ± 0.8 | 94.4 ± 0.9 | 98.8 ± 0.4 | 1.2 ± 0.4 |
| 2006 | 2.5 ± 0.9 | 0.5 ± 0.1 | 58.6 ± 5.6 | 0.8 ± 0.1 | 62.5 ± 5.9 | 93.8 ± 1.2 | 98.7 ± 0.3 | 1.3 ± 0.3 | ||
| 2008 | 0.7 ± 0.4 | n.d. | 36.8 ± 3.6 | 0.5 ± 0.1 | 38.1 ± 3.9 | 96.7 ± 0.7 | 98.6 ± 0.3 | 1.4 ± 0.3 | ||
| 2009 | 0.8 ± 0.4 | n.d. | 36.1 ± 2.9 | 0.7 ± 0.1 | 37.6 ± 3.2 | 96.1 ± 0.9 | 98.3 ± 0.1 | 1.7 ± 0.1 | ||
| YR Tengu | F1 | 2005 | 1.0 ± 0.3 | 0.4 ± 0.3 | 53.6 ± 10.8 | 0.7 ± 0.1 | 55.8 ± 11.3 | 96.1 ± 0.2 | 98.7 ± 0.2 | 1.3 ± 0.2 |
| 2008 | 1.0 ± 0.4 | n.d. | 33.6 ± 4.3 | 0.7 ± 0.1 | 35.3 ± 4.2 | 95.2 ± 1.4 | 98.1 ± 0.3 | 1.9 ± 0.3 | ||
| YR Kurama | F1 | 2009 | 1.9 ± 0.7 | 0.1 ± 0.2 | 29.6 ± 3.2 | 0.9 ± 0.1 | 32.4 ± 2.6 | 91.0 ± 2.9 | 97.2 ± 0.4 | 2.8 ± 0.4 |
| Oroshi | F1 | 2005 | 3.4 ± 0.4 | 1.5 ± 0.1 | 75.2 ± 5.8 | 1.7 ± 0.1 | 81.7 ± 5.7 | 92.0 ± 0.8 | 97.9 ± 0.1 | 2.1 ± 0.1 |
| Kenka 31 | F1 | 2005 | 1.6 ± 0.4 | 0.3 ± 0.4 | 44.9 ± 6.9 | 0.8 ± 0.3 | 47.7 ± 7.0 | 94.0 ± 1.4 | 98.3 ± 0.9 | 1.9 ± 0.9 |
| Miyashige group | ||||||||||
| Miyashige | OP | 2008 | 1.7 ± 0.6 | 0.1 ± 0.2 | 30.0 ± 10.6 | 0.4 ± 0.1 | 32.1 ± 11.0 | 92.9 ± 1.7 | 98.6 ± 0.6 | 1.4 ± 0.6 |
| Shougoin group | ||||||||||
| Hayabutori-shougoin | F1 | 2005 | 0.9 ± 0.2 | 0.3 ± 0.4 | 53.1 ± 6.3 | 0.7 ± 0.1 | 55.0 ± 7.0 | 96.7 ± 0.8 | 98.8 ± 0.0 | 1.2 ± 0.0 |
| 2009 | 2.2 ± 0.6 | 0.5 ± 0.1 | 41.4 ± 8.2 | 0.8 ± 0.1 | 44.9 ± 7.9 | 92.0 ± 2.2 | 98.2 ± 0.4 | 1.8 ± 0.4 | ||
| Nerima group | ||||||||||
| Nishimachi-risou | OP | 2005 | 0.8 ± 0.2 | 1.0 ± 0.9 | 57.7 ± 12.9 | 0.4 ± 0.1 | 60.0 ± 13.0 | 96.2 ± 1.9 | 99.2 ± 0.3 | 0.8 ± 0.3 |
| 2008 | 0.2 ± 0.3 | 1.1 ± 0.4 | 57.5 ± 12.1 | 0.6 ± 0.2 | 59.4 ± 11.8 | 96.7 ± 1.6 | 99.9 ± 0.6 | 1.0 ± 0.6 | ||
| 2009 | 0.6 ± 0.7 | 0.7 ± 0.2 | 52.7 ± 10.7 | 0.4 ± 0.2 | 54.4 ± 10.5 | 96.8 ± 1.4 | 99.2 ± 0.5 | 0.8 ± 0.5 | ||
| Miura | OP | 2008 | 0.8 ± 0.2 | 0.7 ± 0.2 | 54.7 ± 5.3 | 0.8 ± 0.1 | 57.0 ± 5.5 | 96.0 ± 0.3 | 98.7 ± 0.2 | 1.3 ± 0.2 |
| Hayabutori-ookura | F1 | 2009 | 1.3 ± 0.3 | n.d. | 41.9 ± 5.5 | 0.9 ± 0.2 | 44.0 ± 5.2 | 95.0 ± 1.4 | 97.9 ± 0.6 | 2.1 ± 0.6 |
| Fuyudori-ookura | F1 | 2009 | 0.6 ± 0.1 | n.d. | 30.3 ± 3.3 | 0.5 ± 0.1 | 31.5 ± 3.2 | 96.2 ± 0.8 | 98.3 ± 0.5 | 1.8 ± 0.5 |
| Akimasari 2 | F1 | 2008 | 1.4 ± 0.7 | 0.4 ± 0.3 | 51.3 ± 6.3 | 0.7 ± 0.3 | 53.8 ± 7.0 | 95.5 ± 0.9 | 98.7 ± 0.6 | 1.3 ± 0.6 |
| 2009 | 1.1 ± 0.7 | 0.0 ± 0.0 | 43.9 ± 8.8 | 0.8 ± 0.1 | 45.8 ± 8.8 | 95.7 ± 1.7 | 98.2 ± 0.3 | 1.8 ± 0.3 | ||
| Shinhatusuu | F1 | 2008 | 1.3 ± 0.5 | 0.4 ± 0.5 | 33.7 ± 9.3 | 0.8 ± 0.3 | 36.2 ± 10.3 | 93.4 ± 1.2 | 97.9 ± 0.6 | 2.1 ± 0.6 |
| Kogarashi | F1 | 2008 | 1.9 ± 0.9 | 0.6 ± 0.1 | 64.6 ± 14.6 | 1.0 ± 0.2 | 68.2 ± 14.2 | 94.5 ± 2.1 | 98.4 ± 0.6 | 1.6 ± 0.6 |
|
| ||||||||||
| Japanese pungent radish type | ||||||||||
