Abstract
The photoinitiated radical reactions between thiols and alkenes/alkynes (thiol-ene and thiol-yne chemistry) have been applied to a functionalization methodology to produce carbohydrate-presenting surfaces for analyses of biomolecular interactions. Polymer-coated quartz surfaces were functionalized with alkenes or alkynes in a straightforward photochemical procedure utilizing perfluorophenylazide (PFPA) chemistry. The alkene/alkyne surfaces were subsequently allowed to react with carbohydrate thiols in water under UV-irradiation. The reaction can be carried out in a drop of water directly on the surface without photoinitiator and any disulfide side products were easily washed away after the functionalization process. The resulting carbohydrate-presenting surfaces were evaluated in real-time studies of protein-carbohydrate interactions using a quartz crystal microbalance flow-through system with recurring injections of selected lectins with intermediate regeneration steps using low pH buffer. The resulting methodology proved fast, efficient and scalable to high-throughput analysis formats, and the produced surfaces showed significant protein binding with expected selectivities of the lectins used in the study.
Keywords: Photochemistry, Click chemistry, Thiol-ene/-yne, Carbohydrates, Lectins, Quartz Crystal Microbalance
Introduction
More and improved strategies towards the synthesis of oligosaccharides, glycoproteins, and glycoconjugates, as well as an increased number of high-throughput analysis methods, have led to increased knowledge of the biological functions of glycans. (Kiessling and Splain 2010; Lepenies and Seeberger 2010; Li and Richards 2010; Seeberger 2009; Varki et al. 2009; Wu and Wong 2011) For example, different carbohydrate array methodologies have recently been designed and evaluated for high-throughput analysis of protein-carbohydrate interactions. (Horlacher and Seeberger 2008; Krishnamoorthy and Mahal 2009; Lee and Shin 2005; Oyelaran and Gildersleeve 2009; Park et al. 2008; Park and Shin 2007; Pei et al. 2007c; Tyagi et al. 2010; Wu et al. 2009) Glycan arrays have thus been used to identify proteins involved in cancer metastasis, (Hatakeyama et al. 2009) enzymes involved in wound healing, (Saravanan et al. 2010) and glycans modulating T cell death; (Earl et al. 2010) to evaluate blood serum glycan binding, (Huflejt et al. 2009) antibodies towards HIV, (Luallen et al. 2010) and antibodies for use in cancer treatment; (Huang et al. 2006; Nagre et al. 2010; Sawada et al. 2011) to evaluate the binding specificity of glycan-binding proteins and receptors; (Feinberg et al. 2010; Gout et al. 2010; Hoorelbeke et al. 2011; Horlacher et al. 2011; Pipirou et al. 2011; Porter et al. 2010; Singh et al. 2009) to investigate the binding specificities of disease causing bacteria, (Hu et al. 2011) viruses, (Krishnamoorthy et al. 2009; Neu et al. 2010; Nilsson et al. 2011) and fungi;(Chachadi et al. 2011) as well as for the in-depth investigation of avian and swine influenza viruses.(de Vries et al. 2011; Lao et al. 2011; Liao et al. 2010; Pappas et al. 2010; Stevens et al. 2010; Xu et al. 2010) Although such glycan array methodologies yield significant knowledge of glycan interactions, there are still obstacles to overcome in the production of universally valid array methodologies. Since the produced glycan surfaces aim to mimic those of cells, proteins or other biomolecules, the glycan presentation is important to achieve correct binding. The ligation techniques therefore need to be selective, adaptable, and compatible with the target glycans, and since most glycans are polar entities, it is highly desirable that the coupling chemistry can be effectuated in water.
These issues have been addressed in the present study, where the thiol-alkene (thiol-ene) and thiol-alkyne (thiol-yne) photoradical reactions have been evaluated for the fabrication of carbohydrate-functionalized surfaces. Although the radical reaction between thiols and alkenes/alkynes has been known since the beginning of the last century, (Posner 1905) it is only recently that it has been applied to functionalization methods with biomolecules.(Bader 1956; Bader et al. 1949) The thiol-ene reaction has since its discovery been thoroughly investigated in the field of polymer chemistry, but the thiol-yne reaction has only recently been explored. (Chan et al. 2009; Fairbanks et al. 2009; Fairbanks et al. 2010; Hensarling et al. 2009; Hoogenboom 2010; Konkolewicz et al. 2009; Yu et al. 2009) In an article from 2010, Fairbanks et al. reported a detailed study of reaction rates and mechanisms for various thiol-yne reactions, pinpointing important factors when designing the reaction. (Fairbanks et al. 2010) Since then, the thiol-yne reaction, along with the thiol-ene reaction, has been investigated for glycan coupling, and biomolecular functionalization of diverse materials. (Gupta et al. 2010; Lo Conte et al. 2010; Semsarilar et al. 2010; Wang et al. 2011; Wendeln et al. 2010)
Development of new glycan array methodologies is usually accomplished using glycans/proteins with known and complimentary specificities, to ensure accurate binding results and good validation of the systems. Glycan immobilization techniques for glycan array purposes are typically evaluated by biosensors, such as Quartz Crystal Microbalance (QCM),(Lyu et al. 2008; Mahon et al. 2010; Norberg et al. 2009b; Pei et al. 2007a) and Surface Plasmon Resonance (SPR), (de Boer et al. 2008; Dhayal and Ratner 2009; Linman et al. 2008; Muñoz et al. 2009; Uzawa et al. 2008; Zhang et al. 2006) or by high quality imaging using FITC-labeled proteins. (Branderhorst et al. 2008; Michel and Ravoo 2008; Park et al. 2009; Park and Shin 2007; Sun et al. 2006) QCM and SPR, which rely on the physical properties of quartz and gold to detect target binding, have the advantage of enabling the use of unlabeled proteins in the analyses. Conversely, both QCM and SPR are relatively low-throughput analysis tools compared to traditional microarrays, and are primarily used to obtain advanced kinetic data rather than large scale analysis of protein specificity.
Herein, we explore the thiol-ene/-yne reactions as surface functionalization approaches for the fabrication of carbohydrate-presenting surfaces. Carbohydrate-thiols with and without short spacers were coupled to alkene-/alkyne-functionalized surfaces in aqueous solution, and unlabeled lectins of corresponding known specificities were used to evaluate the methodology using a QCM flow-through instrumentation.
