Abstract
Breast cancer is the leading cancer among women world-wide, affecting 1 of 8 women during their lifetimes. In the US alone, some 2 million breast cancer survivors comprise 20% of all cancer survivors. Conservatively, it is estimated that some 20-40% of all breast cancer survivors will develop the health deviation of lymphedema or treatment-related limb swelling over their lifetimes. This chronic accumulation of protein-rich fluid predisposes to infection, leads to difficulties in fitting clothing and carrying out activities of daily living, and impacts self-esteem, self-concept, and quality of life. Lymphedema is associated with self-care deficits (SCD) and negatively impacts self-care agency (SCA) and physiological and psychosocial well-being. Objectives of this report are two-fold: (1) to explore four approaches of assessing and diagnosing breast cancer lymphedema, including self-report of symptoms and the impact of health deviations on SCA; and (2) to propose the development of a clinical research program for lymphedema based on the concepts of Self-Care Deficit Nursing Theory (SCDNT). Anthropometric and symptom data from a National-Institutes-of-Health-funded prospective longitudinal study were examined using survival analysis to compare four definitions of lymphedema over 24 months post-breast cancer surgery among 140 of 300 participants (all who had passed the 24-month measurement). The four definitions included differences of 200 ml, 10% volume, and 2 cm circumference between pre-op baseline and/or contralateral limbs, and symptom self-report of limb heaviness and swelling. Symptoms, SCA, and SCD were assessed by interviews using a validated tool. Estimates of lymphedema occurrence varied by definition and time since surgery. The 2 cm girth change provided the highest estimation of lymphedema (82% at 24 months), followed by 200 ml volume change (57% at 24 months). The 10% limb volume change converged with symptom report of heaviness and swelling at 24 months (38-39% lymphedema occurrence), with symptom report being the earliest predictor of lymphedema occurrence than any other measurement. Findings verify the importance of subjective assessment by symptom report of limb changes and SCD following breast cancer treatment as an essential tool in early detection and treatment of lymphedema. Findings also support the importance of pre-operative baseline measurements, symptom history, and SCA for later post-op comparisons. These preliminary findings underscore the importance of strengthening SCA by educating breast cancer survivors. Self assessment, early detection, and early treatment hold the best promise for optimal management of this chronic condition, limiting detrimental effects on SCA, and improving quality of life and physiological and psychosocial well-being. These findings lay the foundation for a clinical research program in breast cancer lymphedema based on SCDNT in which education in and awareness for self-report of lymphedema-associated symptoms is a first step in screening. Increasing patient knowledge through education will increase SCA by identifying ane providing information to meet self-care requisites (SCR) related to the health deviation of lymphedema. The nurse has the opportunity to assist patients in developing self-care actions as needed to meet universal and health deviation therapeutic requisites to address self-care demands following breast cancer treatment.
Keywords: breast cancer, lymphedema, self-care agency, self-care deficit, Orem
Over the last decade, major advances have been made in the prevention and treatment of breast cancer with minimal attention being given to the treatment side effect of lymphedema. More than 2 million U.S. breast cancer survivors are at a lifetime risk of developing lymphedema (LE), a chronic condition which can cause debilitating side effects and diminish self-care agency (SCA) (ACS, 2007; Armer, 2006).
According to the Self-Care Deficit Nursing Theory (SCDNT) of nursing, developed by the late Dorothea Orem (1980, 2001)…, “self-care requires both learning and use of knowledge as well as enduring motivation and skill. The learning process includes the individual's gradual development of a repertoire of self-care practices and related skills” (p. 271). She emphasized that:
… if a nurse sees only movements toward health, toward more effective living, without seeing the demands and burdens that injury, illness, and health care place upon a patient, the basis for nursing diagnosis and prescription is incomplete. The nursing perspective will be inaccurate, and the nurse will not have a sound basis for proceeding toward the nursing goal of assisting the patient in responsible action in matters of self-care (Orem, 1980, p. 167).
The purpose of this report is to explore four approaches of assessing and diagnosing breast cancer LE occurrence. This includes self-report of symptoms (being aware of and taking care of the effect of LE) and the impact of health deviations on self-care agency. The second goal is to propose the development of a clinical research program for LE based on the SCDNT. This involves assessing the patient's universal, developmental and health deviation related requisites, determining the patient's therapeutic self-care demands (TSCD) related to these requisites, and assisting the patient as required in a supportive-educative and partially compensatory nurse role.