| Karami 199 | F1 | 2005 | 5.7 ± 0.5 | 1.5 ± 0.3 | 135.7 ± 12.7 | 2.5 ± 0.4 | 145.5 ± 12.9 | 93.3 ± 0.6 | 98.3 ± 0.3 | 1.7 ± 0.3 |
| 2006 | 6.6 ± 0.9 | 1.4 ± 0.1 | 134.9 ± 8.9 | 3.0 ± 0.3 | 145.9 ± 9.9 | 92.5 ± 0.4 | 97.9 ± 0.2 | 2.1 ± 0.2 | ||
| 2008 | 7.5 ± 1.5 | 0.8 ± 0.3 | 113.4 ± 3.4 | 1.9 ± 0.3 | 123.7 ± 4.4 | 91.7 ± 0.9 | 98.4 ± 0.3 | 1.6 ± 0.3 | ||
| 2009 | 7.1 ± 0.6 | 0.9 ± 0.2 | 91.7 ± 14.4 | 2.3 ± 0.7 | 102.0 ± 15.3 | 89.9 ± 0.8 | 97.7 ± 0.7 | 2.3 ± 0.7 | ||
| Karaine | F1 | 2005 | 3.5 ± 0.3 | 1.8 ± 0.7 | 122.6 ± 16.5 | 2.1 ± 0.4 | 130.0 ± 16.8 | 94.3 ± 0.5 | 98.4 ± 0.2 | 1.6 ± 0.2 |
| 2008 | 6.7 ± 2.7 | 0.8 ± 0.2 | 83.3 ± 15.4 | 1.2 ± 0.3 | 92.0 ± 13.9 | 90.2 ± 4.7 | 98.6 ± 0.4 | 1.4 ± 0.4 | ||
| Karaine Red | F1 | 2005 | 2.3 ± 0.6 | 2.4 ± 0.1 | 117.9 ± 4.4 | 2.0 ± 0.1 | 124.7 ± 5.0 | 94.6 ± 0.3 | 98.4 ± 0.1 | 1.6 ± 0.1 |
| 2008 | 5.1 ± 1.5 | 1.4 ± 0.3 | 78.2 ± 10.6 | 2.0 ± 0.1 | 86.7 ± 10.9 | 90.2 ± 1.9 | 97.7 ± 0.3 | 2.3 ± 0.3 | ||
| Karamaru | F1 | 2005 | 5.8 ± 0.6 | 0.3 ± 0.3 | 62.2 ± 6.7 | 1.8 ± 0.2 | 70.1 ± 7.6 | 88.8 ± 0.7 | 97.4 ± 0.2 | 2.6 ± 0.2 |
| 2006 | 6.1 ± 0.8 | 0.7 ± 0.1 | 92.8 ± 8.3 | 1.8 ± 0.1 | 101.4 ± 8.6 | 91.5 ± 0.8 | 98.2 ± 0.2 | 1.8 ± 0.2 | ||
|
| ||||||||||
| Chinese radish type | ||||||||||
| Northern China group | ||||||||||
| Koushin | OP | 2008 | 1.9 ± 1.1 | n.d. | 8.6 ± 0.9 | 1.0 ± 0.3 | 11.5 ± 1.7 | 75.4 ± 7.7 | 91.1 ± 1.7 | 8.9 ± 1.7 |
| 2009 | 3.9 ± 1.3 | n.d. | 12.5 ± 1.1 | 1.5 ± 0.2 | 17.9 ± 1.5 | 70.0 ± 6.8 | 91.6 ± 1.3 | 8.4 ± 1.3 | ||
| Tenan-koushin | OP | 2005 | 1.5 ± 0.0 | 0.7 ± 0.0 | 40.0 ± 0.1 | 0.8 ± 0.0 | 42.9 ± 0.1 | 93.1 ± 0.0 | 98.1 ± 0.0 | 1.9 ± 0.0 |
| Southern China group | ||||||||||
| Everest | F1 | 2005 | 1.7 ± 0.3 | 0.3 ± 0.3 | 36.0 ± 5.0 | 5.0 ± 0.1 | 38.9 ± 5.6 | 92.7 ± 1.4 | 97.8 ± 0.2 | 2.3 ± 0.2 |
| Whitestick | OP | 2005 | 1.6 ± 0.1 | n.d. | 29.9 ± 2.7 | 1.1 ± 0.1 | 32.6 ± 2.6 | 91.6 ± 1.1 | 96.5 ± 0.6 | 3.5 ± 0.6 |
|
| ||||||||||
| European garden radish type | ||||||||||
| Scarlett 20 | OP | 2005 | 2.1 ± 0.4 | 0.9 ± 0.1 | 20.1 ± 5.2 | 2.0 ± 0.2 | 25.1 ± 5.5 | 79.8 ± 3.0 | 91.9 ± 2.0 | 8.1 ± 2.0 |
| 2006 | 1.7 ± 1.0 | n.d. | 26.8 ± 8.2 | 1.9 ± 0.6 | 30.3 ± 9.5 | 88.5 ± 2.6 | 93.6 ± 1.9 | 6.4 ± 1.9 | ||
| Comet | OP | 2005 | 3.0 ± 0.7 | 0.7 ± 0.1 | 15.9 ± 3.7 | 2.3 ± 0.4 | 21.9 ± 3.8 | 72.2 ± 4.5 | 89.7 ± 1.2 | 10.3 ± 1.2 |
| French Breakfast | OP | 2005 | 1.9 ± 0.4 | 0.6 ± 0.4 | 26.1 ± 4.1 | 1.5 ± 0.3 | 30.0 ± 4.6 | 86.9 ± 0.8 | 95.0 ± 0.6 | 5.0 ± 0.6 |
| White Icicle | OP | 2005 | 0.3 ± 0.4 | n.d. | 17.3 ± 2.8 | 0.7 ± 0.1 | 18.3 ± 3.0 | 94.5 ± 1.4 | 96.1 ± 0.6 | 3.9 ± 0.6 |
| Isabel | F1 | 2006 | 1.6 ± 0.4 | n.d. | 40.6 ± 8.1 | 2.0 ± 0.4 | 44.1 ± 7.5 | 91.5 ± 3.2 | 95.3 ± 1.8 | 4.7 ± 1.8 |
| Linda Red | F1 | 2006 | 2.7 ± 0.7 | n.d. | 41.9 ± 8.8 | 2.7 ± 0.1 | 47.4 ± 9.1 | 88.3 ± 2.4 | 94.2 ± 1.3 | 5.8 ± 1.3 |
Data are presented as a means ± SD.