Materials and Methods
General
All commercially available starting materials were of reagent grade and used as received. Ricinus communis Agglutinin I (RCA-I), Concanavalin A (Con A) and bovine serum albumin (BSA) were purchased from Vector Labs and Sigma-Aldrich. Polystyrene-coated QCM crystals were obtained from Attana AB. 1H and 13C NMR data were recorded on a Bruker Avance 400 instrument at 400 MHz (1H) or a Bruker DMX 500 instrument at 500 MHz (1H) or 125 MHz (13C). Chemical shifts are reported as δ values (ppm) with either CDCl3 (1H: δ = 7.26, 13C = 77.16) or D2O (1H: δ = 4.79) as internal standard. J values are given in Hz. 1H peak assignments were made by first order analysis of the spectra supported by standard 1H-1H correlation spectroscopy (COSY). Thin layer chromatography (TLC) was performed on precoated Cromatofolios AL Silica gel 60 F254 plates (Merck). Flash column chromatography was performed on silica gel 60, 0.040–0.063 mm (SDS). Mass Spectrometry was performed in positive mode on a ThermoElectron LTQ-Orbitrap XL from Thermo Scientific, Bremen, Germany. Spincoating was performed using a Cookson Electronics Specialty Coating Systems Spincoater model P6708D. Photoreactions were performed at either 254, 300 or 350 nm using a photochemical reactor from Rayonet Srinivasan - Griffin. QCM analyses were performed using a flow-through Attana A100 C-Fast QCM system.
Synthesis (cf. Scheme 1)
Scheme 1.
Synthesis of thio-functionalized carbohydrates and alkene-linker for photo-click immobilization; a) 2- bromoethanol, BF3·Et2O, DCM, 0 °C, 23 h; b) KSAc, DMF, 45 °C, 17 h (72 %); c) NaOMe, MeOH, rt, 3 h (quant.); d) 2-bromoethanol, BF3·Et2O, DCM, −40 °C, 22 h (67 %); e) KSAc, DMF, 45 °C, 7 h (56 %); f) NaOMe, MeOH, rt, 4 h (70 %); g) NaOH, DMF, 45 °C, 2 h (30 %); h) TsCl + KOH, DCM, 0 °C, 5 h (quant.); i) NaN3 + TBAI, DMF, 45 °C, 22 h (77 %); j) PPh3, THF + H2O, 0 °C, 24 h (32 %).
Compound 2 and 6 were synthesized according to Davis et al.(Davis et al. 2000) Compound 4 and 8 were synthesized according to slightly modified procedures by Revell et al.(Revell et al. 1998) and Schofield et al. (Schofield et al. 2008) respectively. Compounds 14 was synthesized according to a modified procedure of Lim et al. (Lim et al. 2007) Compounds 15–18 were synthesized as previously reported. (Norberg et al. 2009b; Pei et al. 2007b; Pei et al. 2006) More details about the synthesis along with spectroscopic data can be found in supplementary information.
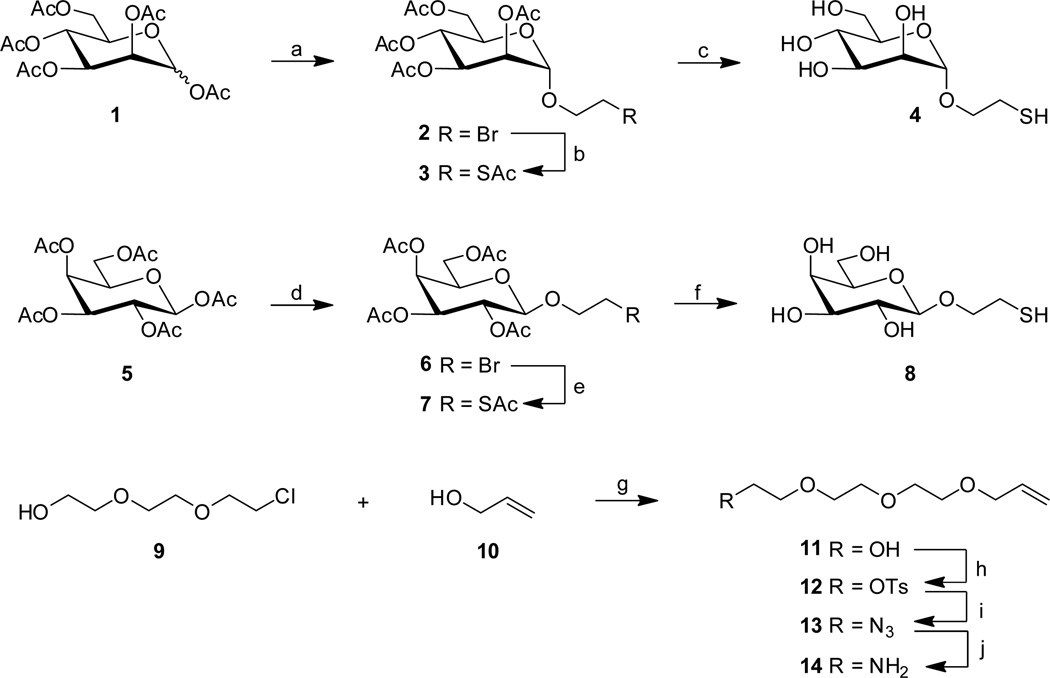
Surface functionalization (cf. Figure 1)
Figure 1.
General surface functionalization procedure. The polymeric surfaces were spincoated with a solution of compound 15 and irradiated with UV-light (254 nm) for 5 min. The NHS-activated ester surfaces were linked through an amidation process with amine 14 or 16 to produce the alkene/alkyne functionalized surfaces. The alkene/alkyne surfaces were then differentiated with thiol-functionalized molecules (4, 8, 17, 18 or 19) through radical photo-click chemistry.
The polystyrene-coated QCM-crystals were spincoated with a solution of PFPA-NHS (15, 10 mM, 10 µL) in ethanol at 1500 rpm for 180 s followed by UV-irradiation at 254 nm for 5 min. The crystals were then immersed in a solution of either alkene-linker 14 or alkyne-linker 16 (200 µL, 53 mM) in acetonitrile for 8 h and subsequently washed with acetonitrile and dried under a gentle stream of nitrogen. The crystal surfaces were then functionalized by applying solutions of thiols 4, 8, 17, 18 or 19 (50 µL, 312 mM) directly on the surface, followed by UV-irradiation at 350 nm for 30 min. The crystals were then thoroughly rinsed with water and methanol, dried under a gentle stream of nitrogen and mounted in the QCM flow-through system.