Defining Lymphedema
Lymphedema is the accumulation of protein-rich fluid in the interstitial space in the affected area
The lymphatic system normally returns 10% of the extracellular fluids and plasma proteins from the interstitial spaces to the heart. Secondary LE results when protein-rich lymph fluid becomes trapped in the extracellular spaces due to identifiable external damage to this system. This is inherently where the problem lies as the ineffective function and/or damaged structure of the lymphatic system impairs movement of the protein-rich lymph fluid from the interstitial space into the lymphatic system for re-distribution into the arterio-venous system at the subclavian vein (Lasinski, 2007). The condition lends itself to infection, sometimes life-threatening when spreading systemically, leads to difficulties in clothing fit and activities of daily living, and also affects self-esteem, self-identity, and quality of life (Armer, 2006; Radina & Armer, 2004). Potential contributing factors to this condition include surgical removal of lymph nodes, traumatic injury, radiation, infection, underlying or pre-existing impaired lymphatic structure and function, and/or individual predisposition(s) and basic conditioning factors yet to be fully understood (Földi, 2003).
Types of lymphedema include primary and secondary and these may exist as acute or chronic conditions (American Cancer Society, 2007). LE may exist in mild, moderate, or severe states. Földi (2003) identifies these stages (phases) of LE. Secondary lymphedema may affect the arm, breast, and chest in the person treated for breast cancer, while most commonly affecting the ipsilateral arm.
Breast cancer treatment is the leading cause of secondary (acquired) lymphedema in developed countries of the world (ACS, 2003; Lymphedema in the developing and developed world: Contrasts and prospects, 1998). Scientific literature reports 3-87% of the breast cancer population may experience lymphedema (Coen, Taghian, Kachnic, Assaad & Powell, 2003; Deutsch & Flickinger, 2003; Ozaslan & Kuru, 2003; Voogd et al., 2003) while medical literature estimates 15-20% LE occurrence in breast cancer survivors (Disa & Petrek, 2001). Recent figures indicate an estimation of 20-40% overall (Armer & Whitman, 2002; Armer & Stewart, 2005). Reported incidence of LE varies greatly among each patient group at risk for LE (Armer & Stewart, 2005; Armer, 2005; Armer, Fu et al., 2004). Discrepancies in LE occurrence are due to inconsistent definitions of lymphedema, lack of definitive subjective and objective measurement, medical procedures (e.g. sentinel lymph node biopsy/axillary node dissection, radiation and surgery) that are not always accounted for in analyses and research findings, and varying periods of follow-up. These discrepancies often lead to inaccurate or incomplete reports, overlooked diagnosis, and under-treatment of LE in the health care setting.
Research in Lymphedema
Dr. Jane Armer, PhD, RN, and her multidisciplinary team at the University of Missouri-Columbia are currently addressing this problem by leading a National-Institutes-of-Health-funded prospective study, the first of its kind, and one which builds upon a series of studies of increasing sophistication and rigor (Table 1).
Table 1. Post-Breast Cancer Lymphedema Research Chronology at University of Missouri-Columbia (1998-present).
|
Lymphedema Measurement
The Lymphedema and Breast Cancer Questionnaire (LBCQ), an assessment tool for LE signs and symptoms, is a 57-item questionnaire examining 19 signs and symptoms of LE (Armer et al., 2003). This tool was developed following a comprehensive literature review and with the participation of expert oncology nursing clinicians and the multidisciplinary lymphedema research team. It was piloted with patients with known breast cancer lymphedema and healthy women with no history of breast cancer or lymphedema. The LBCQ in its present form has been used in more than six studies at the University of Missouri-Columbia and is in use at multiple sites in the US and internationally. The tool has 2-hour test-retest reliability with healthy women (0.98), with two less stable items (∼0.75): self reported weight and report of repetitive movement in daily activity.
Using LBCQ Symptom Report to Predict LE
In a secondary analysis designed to test the predictive validity of the LBCQ, data from two samples were utilized. The first sample (Sample A) was comprised of 2 distinct groups of women, 40 of whom were healthy women with no breast cancer or LE history. The other 40 women had known post-breast cancer LE of one upper extremity. The second (Sample B) consisted of 100 breast cancer survivors with and without LE, with data collected cross-sectionally from survivors less than one year to more than 20 years after treatment. Hierarchical multiple regression was used to assess the contribution of the 19 symptoms of the LBCQ to a prediction of lymphedema occurrence, defined as 2 cm or greater difference between limbs at matched anatomical points, in the absence of the preferred pre-op baseline comparisons. Symptoms were clustered and entered based on theoretical and clinical best judgments by the PI and team (Table 2).