F1; Hybrid cultivar, OP; Open pollinated cultivar
n.d.; not detected
To examine the palladium colorimetric method for quantifying the total GSLs, 136 samples were prepared from 66 plants of 5 commercial cultivars, i.e., cv. ‘Taibyousobutori’, ‘Akimasari 2’, ‘Shinhatusuu’, ‘Nishimachi-risou’ and ‘Karami 199’ and 13 breeding lines derived from these five commercial cultivars.
Plants were grown in the field of NARO Institute of Vegetable and Tea Science, Tsu, Japan from the beginning of September through November in 2005, 2006, 2008 and 2009 using conventional culture methods. The basal fertilizer contained 80 kg/ha N, 80 kg/ha K2O, 185 kg/ha P2O5, 350 kg/ha Ca and 175 kg/ha Mg. Top dressing was 40 kg/ha each of N, P2O5 and K2O. Seeds were sown at 30 cm spacing in a single row with 60 cm spacing and plants were thinned out to leave a single plant. In each line, 30 plants were grown. Roots were harvested 60 days after sowing seeds in 2005 and 2006 and 80 days after sowing in 2008 and 2009. They were subjected to GSL analysis.
Extraction of GSLs
Because it has been revealed that GSL contents in parts near the root tip are higher than in parts near a shoot (Ishii 1991), roots were cut longitudinally to mix every part of a root in samples. For evaluation of the palladium colorimetric method, roots cut horizontally (n = 50) into five equal pieces were also used as samples, as well as roots cut longitudinally (n = 86). Four Japanese radish cultivars with high GSL contents (90–100 μmol/g), medium GSL contents (50–70 μmol/g) and low GSL contents (30–40 μmol/g), which had been preliminary selected using HPLC analysis, were used for these analyses. Small pieces of roots were lyophilized and pulverized using a grinder (Multibeads Shocker MB831, Yasui Kikai Corp., Osaka, Japan). Then 0.1 g powder was transferred to a 10 ml glass tube with a lid.
Using the method described by Ishida et al. (2011), GSLs were extracted as follows. To the powder in glass tubes, 4.8 ml of 80% methanol kept at room temperature was added. After addition of 0.2 ml of 5 mM sinigrin (Sigma-Aldrich Corp., St. Louis, USA) as an internal standard, the tubes were kept at 25°C for 30 min and then shaken reciprocally (120 r/min) for 30 min in a shaker (Reciproshaker SR-2s, Taitec Co., Saitama, Japan). The tubes were centrifuged at 1,600 × g for 10 min. The supernatant was used as a crude extract.
HPLC analysis
Contents and composition of the GSLs were analyzed using HPLC (LC-10A, Shimadzu Corp., Kyoto, Japan) with a column (150 × 4.6 mm, COSMOSIL 5C18-II, Nacalai Tesque Inc., Kyoto, Japan) following desulfation of GSLs with sulfatase (Sigma-Aldrich Corp.) using the methods described by Bjerg and Sørensen (1987) and Ishida et al. (2003). To quantify each GSL, relative response factors to the internal standard of sinigrin were used according to the International Organization for Standardization (1992). The GSL contents were inferred as the average of four plants in each cultivar or line. For comparison with values of the total GSLs obtained using the palladium colorimetric method, an average of duplicated analyses for each plant was used.
Palladium colorimetric analysis of the total GSL content
Colorimetric analysis of the total GSL content was performed by simplifying the method described by Møller et al. (1985). Purification with ion-exchange chromatography was omitted. To 0.2 ml of crude GSL extract, 0.3 ml of distilled water and 3 ml of 2 mM palladium (II) chloride (Sigma-Aldrich Corp.) reagent, in which 3.54 g PdCl2 had been dissolved in 1.68 ml concentrated hydrochloric acid and diluted into 1,000 ml with distilled water, were added and mixed. After incubation at 25°C for 1 hr, absorbance at 425 nm was measured using a spectrophotometer (Hitachi U-3310, Hitachi Ltd., Tokyo, Japan). Absorbance was shown by an average of three measurements. After subtraction of the value of a blank, as obtained using 0.2 ml of distilled water as a sample, differences of absorbance were used for estimation of total GSL contents. According to the method used by Nagata (2009) and Nagata et al. (2007), the 136 samples were divided into two groups by choosing one alternately from the sample with the highest GSL content. One group was used as the calibration dataset. The other group was used as the evaluation dataset. When a calibration curve was obtained, the coefficient of determination (R2) and the standard error of calibration (SEC), referred to as the root-mean-square error of calibration, were calculated. R2 between predicted GSL content and true content, the regression equation and standard error of prediction (SEP) were used to evaluate reliability of the calibration model.