General procedure for QCM analysis
A continuous flow of running buffer (TRIS 10 mM, pH 7.4, 25 µL/min) was used throughout the experiments, and samples of RCA-I and Con A were prepared in the same buffer. The protein solutions were desalted on PD-10 columns and the final protein concentrations were determined by UV-Vis. The crystals were washed/equilibrated with buffer solution prior to manipulations/measurements. After equilibration of the crystals in the flow-through system, they were subjected to ten injections of BSA (20 µM), three injections of low pH buffer (pH 1.5) and finally two additional injections of BSA (20 µM) to block non-functionalized surfaces. Solutions of lectins were then injected on the system. Binding to the surfaces was monitored by frequency logging with Attester 3.3.4 (Attana), and adsorption/desorption to the surface recorded as the resulting frequency shifts. Bound lectins were released from the surfaces between measurements by two successive injections of low pH buffer (TRIS 10 mM, pH 1.5). The procedure was then repeated three times for each lectin concentration (32 nM - 1 µM) to give an average value and determine the surface stability over time.
Results and Discussion
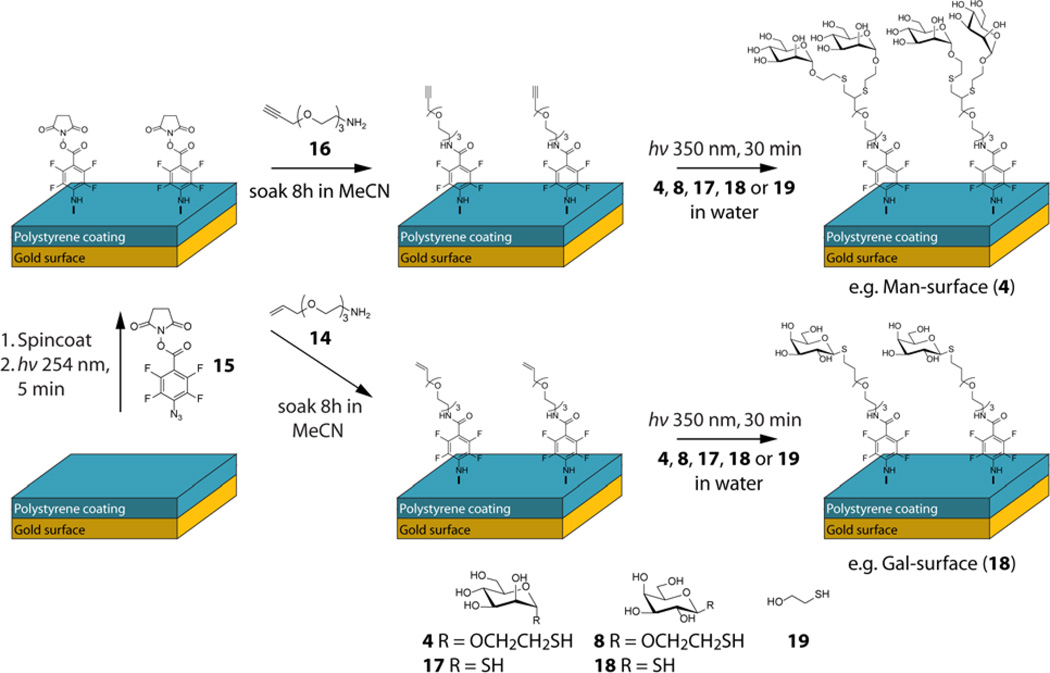
The reported reaction conditions for both the thiol-ene and thiol-yne reactions, i.e. nonpolar solvents or solvent-free systems, conventional nonpolar radical initiators and commonly high temperatures, were not applicable in the present study due to the instability of carbohydrates at elevated temperatures and their poor solubility in organic solvents. Therefore, model starting materials, allyl/propargyl alcohol and 2-mercaptoethanol (Figure 2) were initially evaluated to test the reactions in aqueous solutions and at room temperature. The reactions were carried out under UV-irradiation at 254, 300 or 350 nm, of which 350 nm proved optimal and was deemed the most suitable for the forthcoming applications. The results showed that the reactions proceeded smoothly in water (from pH 3 to pH 10) and, as expected, that the thiol-ene reaction proceeds at a higher rate than the thiol-yne reaction. NMR-analyses for the model systems are displayed in Figure 2, showing the expected mono- and difunctionalized alkene/alkynes. Reactions carried out in the presence or absence of a water-soluble photoinitiator (4,4'-azobis(4- cyanovaleric acid), ACVA) showed similar reaction times, in principle allowing for initiator-free reaction conditions. In some cases, disulfide side products were obtained in varying concentrations, a side effect that is insignificant in the surface reactions where the disulfide can be easily washed away.
Figure 2.
1H-NMR-spectra of model thiol-ene and thiol-yne reactions in water; a) 2-mercaptoethanol; b) allyl alcohol; c) thiol-ene reaction mixture (1:1 ratio) after 30 min UV-irradiation at 350 nm; d) propargyl alcohol; e) thiol-yne reaction mixture (1:2.1 alkyne:thiol) after 2 h UV-irradiation at 350 nm.
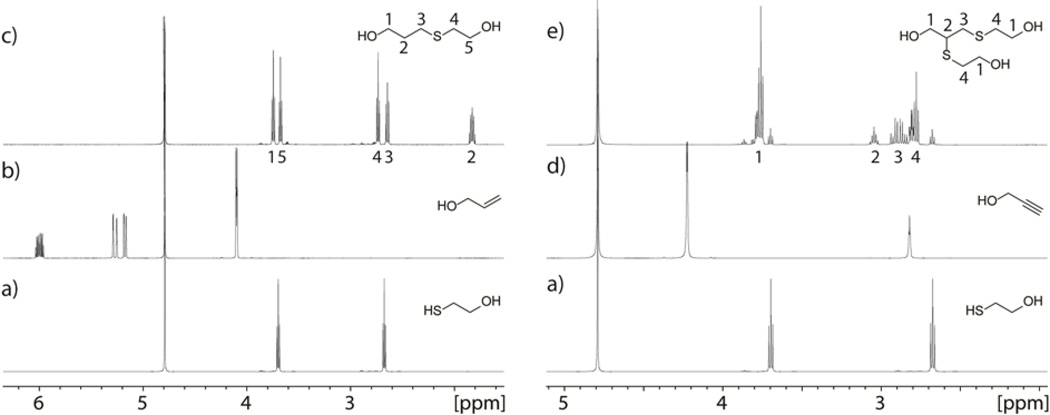
The photo-click immobilization method presented in Figure 1 is based on initial photochemical insertion of perfluorophenyl azides (PFPAs) into the polymeric material, as previously reported, (Norberg et al. 2011; Norberg et al. 2009a) and subsequent amide coupling to produce the corresponding active materials. In the present case, the polystyrene surfaces were functionalized with triethyleneglycol-linked alkynes or alkenes in a two-step procedure, starting with spincoating of PFPA-NHS structure 15 from an ethanol solution, and subsequent nitrene-mediated photoligation under UV-irradiation at 254 nm. The resulting activated ester-functionalized surfaces were then submerged in solutions of alkyne- (16) or alkene-linkers (14) in acetonitrile, where stable amide bonds were formed. Carbohydrate-thiols were then coupled to the surfaces by photoinitiated radical addition in aqueous solution, yielding the corresponding carbohydrate-presenting surfaces. The crystals were thus placed on a horizontal surface and a drop of water containing the respective carbohydrate-thiol was placed directly on the surface, followed by UV-irradiation at 350 nm (Figure 3).