Table 2. LBCQ Symptom Report to Predict LE using Hierarchical Regression (Armer et al., 2003).
| Sample A: LE-nonLE (n=80) | Sample B: BrCA+/-LE (n=100) | |
|---|---|---|
| Group#1 – Heaviness | → | Heaviness |
| (c=0.775) p<0.0001 | ||
| ↓ | ||
| Group#2 – Swelling (general) | → | Heaviness and Swelling (general) |
| (c=0.919) p<0.0001 | ||
| ↓ | ||
| Group#3,4,5 – Swelling (trunk), Infection-related, Aching | → | Heaviness and Swelling (general) |
| (c=0.919) p<0.0001 | ||
| ↓ | ||
| Group#6 – Numbness, Neurological-type | → | Heaviness, Swelling (general) |
| (c=0.952) p=0.0080 |
In Study A, three self-reported symptoms predicted lymphedema in the model of best fit (c = .952):
“Heaviness in the past year,”
“Swelling now,” and
“Numbness in the past year.”
In Study B, prediction of absolute maximal circumferential limb difference (i.e., ≥ 2 cm differences) revealed two symptoms: “Heaviness in the past year” (p = .0279) and “Swelling now” (p = .0007) were predictive of lymphedema. “Numbness in the past year” was not predictive of lymphedema. However, interestingly, those with lesser limb differences reported the symptom of numbness more often.
Thus, “heaviness in the past year” and “swelling now” were found to be most predictive of limb swelling at the 2 cm bilateral comparison criteria, and numbness and other symptoms did not significantly improve the prediction of LE.
Aims of the Parent Study
The primary aim of the parent study was to compare reliability of two limb volume (LV) measurement methods in assessing lymphedema. The secondary aim involved exploring psychosocial aspects of coping and social support; LV and LE management on adjustment, functional health, and QOL. The study design included 200 persons newly-diagnosed with breast cancer who consented and were enrolled and assessed at pre-op, post-op, and every 3-6 months through 30 months after diagnosis. One hundred additional persons enrolled within 3 months post-op, and were followed for 30 months using the internal research match money at the University of Missouri. At the time of this analysis, 287 individuals were actively enrolled and followed, with 143 participants at 24 months data completion.
Methods
At each lab visit, anthropometric (circumferences and perometer) assessment of the limb and limb volume were completed, as follows:
-
Anthropometric Measurements – each visit
Circumferences
Perometry
-
Interview – each visit
Signs and symptoms (LBCQ)
Symptom management
-
Mail Back Survey – post-op, 12 months, 24 months
Problem-solving (PSI)
Social support (SPS)
Adjustment to chronic illness (PAIS-SR)
Quality of life (FLIC)
Functional status (SF-36)
Symptom distress (BSI)
The First 24 Months: Preliminary Analysis of Lymphedema Occurrence 24 Months Following Breast Cancer Surgery
Survival Analysis
Survival analysis was chosen as the analytic technique to support the examination of data for the occurrence of LE at selected time points (Machin, Cheung & Parmar, 2006). Survival curves were estimated using the LIFETEST procedure in SAS (SAS Institute Inc., Cary, NC, USA). ☺With this approach, these conditions were accepted:
There are different follow-up times for different people, since participants are enrolled over time.
We measure time until LE is diagnosed.
If no LE is diagnosed, time is used as a censored observation, (that is, time until LE is at least as long as has been observed, but actual time to LE is unknown).
Time points for LE are estimations with limb volume measured every 3 months (for one year) and every six months (to 30 months), not daily.
Definitions of LE
The four definitions of LE selected from the literature and clinical practice are:
200 ml LV difference from baseline or other limb
10% LV difference from baseline or other limb
2 cm circumference difference from baseline or other limb
Symptom report of swelling or heaviness in affected limb
Results
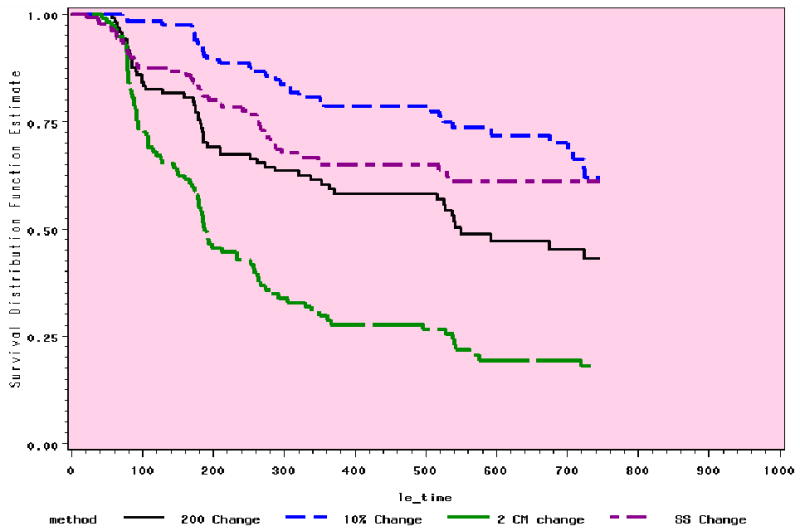
The range of occurrence at 6 to 24 months with the 4 definitions is from 7% to 82% (Table 3), depending on time and definition. Figures 1 and 2 demonstrate the relative stability of the trends in the preliminary analyses at 12 and 24 months.