Results
Varietal differences of GSL contents
In the 28 examined cultivars, three aliphatic GSLs (glucoraphenin, glucoerucin and 4MTB-GSL) and three indolyl GSLs (4-hydroxyglucobrassicin, glucobrassicin, and 4-methoxyglucobrassicin) were detected (Fig. 1 and Table 1). Although glucoerucin and glucobrassicin were not detected in some cultivars, no cultivar-specific GSL was found. ANOVA analyses of varietal differences in 2005, 2006, 2008 and 2009 revealed significant differences at the 1% level in every year, the result in 2005 is shown in Table 2.
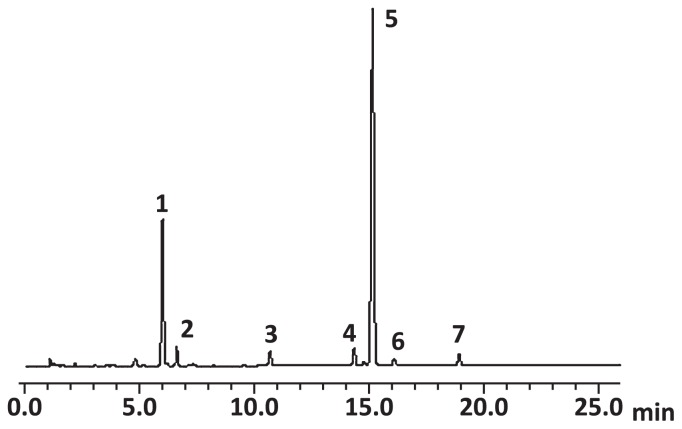
Fig. 1.
HPLC chromatograms of desulfoglucosinolates of Japanese radish cv. Nishimachi-risou. Detection wavelength: 229 nm. Six peaks of glucosinolates were identified as the following: 1, standard (sinigrin, 5 mM); 2, glucoraphenin; 3, 4-hydroxyglucobrassicin; 4, glucoerucin; 5, 4-methylthio-3-butenylglucosinolate; 6, glucobrassicin; 7, 4-methoxyglucobrassicin.
Table 2.
Varietal differences of glucosinolate contents
| Factor | Degree of freedom | Glucoraphenin | Glucoerucin | 4MTB-GSL | Total aliphatic GSL | Total indolyl GSL | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Mean square | F value | Mean square | F value | Mean square | F value | Mean square | F value | Mean square | F value | ||
| Cultivar | 16 | 16.56 | 3.48** | 1.94 | 14.34** | 5865.68 | 44.92** | 6201.23 | 57.40** | 1.85 | 17.30** |
| Error | 51 | 4.75 | 0.14 | 130.57 | 108.04 | 0.11 | |||||
| Factor | Degree of freedom | Total GSL | 4MTB-GSL/Total GSL | Aliphatic GSL/Total GSL | Indolic GSL/total GSL | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Mean square | F value | Mean square | F value | Mean square | F value | Mean square | F value | ||
| Cultivar | 16 | 6470.79 | 62.01** | 609.74 | 2.88** | 55.25 | 4.04** | 26.08 | 14.20** |
| Error | 51 | 104.34 | 211.66 | 13.69 | 1.84 | ||||
The data of 17 cultivars obtained in 2005 were used.
significant at P < 0.01 by 1 way ANOVA.
Using the data of 2005, when the largest number of cultivars was analyzed, the maximum, minimum, and mean values of the contents and percentages of GSLs were compared for different cultivar types (Table 3). In the data of 2005, the cultivar having the highest amount of 4MTB-GSL was ‘Karami 199’, the amount being 135.7 μmol/g, and the cultivar having the lowest amount of 4MTB-GSL was ‘Comet’, the amount being 15.9 μmol/g. The Japanese pungent radish type had 109.6 μmol/g 4MTB-GSL on average, which is significantly higher than the other types, but ‘Karamaru’ had an intermediate amount, i.e. 62.2 μmol/g, among the cultivars used. The Japanese common radish type including ‘Taibyou-soubutori’, the most popular type in Japanese markets, had 4MTB-GSL contents from 44.1 μmol/g to 75.2 μmol/g, with 54.8 μmol/g on average. Averages of 4MTB-GSL contents in Chinese radish and European garden radish were 35.3 μmol/g and 19.9 μmol/g, respectively, and lower than the Japanese common radish type. The glucoraphenin content was significantly higher in the Japanese pungent radish type than in the other types, but the glucoerucin content and the percentage of indolyl GSLs were not significantly different. In the European garden radish type, the percentage of indolyl GSLs was significantly higher than those in the other types, and the percentages of 4MTB-GSL and aliphatic GSLs were significantly lower than those in the Japanese common radish type.
Table 3.