Figure 3.
Illustration of thiol-ene/-yne reactions performed directly on alkene/alkyne functionalized quartz crystals in a drop of pure water.
The overall methodology makes use of relatively simple organic molecules which can be synthesized in few steps in good yields. The compounds used to produce the alkene-/alkyne-surfaces can also be stored in prepared solutions at low temperature for repeated use over long time. The procedure is in addition fast and straightforward and can easily be expanded to high-throughput analysis methods using fluid robotics. The photochemical steps furthermore enable spatial and temporal control of the ligation process, in principle allowing for various patterning applications.
Initial surface experiments were carried out in the presence and absence of the water-soluble ACVA photoinitiator, with very similar product outcome in accordance with the solution-phase results. Thus, the surfaces could be efficiently prepared also in the absence of any photoinitiator, albeit requiring somewhat longer reaction times. It was also found that carbohydrate-thiols 4 and 8 were prone to oxidation to disulfides during storage in water-solution, and for this reason fresh solutions of all thiol-ligands were prepared prior to functionalization. In addition to carbohydrate-thiols 4, 8, 17 and 18, 2-mercaptoethanol 19 was used to produce control-surfaces for both alkyne- and alkene-surfaces. The resulting control-surfaces were used to evaluate and correct for non-specific binding of each lectin in the binding analysis.
The carbohydrates d-mannose and d-galactose, along with the lectins Con A, specific for α-d-mannosides, and RCA-I, specific for β-d-galactosides, were used as model pairs for evaluation of the method. These lectins were chosen based on their binding specificities towards the chosen carbohydrates, their sizes and physical properties. RCA-I and Con A thus display similar molecular weights and pI values, (Bhattacharyya and Brewer 1990; Norberg et al. 2011; Ready et al. 1984; Sweeney et al. 1997) leading to similar behavior towards the surfaces in the QCM setup. Furthermore, two different types of carbohydrate thiols were utilized to investigate the versatility of the method as well as the potential implications of different divalent ligands on protein binding: 1-thio-carbohydrates 17–18 and thioethyl-linked carbohydrates 4 and 8. The methodology was evaluated using a QCM flow-through instrumentation which allowed for repetitive injections of different proteins/concentrations of proteins and subsequent binding analysis to the same surface. BSA was used to block any potential unreacted/blank surface and consequently minimize the non-specific binding of the lectins in the study. BSA was injected only in the initial phase of the flow-through experiment, which was found to be sufficient to obtain stable blocking throughout the whole experiment. (Norberg et al. 2009b; Pei et al. 2005; Pei et al. 2006) Regeneration of the carbohydrate surfaces was easily accomplished by injections of low-pH buffer (pH 1.5), and the resulting binding was found to be very stable and reproducible over time (Figures 4 and 5).
Figure 4.
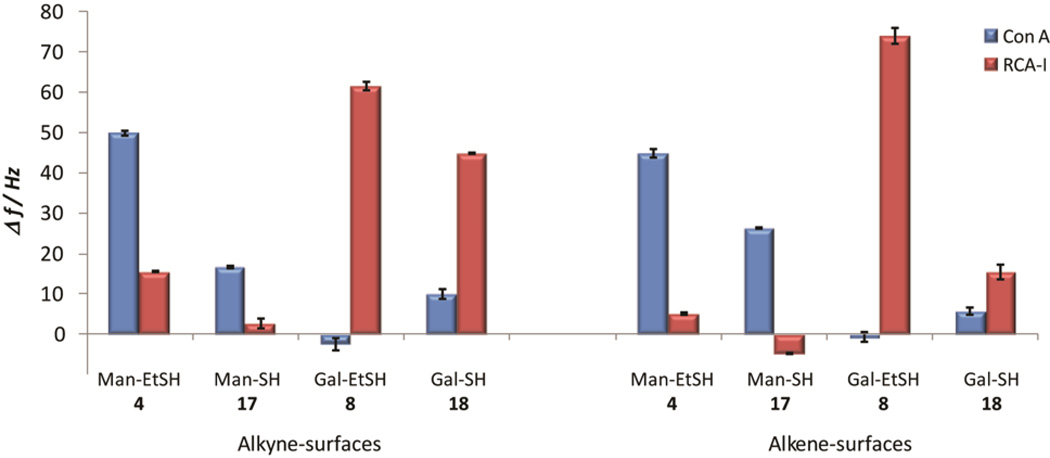
Corrected lectin binding to all functionalized surfaces. Negative values are due to slightly higher binding to control surfaces. Error bars represent the standard errors of the mean (SEM) of triplicate injections of lectin at the same concentration (1 µM).
Figure 5.
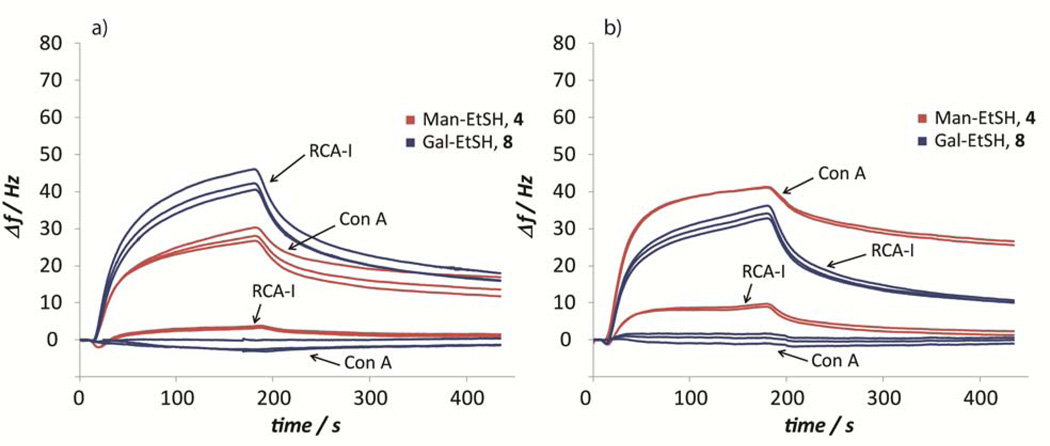
Illustration of referenced binding curves of triplicate injections of Con A and RCA-I to mannose-/galactose-surfaces on a) alkyne-based surfaces and b) alkene-based surfaces.