Table 3. Comparison of 4 Methods: Defining Lymphedema at 6, 12, 18, and 24 months (CI = 95).
|
Figure 1. Comparison of 4 Methods: 12 Month Trends (n=118).
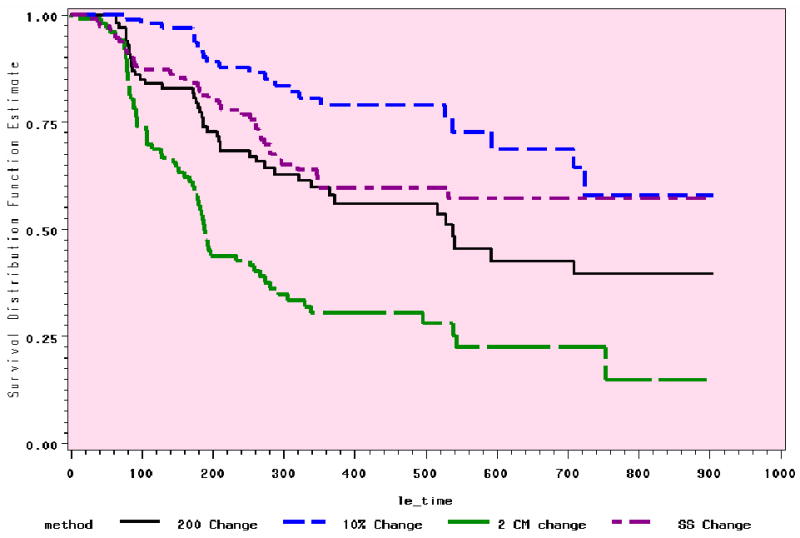
Figure 2. Comparison of 4 Methods: 24 Month Trends (n=143).
Preliminary Conclusions
Preliminary Anthropometric Conclusion
Based on this preliminary analysis, the following conclusions were drawn:
Discrepancies in literature reports of LE are due to definition and measurement issues.
Post-surgical LV changes are assessable by circumferences and perometry.
Baseline (preferably pre-op) bilateral LV assessments are essential for evaluation of LV change over time.
Self-report of symptoms is an important source of data in evaluating limb volume changes, LE, and treatment effects.
Orem's Self Care Deficit Nursing Theory
As a foundational step in the development of a clinical nursing research program in lymphedema, universal self-care requisites were reviewed for relevance to the imbalance associated with lymphedema occurrence (Orem, 2001, p. 225). Health deviation self-care requirements and self-care limitations in lymphedema were identified (Table 4) and universal health deviation self-care requisites were reviewed for relevance (Table 5) as applied to lymphedema (Orem, 2001, 6th edition, p. 235). Nursing and patient actions to maintain or return the patient with LE to optimal well-being are outlined (Table 7).
Table 4. Selected Universal Self-Care Requisites as Related to Lymphedema.
| Maintain Balance Between Activity and Rest |
|
| National Lymphedema Network [NLN], 2005 |
| Prevent Hazards to Life, Functioning, and Well-Being |
|
| NLN, 2005 |
Table 5. Health Deviation Self-Care Requisites.
| Be Aware and Take Care of Effects of Pathological Conditions |
|
| NLN, 2005 |
| Effectively Carry Out Prescribed Diagnostic, Therapeutic, or Rehabilitative Measures |
|
| NLN, 2005 |
|
| Learn to Live with Effects of Pathological Conditions and Medical Care Measures |
|
| NLN, 2005 |
| Opportunities for Growth due to Patient Self-Care Limitations Secondary to LE |
| Self-care limitations in lymphedema are opportunities for both the nurse and patient. It is an opportunity for the nurse to provide and for the patient to seek information regarding the pathological condition and how to manage self-care of the condition through development of the patient's personal self-care practices and individualized skills (Orem, 2001, p. 270). |
Table 7. Nursing and Patient Actions in the Presence of Lymphedema.