Maximum, minimum and mean contents of glucosinolates in 17 radish
| Cultiver type | Glucosinolatets (μmol/g) | % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Aliphatic GSL | Total of indolyl GSL | Total | 4MTB-GSL/Total GSL | Aliphatic GSL/Total GSL | indolyl GSL/total GSL | |||||
|
| ||||||||||
| Gluco-raphenin | Gluco-erucin | 4MTB-GSL | Total | |||||||
| 6 Japanese common radish cultivars | Max | 3.4 | 1.5 | 75.2 | 80.1 | 1.7 | 81.7 | 96.7 | 99.2 | 2.1 |
| Min | 0.8 | 0.2 | 44.1 | 46.1 | 0.4 | 46.8 | 92.0 | 97.9 | 0.8 | |
| Mean ± SD | 1.6 ± 1.0 aa | 0.6 ± 0.5 | 54.8 ± 11.3 b | 57.0 ± 12.4 b | 0.8 ± 0.5 | 57.8 ± 12.7 b | 94.9 ± 1.8 a | 98.6 ± 0.4 a | 1.4 ± 0.5 a | |
|
| ||||||||||
| 4 Japanese pungent radish cultivars | Max | 5.7 | 2.4 | 135.7 | 142.9 | 2.5 | 145.5 | 94.6 | 98.4 | 2.6 |
| Min | 2.3 | 0.3 | 62.2 | 68.3 | 1.8 | 70.1 | 88.8 | 97.4 | 1.6 | |
| Mean | 4.3 ± 0.1 b | 1.5 ± 0.3 | 109.6 ± 5.5 a | 115.4 ± 32.6 a | 2.1 ± 0.2 | 117.6 ± 5.3 a | 92.8 ± 0.2 ab | 98.1 ± 0.1 a | 1.9 ± 0.1 a | |
|
| ||||||||||
| 3 Chinese radish cultivars | Max | 1.7 | 0.7 | 40.0 | 42.2 | 5.0 | 42.9 | 93.1 | 98.1 | 3.5 |
| Min | 1.5 | 0.0 | 29.9 | 31.5 | 0.8 | 32.6 | 91.6 | 96.5 | 1.9 | |
| Mean | 1.6 ± 0.2 a | 0.3 ± 0.2 | 35.3 ± 2.5 bc | 37.2 ± 5.4 bc | 2.3 ± 0.1 | 38.1 ± 2.8 bc | 92.5 ± 0.7 ab | 97.5 ± 0.3 a | 2.6 ± 0.3 a | |
|
| ||||||||||
| 4 European garden radish cultivars | Max | 3.0 | 0.9 | 26.1 | 28.6 | 2.2 | 30.1 | 94.5 | 96.2 | 10.1 |
| Min | 0.3 | 0.0 | 15.9 | 17.6 | 0.7 | 18.3 | 72.9 | 89.9 | 3.8 | |
| Mean | 1.8 ± 0.2 a | 0.6 ± 0.2 | 19.9 ± 1.0 c | 22.2 ± 4.8 c | 1.6 ± 0.1 | 23.8 ± 1.1 c | 83.4 ± 1.7 b | 93.2 ± 0.7 b | 6.8±0.7 b | |
The data of 17 cultivars obtained in 2005 were used.
Different letters represent significant differences according to Tukey-Kramer’s test (P < 0.05).
To evaluate the consistency of GSL contents in different years, the contents of 4MTB-GSL, aliphatic GSLs and indolyl GSLs of different cultivars were compared between 2005 and 2006, between 2008 and 2009 and between 2005 and 2008, in which the culture periods were nearly the same as those between 2005 and 2006 and between 2008 and 2009, which had different parameters from those used between 2005 and 2008. Remarkably high correlation was found in all combinations: correlation coefficients were higher than 0.939 (Table 4).
Table 4.
Correlation coefficients between glucosinolate contents of two years
| Year | No. of cultivars | 4MTB-GSL | Aliphatic GSL | Indolyl GSL | Total GSL |
|---|---|---|---|---|---|
| 2005, 2006 | 4 | 0.964** | 0.964** | 0.947** | 0.966** |
| 2008, 2009 | 5 | 0.998** | 0.998** | 0.973** | 0.998** |
| 2005, 2008 | 6 | 0.939** | 0.953** | 0.938** | 0.955** |
Significant correlation coefficients are indicated by asterisks (*P < 0.05, ** P < 0.01).
Evaluation using palladium colorimetric analysis
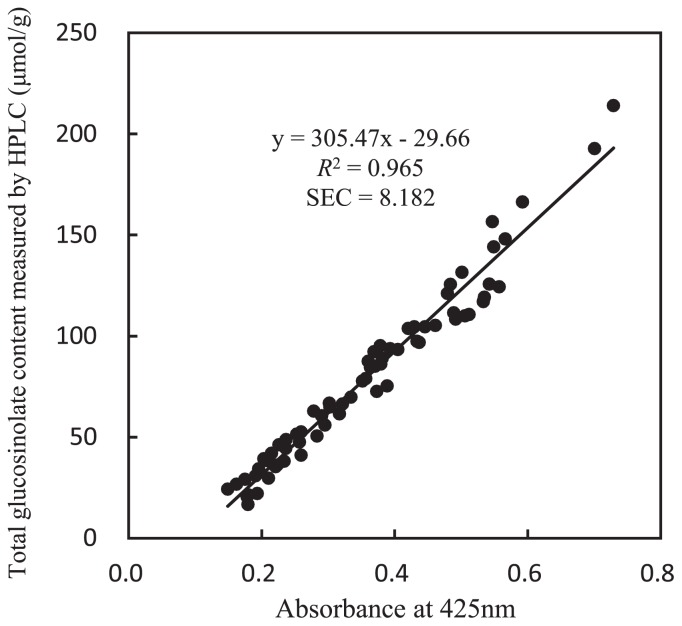
The total GSL contents of 136 samples were determined using HPLC analysis and the samples were divided into two groups: one as a calibration dataset and the other as an evaluation dataset. Absorbance at 425 nm of the 68 samples of one group by palladium colorimetric analysis of the total GSL content was compared with the content determined using HPLC analyses. High linearity was observed between the absorbance at 425 nm and HPLC analyses (Fig. 2). A single regression analysis revealed an equation for prediction as Y = 305.47 X − 29.66, in which X is absorbance at 425 nm and Y (μmol/g) is the content determined using HPLC analyses. SEC was 8.182. R2 was 0.965.