The analysis resulted in binding well in accordance with the known specificities for both lectins. The quantitative binding was found to be similar for both alkyne- and alkene-originated surfaces, although varying slightly within series of the two types of carbohydrate thiols. In general, higher binding signals were found for carbohydrate surfaces produced from the thioethyl-linked carbohydrates (4 and 8) compared to surfaces based on the corresponding 1-thio-carbohydrates 17–18 (Figure 4), an effect which may be due to differences in lectin affinity towards 1-O- or 1-S-carbohydrates or differences in reactivities between the carbohydrate-thiols in the study. Interestingly, analysis of the binding curves furthermore suggested similar residual binding to both the alkyne- and the alkene-based surfaces (Figure 5), indicating comparable presentation patterns of the surfaces and potential monofunctionalization of the alkyne-based surfaces. This effect, together with the fact that the thiol-ene/-yne reactions proceed at higher rates, render the alkene-based surfaces more appropriate under the present conditions. More detailed studies are however of interest in order to elucidate the substrate dependency in the alkyne-thiol photoradical reaction when utilizing carbohydrate thiols for future applications.
Conclusions
A methodology to functionalize polymeric materials with carbohydrates using thiol-ene and thiol-yne photoinitiated radical chemistry has been demonstrated. The functionalization of intermediate alkene/alkyne surfaces was successfully performed with both 1-thio-carbohydrates and thioethyl-linked carbohydrate structures in pure water, and at room temperature using long-range UV-irradiation. The results showed that no addition of radical initiator was necessary, and that the reactions proceeded well in aqueous solutions. The method is fast and straightforward, and can easily be employed in other biosensing/glycan array formats. As expected, the produced carbohydrate-presenting surfaces showed good selectivity towards the model lectins used in the study. Generally, the surfaces produced from 1-thio-carbohydrates showed slightly lower responses compared to the corresponding thioethyl-linked structures in the interaction studies. Inexpensive materials and relatively simple organic compounds were used throughout the study and the photochemical steps of the method enable the use of photolithography to produce patterned functionalized surfaces, important factors which further prompt the potential of the method in high-throughput analysis formats.
Supplementary Material
Acknowledgements
This study was supported in part by the Swedish Research Council (contract no. 621-2007-4709), the Royal Institute of Technology, Attana AB, and the National Institutes of General Medical Science (NIGMS) under NIH Award Numbers R01GM080295 and 2R15GM066279.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bader H. 23. The addition of thiolacetic acid to ethynylcarbinols and the conversion of the adducts into aldols and [small alpha][small beta]-unsaturated aldehydes. J. Chem. Soc. 1956:116–121. [Google Scholar]
- Bader H, Cross LC, Heilbron I, Jones ERH. 132. Researches on acetylenic compounds. Part XVIII. The addition of thiolacetic acid to acetylenic hydrocarbons. The conversion of monosubstituted acetylenes into aldehydes and 1 : 2-dithiols. J. Chem. Soc. 1949:619–623. [Google Scholar]
- Bhattacharyya L, Brewer CF. Isoelectric focusing studies of concanavalin A and the lentil lectin. J. Chromatogr. A. 1990;502:131–142. doi: 10.1016/s0021-9673(01)89570-4. [DOI] [PubMed] [Google Scholar]
- Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. Multivalent Carbohydrate Recognition on a Glycodendrimer-Functionalized Flow-Through Chip. ChemBioChem. 2008;9(11):1836–1844. doi: 10.1002/cbic.200800195. [DOI] [PubMed] [Google Scholar]
- Chachadi VB, Inamdar SR, Yu L-G, Rhodes JM, Swamy BM. Exquisite binding specificity of Sclerotium rolfsii lectin toward TF-related O-linked mucin-type glycans. Glycoconjugate J. 2011;28(1):49–56. doi: 10.1007/s10719-011-9323-8. [DOI] [PubMed] [Google Scholar]
- Chan JW, Hoyle CE, Lowe AB. Sequential Phosphine-Catalyzed, Nucleophilic Thiol-Ene/Radical-Mediated Thiol-Yne Reactions and the Facile Orthogonal Synthesis of Polyfunctional Materials. J. Am. Chem. Soc. 2009;131(16):5751–5753. doi: 10.1021/ja8099135. [DOI] [PubMed] [Google Scholar]
- Davis BG, Maughan MAT, Green MP, Ullman A, Jones JB. Glycomethanethiosulfonates: powerful reagents for protein glycosylation. Tetrahedron: Asymmetry. 2000;11(1):245–262. [Google Scholar]
- de Boer A, Hokke C, Deelder A, Wuhrer M. Serum antibody screening by surface plasmon resonance using a natural glycan microarray. Glycoconjugate J. 2008;25(1):75–84. doi: 10.1007/s10719-007-9100-x. [DOI] [PubMed] [Google Scholar]
- de Vries RP, de Vries E, Moore KS, Rigter A, Rottier PJM, de HCAM. Only Two Residues Are Responsible for the Dramatic Difference in Receptor Binding between Swine and New Pandemic H1 Hemagglutinin. J. Biol. Chem. 2011;286(7):5868–5875. doi: 10.1074/jbc.M110.193557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhayal M, Ratner DM. XPS and SPR Analysis of Glycoarray Surface Density. Langmuir. 2009;25(4):2181–2187. doi: 10.1021/la8031122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl LA, Bi S, Baum LG. N- and O-Glycans Modulate Galectin-1 Binding, CD45 Signaling, and T Cell Death. J. Biol. Chem. 2010;285(4):2232–2244. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks BD, Scott TF, Kloxin CJ, Anseth KS, Bowman CN. Thiol-Yne Photopolymerizations: Novel Mechanism, Kinetics, and Step-Growth Formation of Highly Cross-Linked Networks. Macromolecules. 2009;42(1):211–217. doi: 10.1021/ma801903w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks BD, Sims EA, Anseth KS, Bowman CN. Reaction Rates and Mechanisms for Radical, Photoinitated Addition of Thiols to Alkynes, and Implications for Thiol-Yne Photopolymerizations and Click Reactions. Macromolecules. 2010;43(9):4113–4119. [Google Scholar]