Fig. 2.
Relation between measured total glucosinolate contents using HPLC and colorimetric measurement by palladium-glucosinolate complexes in 68 calibration datasets of radish. SEC: standard error of calibration
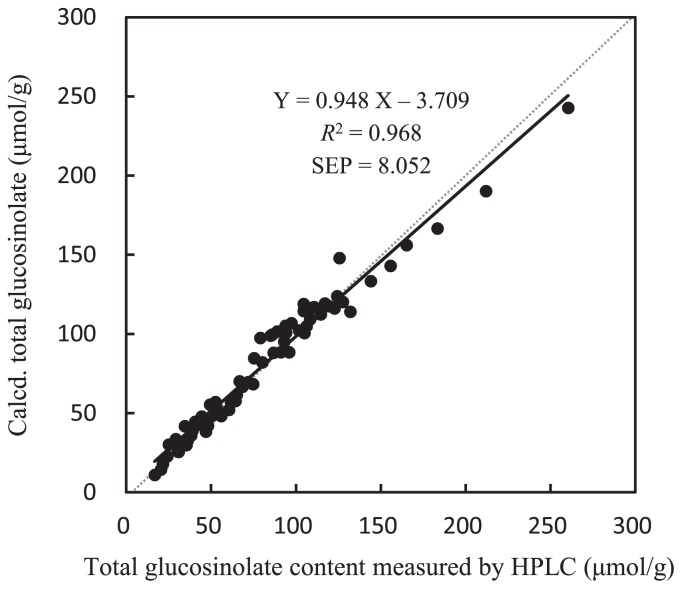
To evaluate the prediction accuracy, another set of data was applied to the equation for prediction (Fig. 3). The regression equation of Y = 0.948 X − 3.709 was inferred, with X representing values determined using HPLC analysis and Y being predicted values calculated using the equation for the prediction above. SEP was 8.052 and R2 was 0.968.
Fig. 3.
Relation between calculated total glucosinolate contents with the equation for prediction (GSL = 305.47 × A425 −29.66) and measured total glucosinolate contents using HPLC in 68 samples of radish. SEP: Standard error of prediction
Discussion
In this study, the variation of GSLs and their contents were investigated using 28 radish cultivars including mainly Japanese F1 hybrid cultivars. There were variations of 4MTB-GSL contents within cultivars in both F1 hybrid cultivars and open-pollinated cultivars, and standard deviations (SD) of 4MTB-GSL contents did not depend on seed production methods. All cultivars in the Japanese pungent radish type had higher SD, suggesting cultivars having high 4MTB-GSL contents to have high variations of 4MTB-GSL contents.
The cultivars that were used differed between years. The days from sowing to harvest in 2005 and 2006 were also different from those in 2008 and 2009. Because the contents of GSLs in radish roots change depending on growing conditions and the growth stage (Ishii 1991), it is difficult to compare them simply between different cultivars. In fact, the total GSL contents of ‘Taibyou-soubutori’ and ‘Karami 199’ in 2008, root harvesting time being three weeks later than that in 2005, were 15–20% lower than those in 2005. Ishii (1991) has reported that 4MTB-GSL contents in radish roots decrease gradually from ca. 40 days after sowing seeds. Such decrease of 4MTB-GSL contents was observed in the present study and range of annual change in 4MTB-GSL contents depended on cultivars. Freeman and Mossadeghi (1972) and Ishii and Saijo (1987) have reported that isothiocyanate contents are influenced by the concentration of SO42− ion and water content in soil. Differences of annual change in 4MTB-GSL contents between cultivars are considered to be due to the difference of response to environmental factors between cultivars. However, contents of 4MTB-GSL, aliphatic GSLs and indolyl GSLs in the total GSLs showed high positive correlation, with r = 0.939 between different years, suggesting that the composition ratio of each GSL at the harvesting time is stable and independent of the cultivation period length. In the data of 2005, the percentage of 4MTB-GSL in the total GSLs of the Japanese common radish type was significantly higher than that of the European garden radish type. Although high 4MTB-GSL content is a characteristic of the Japanese pungent radish type and approximately twice the amount of 4MTB-GSL of other cultivar types was detected in the Japanese pungent radish type in this study, the percentage of 4MTB-GSL in the total GSLs of this type was not significantly different from that of the Japanese common radish type. Percentages of indolyl GSLs in the total GSLs of the European garden radish cultivars were significantly higher than those of other types. However, the difference of indolyl GSL contents was not significant between the European garden radish type and other types. It can be inferred that the high composition ratio of indolyl GSLs in the European garden radish cultivars results from a lower amount of 4MTB-GSL than other GSLs in these cultivars. Carlson et al. (1985) have reported a similar tendency. The low composition rate of 4MTB-GSL is inferred to be a genetic trait of the European garden radish type. However, a possibility that delay of sampling time from the best harvesting time resulted in decrease of aliphatic GSLs and increase of percentage of indolyl GSL cannot be ruled out.
Carlson et al. (1985) and Ishii et al. (1989), after respectively analyzing 4MTB-GSL contents and the total GSL contents of 109 cultivars including 41 Japanese cultivars and 20 cultivars including 18 Japanese cultivars, mainly including Japanese cultivars, reported that the Chinese cultivar ‘Tenan-koushin’ has lower amounts of 4MTB-GSL and total GSLs than Japanese cultivars have. Furthermore, Japanese cultivars have higher amounts of 4MTB-GSL than European garden radishes do. These findings were confirmed in 4MTB-ITC analyses using 38 cultivars, mainly including Japanese and Chinese cultivars (Okano et al. 1990). Similar results were obtained for the present study. Although it is difficult to compare the numerical data of these studies in a simple manner because of differences of plant culture conditions, analytical methods and data representations, similar tendencies shown in these different results of studies suggest that these differences of composition ratio of 4MTB-GSL are a stable trait that is controlled genetically. We are going to investigate genetic control of composition ratios of 4MTB-GSL and other GSLs in radish.
In the desulfation method generally used for GSL analysis, crude GSLs extracted from plants are adsorbed onto anion exchange resin and desulfated by sulfatase for one day. Then desulfo-GSLs are analyzed using HPLC. The desulfation method entails onerous and time-consuming pre-treatment and analysis. Therefore, we strove to develop a simple and rapid method for GSL quantitative analysis. For oilseed rape, a palladium colorimetric method has been developed for analysis of GSL contents in seeds. The GSLs in crude extracts are bound with palladium to form a stable complex, resulting in a color change from light brown to dark brown (Thies 1982). In this method, absorbance at 450 nm is influenced strongly by inhibitory substances in the GSL crude extracts. Møller et al. (1985) improved the method described by Thies (1982) by adding a purification step using an ion exchange chromatography column of Ecteola cellulose. This improved method presents the advantage that the analysis is influenced neither by inhibitors of color development nor pigments in crude extracts. Therefore intact GSL can be quantified directly without chemical modification. However, because the purification step used for the study requires much labor and long time, its use is limited.
Although crude extracts of GSL from rapeseed are orange–yellow, extracts from radish roots are colorless and transparent. Radish roots are regarded as having few substances interfering with colorimetric analysis. Furthermore, the variation of the GSL composition in Japanese radish cultivars was slight. Therefore, we applied the simplified method of Møller et al. (1985). To obtain an accurate equation for prediction, it is important to use data with widely various GSL contents. Therefore, in this study, we used 68 samples of different plants and different positions of roots to obtain the equation for prediction for the total GSL contents: Y = 305.47 X − 29.66, in which X is absorbance at 425 nm of a colored sample and Y (μmol/g) is the total GSL content determined using HPLC analysis. Evaluation of this equation for prediction using the other 68 samples revealed high accuracy for prediction. The regression equation was Y = 0.948 X − 3.709 and the correlation coefficient between X and Y was 0.984, where X denotes values determined using HPLC analysis and Y signifies predicted values calculated using the equation for prediction. The SEP was 8.173, indicating that estimated values have a prediction error of approximately 16 μmol/g with 95% confidence. Such an error might be attributed to two causes. One is the pigments of the crude extracts, such as polyphenols or chlorophylls, which have absorption around 425 nm. In our preliminary investigation, analysis of red or green extracts from red radish or Chinese dark green color cultivars caused estimation of higher amounts than the values determined using HPLC (data not shown). Another is a difference of absorption maximum wavelength of different GSLs (Thies 1982). Although the composition ratios of GSLs in Japanese radish cultivars were constant, there might be slight variation among cultivars. Such differences might bring about errors. Furthermore, there might be differences of endogenous inhibitors of color development between cultivars.
The method developed in this study is intended to calculate the total GSL content by substituting A425 values to the following formula: Total GSL (μmol/g) = 305.47 × A425 −29.66. It is not necessary to calibrate it each time using a standard such as sinigrin. In this study, extremely high percentages of 4MTB-GSL in the Japanese common radish type and the Japanese pungent radish type were confirmed. Therefore, this method is regarded as highly useful for analyzing the total GSL contents of Japanese radish cultivars and breeding lines developed from a cross between Japanese radish cultivars. In the desulfation method, desulfation of 50 samples takes 24 hr, and HPLC analysis requires ca. 36 hr. In contrast, using the colorimetric method described herein, more than 200 samples can be analyzed in a single day. We previously reported a simple method for extraction of GSLs from lyophilized radish roots using methanol at room temperature without hot methanol or hot water (Ishida et al. 2011). Combining this extraction method and the colorimetric analysis developed in this study will enable rapid analysis of the total GSLs in radish roots.
The method developed in the present study can be used not only in evaluation of GSL contents in radish cultivars and lines but also in F1 hybrid breeding for pungency. Radish breeding for pungency has been limited to the breeding for condiment cultivars with high pungency. Radish breeding based on quantitative analysis of 4MTB-GSL will enable development of new cultivars having high processability or functionality. It will be useful for radish breeding for high or low GSL contents and for estimation of pungency in food processing.
Acknowledgments
We thank Dr. Yosuke Yoshioka of the NARO Institute of Vegetable and Tea Science for his advice on statistical analysis. This study was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN), Japan.