- Feinberg H, Powlesland AS, Taylor ME, Weis WI. Trimeric Structure of Langerin. J. Biol. Chem. 2010;285(17):13285–13293. doi: 10.1074/jbc.M109.086058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Garlatti V, Smith DF, Lacroix M, Dumestre-Perard C, Lunardi T, Martin L, Cesbron J-Y, Arlaud GJ, Gaboriaud C, Thielens NM. Carbohydrate Recognition Properties of Human Ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J. Biol. Chem. 2010;285(9):6612–6622. doi: 10.1074/jbc.M109.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Lin BF, Campos LM, Dimitriou MD, Hikita ST, Treat ND, Tirrell MV, Clegg DO, Kramer EJ, Hawker CJ. A versatile approach to high-throughput microarrays using thiol-ene chemistry. Nat. Chem. 2010;2(2):138–145. doi: 10.1038/nchem.478. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Sugihara K, Nakayama J, Akama TO, Wong S-MA, Kawashima H, Zhang J, Smith DF, Ohyama C, Fukuda M, Fukuda MN. Identification of mRNA splicing factors as the endothelial receptor for carbohydrate-dependent lung colonization of cancer cells. Proc. Natl. Acad. Sci. USA. 2009;106(9):3095–3100. doi: 10.1073/pnas.0810110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensarling RM, Doughty VA, Chan JW, Patton DL. "Clicking" Polymer Brushes with Thiol-yne Chemistry: Indoors and Out. J. Am. Chem. Soc. 2009;131(41):14673–14675. doi: 10.1021/ja9071157. [DOI] [PubMed] [Google Scholar]
- Hoogenboom R. Thiol-Yne Chemistry: A Powerful Tool for Creating Highly Functional Materials. Angew. Chem. Int. Ed. 2010;49(20):3415–3417. doi: 10.1002/anie.201000401. [DOI] [PubMed] [Google Scholar]
- Hoorelbeke B, Van DEJM, Rouge P, Schols D, Van LK, Fouquaert E, Balzarini J. Differences in the mannose oligomer specificities of the closely related lectins from Galanthus nivalis and Zea mays strongly determine their eventual anti-HIV activity. Retrovirology. 2011;8:10. doi: 10.1186/1742-4690-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlacher T, Noti C, de PJL, Bindschadler P, Hecht ML, Smith DF, Fukuda MN, Seeberger PH. Characterization of Annexin A1 Glycan Binding Reveals Binding to Highly Sulfated Glycans with Preference for Highly Sulfated Heparan Sulfate and Heparin. Biochemistry. 2011;50(13):2650–2659. doi: 10.1021/bi101121a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlacher T, Seeberger PH. Carbohydrate arrays as tools for research and diagnostics. Chem. Soc. Rev. 2008;37(7):1414–1422. doi: 10.1039/b708016f. [DOI] [PubMed] [Google Scholar]
- Hu H, Armstrong PCJ, Khalil E, Chen Y-C, Straub A, Li M, Soosairajah J, Hagemeyer CE, Bassler N, Huang D, Ahrens I, Krippner G, Gardiner E, Peter K. GPVI and GPIbα mediate staphylococcal superantigen-like protein 5 (SSL5) induced platelet activation and direct toward glycans as potential inhibitors. PLoS One. 2011;6(4):e19190. doi: 10.1371/journal.pone.0019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-Y, Thayer DA, Chang AY, Best MD, Hoffmann J, Head S, Wong C-H. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc. Natl. Acad. Sci. USA. 2006;103(1):15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, Bovin N. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol. Immunol. 2009;46(15):3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kiessling LL, Splain RA. Chemical approaches to glycobiology. Annu. Rev. Biochem. 2010;79:619–653. doi: 10.1146/annurev.biochem.77.070606.100917. [DOI] [PubMed] [Google Scholar]
- Konkolewicz D, Gray-Weale A, Perrier S. Hyperbranched Polymers by Thiol-Yne Chemistry: From Small Molecules to Functional Polymers. J. Am. Chem. Soc. 2009;131(50):18075–18077. doi: 10.1021/ja908206a. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy L, Bess JW, Preston AB, Nagashima K, Mahal LK. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 2009;5(4):244–250. doi: 10.1038/nchembio.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy L, Mahal LK. Glycomic Analysis: An Array of Technologies. ACS Chem. Biol. 2009;4(9):715–732. doi: 10.1021/cb900103n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao W-I, Wang Y-F, Kuo Y-D, Lin C-H, Chang T-C, Su I-J, Wang J-R, Chang C-F. Profiling of influenza viruses by high-throughput carbohydrate membrane array. Future Med. Chem. 2011;3(3):283–296. doi: 10.4155/fmc.10.290. [DOI] [PubMed] [Google Scholar]
- Lee M-r, Shin I. Fabrication of Chemical Microarrays by Efficient Immobilization of Hydrazide-Linked Substances on Epoxide-Coated Glass Surfaces. Angew. Chem. Int. Ed. 2005;44(19):2881–2884. doi: 10.1002/anie.200462720. [DOI] [PubMed] [Google Scholar]
- Lepenies B, Seeberger PH. The promise of glycomics, glycan arrays and carbohydrate-based vaccines. Immunopharm. Immunotoxicol. 2010;32(2):196–207. doi: 10.3109/08923970903292663. [DOI] [PubMed] [Google Scholar]
- Li J, Richards JC. Functional glycomics and glycobiology: an overview. Methods Mol. Biol. 2010;600:1–8. doi: 10.1007/978-1-60761-454-8_1. (Functional Glycomics) [DOI] [PubMed] [Google Scholar]
- Liao H-Y, Hsu C-H, Wang S-C, Liang C-H, Yen H-Y, Su C-Y, Chen C-H, Jan J-T, Ren C-T, Chen C-H, Cheng T-JR, Wu C-Y, Wong C-H. Differential Receptor Binding Affinities of Influenza Hemagglutinins on Glycan Arrays. J. Am. Chem. Soc. 2010;132(42):14849–14856. doi: 10.1021/ja104657b. [DOI] [PubMed] [Google Scholar]
- Lim Y-b, Park S, Lee E, Jeong H, Ryu J-H, Lee MS, Lee M. Glycoconjugate Nanoribbons from the Self-Assembly of Carbohydrate-Peptide Block Molecules for Controllable Bacterial Cell Cluster Formation. Biomacromolecules. 2007;8(5):1404–1408. doi: 10.1021/bm0700901. [DOI] [PubMed] [Google Scholar]