Literature Cited
- Barillari J., Iori R., Broccoli M., Pozzetti L., Canistro D., Sapone A., Bonamassa B., Biagi G.L., Paolini M. (2007) Glucoraphasatin and glucoraphenin, a redox pair of glucosinolates of Brassicaceae, differently affect metabolizing enzymes in rats. J. Agric. Food Chem. 55: 5505–5511 [DOI] [PubMed] [Google Scholar]
- Bjerg B., Sørensen H. (1987) Quantitative analysis of glucosinolates and HPLC of intact glucosinolates. In: Wathelet J.-P. (ed.) Glucosinolates in Rapeseeds. Analytical aspects, Martinus Nijhoff Publishers, Dordrecht. Netherlands, pp. 125–150 [Google Scholar]
- Bjorkqvist B., Hase A. (1988) Separation and determination of intact glucosinolates in rapeseed by high-performance liquid chromatography. J. Chromatogr. 435: 501–507 [DOI] [PubMed] [Google Scholar]
- Carlson D.G., Daxenbichler M.E., Van Etten C.H. (1985) Glucosinolate in radish cultivars. J. Amer. Soc. Hort. Sci. 110: 634–638 [Google Scholar]
- Fahey J.W., Zalcmann A.T., Talalay P. (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56: 5–51 [DOI] [PubMed] [Google Scholar]
- Freeman G.C., Mossadeghi N. (1972) Influence of sulphate nutrition on flavor component of three cruciferous plant: Radish (Raphanus sativus), Cabbage (Brassica oleracea capitata), and White Mustard (Sinapis alba). J. Sci. Food Agri. 23: 387–402 [Google Scholar]
- Friis P., Kjaer A. (1966) 4-Methylthio-3-butenyl isothiocyanate, the pungent principle of radish root. Acta Chem. Scand. 20: 698–705 [Google Scholar]
- Ishida M., Takahata Y., Kaizuma N. (2003) Simple and rapid method for the selection of individual rapeseed plants low in glucosinolates. Breed. Sci. 53: 291–296 [Google Scholar]
- Ishida M., Kakizaki T., Ohara T., Morimitsu Y. (2011) Development of a simple and rapid extraction method of glucosinolates from radish roots. Breed. Sci. 61: 208–211 [Google Scholar]
- Ishii G., Saijo R. (1987) Effect of season, soil type, sulfate level, mulching and plant density on isothiocyanates content in radish root juice (Raphanus sativus L.). J. Japan. Soc. Hort. Sci. 56: 313–320 [Google Scholar]
- Ishii G., Saijo R., Nagata M. (1989) The difference of glucosinolates content in different cultivar of daikon root (Raphanus sativus L.). Nippon Shokuhin Kogyo Gakkaishi 36: 739–742 [Google Scholar]
- Ishii G. (1991) Glucosinolate in Japanese Radish, Raphanus sativus L. JARQ. 24: 273–279 [Google Scholar]
- Mellon F.A., Bennett R.N., Holst B., Willia G. (2002) Intact glucosinolate analysis in plant extracts by programmed cone voltage electrospray LC/MS: Performance and comparison with LC/ MS/MS methods. Analytical Biochemistry 306: 83–91 [DOI] [PubMed] [Google Scholar]
- Møller P., Ploger A., Sørensen H. (1985) Quantitative analysis of total glucosinolate content in concentrated extracts from double low rapeseed by the Pd-glucosinolate complex method. In: Sørensen H. (ed.) Advances in the Production and Utilization of Cruciferous Crop, Martinus Nijhoff/DR W. Junk Publishers, Dordrecht, pp. 97–110 [Google Scholar]
- Mithen R.F., Dekker M., Verkerk R., Rabot S., Johnson L.T. (2000) The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agric. 80: 967–984 [Google Scholar]
- Montaut S., Barillari J., Iori R., Rollin P. (2010) Glucoraphasatin: Chemistry, occurrence, and biological properties. Phytochemistry 71: 6–12 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Iwahashi T., Tanaka A., Koutani J., Matsuo T., Okamoto S., Sato K., Ohtsuki K. (2001) 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus, Japanese white radish). J. Agric. Food Chem. 49: 5755–5760 [DOI] [PubMed] [Google Scholar]
- Nagata M. (2009) A simple spectrophotometric method for the estimation of β-carotene content in spinach acetone extracts. Bull. Natl. Inst. NIVTS. 8: 1–5 [Google Scholar]
- Nagata M., Noguchi Y., Ito H., Imanishi S., Sugiyama K. (2007) A simple spectrophotometric method for the estimation of α-carotene, β-carotene and lycopene concentrations on carrot acetone extracts. Nippon Shokuhin Kogyo Gakkaishi 54: 351–355 [Google Scholar]
- Okano K., Asano J., Ishii G. (1990) Contents of pungent principle in roots of Japanese radish (Raphanus sativus) cultivars. J. Japan. Soc. Hort. Sci. 59: 551–558 [Google Scholar]
- Ozawa Y., Kawakishi S., Uda Y., Maeda Y. (1990a) Isolation and identification of a novel b-carboline derivative in salted radish roots, Raphanus sativus L. Agr. Biol. Chem. 54: 1241–1245 [Google Scholar]
- Ozawa Y., Uda Y., Kawakishi S. (1990b) Generation of b-carboline derivative, the yellowish precursor of processed radish roots, from 4-methylthio-3-butenyl isothiocyanate and L-tryptophan. Agr. Biol. Chem. 54: 1849–1851 [Google Scholar]
- Papi A., Orlandi M., Bartolini G., Bartolini G., Barillari J., Paolini M., Ferroni F., Fumo M.G., Pedulli G.F., Valgimigli L. (2008) Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (kaiware daikon) sprouts. J. Agric. Food Chem. 56: 875–883 [DOI] [PubMed] [Google Scholar]
- Rangkadilok N., Nicolas M.E., Bennett R.N., Premier R.R., Eagling D.R., Taylor P.W.J. (2002) Determination of sinigrin and glucoraphanin in Brassica species using a simple extraction method combined with ion-pair HPLC analysis. Scientia Horticulturae 96: 27–41 [Google Scholar]
- The International Organization for Standardization(1992) Rapeseed-determination of glucosinolate content. ISO 9167–1: 1992. 1–9 [Google Scholar]
- Thies W. (1982) Complex-formation between glucosinolates and tetrachloropalladate (II) and its utilization in plant breeding. Fette. Seifen. Anstrichm. 84: 338–342 [Google Scholar]
- Uda Y., Hayashi H., Shimizu A. (2000) Mutagenic and anti-mutagenic property of 3-hydroxymethylene-2-thioxopyrrolidine, a major product generating from pungent principle of radish. Lebensm Wiss Technol. 33: 37–43 [Google Scholar]
- Yamasaki M., Omi Y., Fujii N., Ozaki A., Nakama A., Sakakibara Y., Suiko M., Nishiyama K. (2009) Mustard oil in “shibori daikon” a variety of Japanese radish, selectively inhibits the proliferation of H-ras-transformed 3Y1 cells. BBB 73: 2217–2221 [DOI] [PubMed] [Google Scholar]