- Linman MJ, Taylor JD, Yu H, Chen X, Cheng Q. Surface Plasmon Resonance Study of Protein-Carbohydrate Interactions Using Biotinylated Sialosides. Anal. Chem. 2008;80(11):4007–4013. doi: 10.1021/ac702566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Conte M, Pacifico S, Chambery A, Marra A, Dondoni A. Photoinduced Addition of Glycosyl Thiols to Alkynyl Peptides: Use of Free-Radical Thiol-Yne Coupling for Post- Translational Double-Glycosylation of Peptides. J. Org. Chem. 2010;75(13):4644–4647. doi: 10.1021/jo1008178. [DOI] [PubMed] [Google Scholar]
- Luallen RJ, Agrawal-Gamse C, Fu H, Smith DF, Doms RW, Geng Y. Antibodies against Manα-1,2-Manα-1,2-Man oligosaccharide structures recognize envelope glycoproteins from HIV-1 and SIV strains. Glycobiology. 2010;20(3):280–286. doi: 10.1093/glycob/cwp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y-K, Lim K-R, Lee BY, Kim KS, Lee W-Y. Microgravimetric lectin biosensor based on signal amplification using carbohydrate-stabilized gold nanoparticles. Chem. Commun. 2008;(39):4771–4773. doi: 10.1039/b807438k. [DOI] [PubMed] [Google Scholar]
- Mahon E, Aastrup T, Barboiu M. Dynamic glycovesicle systems for amplified QCM detection of carbohydrate-lectin multivalent biorecognition. Chem. Commun. 2010;46(14):2441–2443. doi: 10.1039/b924766a. [DOI] [PubMed] [Google Scholar]
- Michel O, Ravoo BJ. Carbohydrate Microarrays by Microcontact "Click" Chemistry. Langmuir. 2008;24(21):12116–12118. doi: 10.1021/la802304w. [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Pérez J, Rumbero Á, Santos JI, Cañada FJ, André S, Gabius H-J, Jiménez-Barbero J, Sinisterra J, Hernáiz MJ. Glycan Tagging to Produce Bioactive Ligands for a Surface Plasmon Resonance (SPR) Study via Immobilization on Different Surfaces. Bioconjugate Chem. 2009;20(4):673–682. doi: 10.1021/bc800350q. [DOI] [PubMed] [Google Scholar]
- Nagre NN, Chachadi VB, Sundaram PM, Naik RS, Pujari R, Shastry P, Swamy BM, Inamdar SR. A potent mitogenic lectin from the mycelia of a phytopathogenic fungus, Rhizoctonia bataticola, with complex sugar specificity and cytotoxic effect on human ovarian cancer cells. Glycoconjugate J. 2010;27(3):375–386. doi: 10.1007/s10719-010-9285-2. [DOI] [PubMed] [Google Scholar]
- Neu U, Maginnis MS, Palma AS, Stroeh LJ, Nelson CDS, Feizi T, Atwood WJ, Stehle T. Structure-Function Analysis of the Human JC Polyomavirus Establishes the LSTc Pentasaccharide as a Functional Receptor Motif. Cell Host Microbe. 2010;8(4):309–319. doi: 10.1016/j.chom.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EC, Storm RJ, Bauer J, Johansson SMC, Lookene A, Aangstroem J, Hedenstroem M, Eriksson TL, Fraengsmyr L, Rinaldi S, Willison HJ, Domelloef FP, Stehle T, Arnberg N. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011;17(1):105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- Norberg O, Deng L, Aastrup T, Yan M, Ramström O. Photo-Click Immobilization on Quartz Crystal Microbalance Sensors for Selective Carbohydrate-Protein Interaction Analyses. Anal. Chem. 2011;83(3):1000–1007. doi: 10.1021/ac102781u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg O, Deng L, Yan M, Ramström O. Photo-Click Immobilization of Carbohydrates on Polymeric Surfaces - A Quick Method to Functionalize Surfaces for Biomolecular Recognition Studies. Bioconjugate Chem. 2009a;20(12):2364–2370. doi: 10.1021/bc9003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg O, Deng L, Yan M, Ramström O. Photo-Click Immobilization of Carbohydrates on Polymeric Surfaces: A Quick Method to Functionalize Surfaces for Biomolecular Recognition Studies. Bioconjugate Chem. 2009b;20(12):2364–2370. doi: 10.1021/bc9003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelaran O, Gildersleeve JC. Glycan arrays: recent advances and future challenges. Curr. Opin. Chem. Biol. 2009;13(4):406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas C, Viswanathan K, Chandrasekaran A, Raman R, Katz JM, Sasisekharan R, Tumpey TM. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011158. No pp. given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee M-R, Shin I. Chemical tools for functional studies of glycans. Chem. Soc. Rev. 2008;37(8):1579–1591. doi: 10.1039/b713011m. [DOI] [PubMed] [Google Scholar]
- Park S, Lee M-R, Shin I. Construction of Carbohydrate Microarrays by Using One-Step, Direct Immobilizations of Diverse Unmodified Glycans on Solid Surfaces. Bioconjugate Chem. 2009;20(1):155–162. doi: 10.1021/bc800442z. [DOI] [PubMed] [Google Scholar]
- Park S, Shin I. Carbohydrate microarrays for assaying galactosyltransferase activity. Org. Lett. 2007;9(9):1675–1678. doi: 10.1021/ol070250l. [DOI] [PubMed] [Google Scholar]
- Pei Y, Yu H, Pei Z, Theurer M, Ammer C, Andre S, Gabius H-J, Yan M, Ramström O. Photoderivatized Polymer Thin Films at Quartz Crystal Microbalance Surfaces: Sensors for Carbohydrate-Protein Interactions. Anal. Chem. 2007a;79(18):6897–6902. doi: 10.1021/ac070740r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Anderson H, Aastrup T, Ramström O. Study of real-time lectin-carbohydrate interactions on the surface of a quartz crystal microbalance. Biosens. Bioelectron. 2005;21(1):60–66. doi: 10.1016/j.bios.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pei Z, Dong H, Caraballo R, Ramström O. Synthesis of Positional Thiol Analogs of β-D-Galactopyranose. Eur. J. Org. Chem. 2007b;2007(29):4927–4934. [Google Scholar]
- Pei Z, Larsson R, Aastrup T, Anderson H, Lehn J-M, Ramström O. Quartz crystal microbalance bioaffinity sensor for rapid identification of glycosyldisulfide lectin inhibitors from a dynamic combinatorial library. Biosens. Bioelectron. 2006;22(1):42–48. doi: 10.1016/j.bios.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Pei Z, Yu H, Theurer M, Waldén A, Nilsson P, Yan M, Ramström O. Photogenerated Carbohydrate Microarrays. ChemBioChem. 2007c;8(2):166–168. doi: 10.1002/cbic.200600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipirou Z, Powlesland AS, Steffen I, Poehlmann S, Taylor ME, Drickamer K. Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology. 2011;21(6):806–812. doi: 10.1093/glycob/cwr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A, Yue T, Heeringa L, Day S, Suh E, Haab BB. A motif-based analysis of glycan array data to determine the specificities of glycan-binding proteins. Glycobiology. 2010;20(3):369–380. doi: 10.1093/glycob/cwp187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 1905;38(1):646–657. [Google Scholar]
- Ready M, Wilson K, Piatak M, Robertus JD. Ricin-like plant toxins are evolutionarily related to single-chain ribosome-inhibiting proteins from Phytolacca. J. Biol. Chem. 1984;259(24):15252–15256. [PubMed] [Google Scholar]
- Revell DJ, Knight JR, Blyth DJ, Haines AH, Russell DA. Self-Assembled Carbohydrate Monolayers: Formation and Surface Selective Molecular Recognition. Langmuir. 1998;14(16):4517–4524. [Google Scholar]
- Saravanan C, Cao Z, Head SR, Panjwani N. Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays. Glycobiology. 2010;20(1):13–23. doi: 10.1093/glycob/cwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada R, Sun S-M, Wu X-H, Hong F, Ragupathi G, Livingston PO, Scholz WW. Human Monoclonal Antibodies to Sialyl-Lewisa (CA19.9) with Potent CDC, ADCC, and Antitumor Activity. Clin. Cancer Res. 2011;17(5):1024–1032. doi: 10.1158/1078-0432.CCR-10-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CL, Mukhopadhyay B, Hardy SM, McDonnell MB, Field RA, Russell DA. Colorimetric detection of Ricinus communis Agglutinin 120 using optimally presented carbohydrate-stabilised gold nanoparticles. Analyst. 2008;133(5):626–634. doi: 10.1039/b715250g. [DOI] [PubMed] [Google Scholar]
- Seeberger PH. Chemical glycobiology: why now? Nat. Chem. Biol. 2009;5(6):368–372. doi: 10.1038/nchembio0609-368. [DOI] [PubMed] [Google Scholar]
- Semsarilar M, Ladmiral V, Perrier Sb. Highly Branched and Hyperbranched Glycopolymers via Reversible Addition-Fragmentation Chain Transfer Polymerization and Click Chemistry. Macromolecules. 2010;43(3):1438–1443. [Google Scholar]
- Singh SK, Streng-Ouwehand I, Litjens M, Weelij DR, García-Vallejo JJ, van Vliet SJ, Saeland E, van Kooyk Y. Characterization of murine MGL1 and MGL2 C-type lectins: Distinct glycan specificities and tumor binding properties. Mol. Immunol. 2009;46(6):1240–1249. doi: 10.1016/j.molimm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Stevens J, Chen L-M, Carney PJ, Garten R, Foust A, Le J, Pokorny BA, Manojkumar R, Silverman J, Devis R, Rhea K, Xu X, Bucher DJ, Paulson J, Cox NJ, Klimov A, Donis RO. Receptor specificity of influenza A H3N2 viruses isolated in mammalian cells and embryonated chicken eggs. J. Virol. 2010;84(16):8287–8299. doi: 10.1128/JVI.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X-L, Stabler CL, Cazalis CS, Chaikof EL. Carbohydrate and Protein Immobilization onto Solid Surfaces by Sequential Diels-Alder and Azide-Alkyne Cycloadditions. Bioconjugate Chem. 2006;17(1):52–57. doi: 10.1021/bc0502311. [DOI] [PubMed] [Google Scholar]
- Sweeney EC, Tonevitsky AG, Temiakov DE, Agapov II, Saward S, Palmer RA. Preliminary crystallographic characterization of ricin agglutinin. Proteins. 1997;28(4):586–589. doi: 10.1002/(sici)1097-0134(199708)28:4<586::aid-prot12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Tyagi A, Wang X, Deng L, Ramström O, Yan M. Photogenerated carbohydrate microarrays to study carbohydrate-protein interactions using surface plasmon resonance imaging. Biosens. Bioelectron. 2010;26(2):344–350. doi: 10.1016/j.bios.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzawa H, Ohga K, Shinozaki Y, Ohsawa I, Nagatsuka T, Seto Y, Nishida Y. A novel sugar-probe biosensor for the deadly plant proteinous toxin, ricin. Biosens. Bioelectron. 2008;24(4):923–927. doi: 10.1016/j.bios.2008.07.049. [DOI] [PubMed] [Google Scholar]
- Wang C, Ren P-F, Huang X-J, Wu J, Xu Z-K. Surface glycosylation of polymer membrane by thiol-yne click chemistry for affinity adsorption of lectin. Chem. Commun. 2011;47(13):3930–3932. doi: 10.1039/c1cc10634a. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. New York: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- Wendeln C, Rinnen S, Schulz C, Arlinghaus HF, Ravoo BJ, editors. Photochemical Microcontact Printing by Thiol-Ene and Thiol-Yne Click Chemistry. Langmuir. 2010;26(20):15966–15971. doi: 10.1021/la102966j. [DOI] [PubMed] [Google Scholar]
- Wu C-Y, Liang P-H, Wong C-H. New development of glycan arrays. Org. Biomol. Chem. 2009;7(11):2247–2254. doi: 10.1039/b902510n. [DOI] [PubMed] [Google Scholar]
- Wu C-Y, Wong C-H. Chemistry and glycobiology. Chem. Commun. 2011;47(22):6201–6207. doi: 10.1039/c0cc04359a. [DOI] [PubMed] [Google Scholar]
- Xu R, McBride R, Paulson JC, Basler CF, Wilson IA. Structure, Receptor Binding, and Antigenicity of Influenza Virus Hemagglutinins from the 1957 H2N2 Pandemic. J. Virol. 2010;84(4):1715–1721. doi: 10.1128/JVI.02162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Chan JW, Hoyle CE, Lowe AB. Sequential thiol-ene/thiol-ene and thiolene/thiol-yne reactions as a route to well-defined mono and bis end-functionalized poly(N-isopropylacrylamide) J. Polym. Sci. A: Polym. Chem. 2009;47(14):3544–3557. [Google Scholar]
- Zhang Y, Luo S, Tang Y, Yu L, Hou K-Y, Cheng J-P, Zeng X, Wang PG. Carbohydrate-protein interactions by "Clicked" carbohydrate self-assembled monolayers. Anal. Chem. 2006;78(6):2001–2008. doi: 10.1021/ac051919+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.