|
| Research Findings and SCDNT |
| Findings from this report confirm the importance of nursing assessment of patient-reported symptom experience in determining occurrence of LE and designing a plan of care to maximize SCA. It is important to note that symptoms preceded changes in objective anthropometric measurement in confirmation of LE. |
| Rationale for Nursing Concern about LE Impact on Self-Care Agency |
| A nursing focus is unrealistic if it does not take into account how the patient views and is personally affected by his illness. The nurse's acceptance of the patient's point of view is essential if the patient is to be assisted through nursing to live with his illness and disability, to cooperate with those who assist him, and above all to be motivated to direct his energies toward recovering a normal or near-normal state of health. (Orem, 2001, 6th edition, p. 194) |
Discussion
The foundations of Orem's nursing theory will help to identify crucial factors in the prevention, early detection, and treatment of lymphedema. A prospective longitudinal intervention study for women preparing for breast cancer treatment (with a pre-operative LV baseline) focuses upon preventive intervention through self-care, self-care agency, and self-care deficits. By understanding the demands placed upon a patient (e.g., resources, injury, illness, environment), the nurse can then better understand factors influencing early LE detection. This is critical in optimal management and treatment of lymphedema. By committing to (1) the evaluation of the efficacy of preventive behaviors and treatment interventions; and (2) the understanding of the psychosocial impact of breast cancer LE, the nurse can greatly improve upon goals of earlier and more effective LE treatment. In order to accomplish these goals, a single site Risk Reduction Intervention (funded by the Lance Armstrong Foundation), is in process, to be followed by a multi-site study. Multi-Site National LE Clinical Trials (e.g. CALGB 70305) are currently underway. The parent grant for this research report has now received funding through the National Institutes of Health for continued follow-up to 7 years for study participants.
Table 6. Areas of Personalized Self-Care Practices and Skills in Lymphedema.
|
Acknowledgments
The data for this project were supported by Grant Number 1 RO1 NR05342-01 from the National Institute for Nursing Research, National Institutes of Health, MU PRIME funds, University of Missouri-Columbia, and Ellis Fischel Cancer Center research funds. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. The authors thank Dr. Richard Madsen, the members of the Lymphedema Research Team (e.g. co-investigators, research nurses, research associates, research assistants), and the study participants.
Contributor Information
Jane M. Armer, Professor, Sinclair School of Nursing, University of Missouri-Columbia, Director, Nursing Research, Ellis Fischel Cancer Center, Columbia, MO USA.
Mary H Henggeler, Research Nurse, Lymphedema Research Project, Sinclair School of Nursing.
Constance W. Brooks, Associate Professor of Teaching, Sinclair School of Nursing.
Eris A. Zagar, Clinical Nurse Specialist, Medial Oncology, Ellis Fischel Cancer Center.
Sherri Homan, Public Health Epidemiologist, Missouri Department of Health and Senior Service, Adjunct Clinical Faculty, Sinclair School of Nursing.
Bob R. Stewart, Adjunct Clinical Faculty, Sinclair School of Nursing, Professor Emeritus, Agricultural Education.
References
- American Cancer Society. Cancer facts & figures – 2007. Atlanta, GA: Author; 2007. [Google Scholar]
- Armer JM. The health deviation of post-breast cancer lymphedema: Symptom assessment and impact on self-care agency. Paper presented at the 9th World Congress Self-Care Deficit Nursing Theory; Johannesburg, South Africa. 2006. Jul, [PMC free article] [PubMed] [Google Scholar]
- Armer JM. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Investigation. 2005;23:76–83. [PubMed] [Google Scholar]
- Armer JM. Case Study: Upper Limb Swelling Following Mastectomy: Lymphedema or Not? ONCOLOGY Nurse Edition. 2007 March;21(4):2–4. [PubMed] [Google Scholar]
- Armer JM, Heckathorn P. Post-breast cancer lymphedema in aging women: Self-management and implications for nursing. Journal of Gerontological Nursing. 2005;31(5):29–39. doi: 10.3928/0098-9134-20050501-07. [DOI] [PubMed] [Google Scholar]
- Armer JM, Fu M, Wainstock J, Zagar E, Jacobs LK. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology. 2004;37(1):73–91. [PubMed] [Google Scholar]
- Armer JM, Culbertson SD, Radina ME, Porock D. Prediction of breast cancer lymphedema based on lymphedema and breast cancer questionnaire (LBCQ) symptom report. Nursing Research. 2003;52(6):370–9. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic Research and Biology. 2005;3(4):208–17. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- Armer JM, Whitman M. The problem of lymphedema following breast cancer treatment: Prevalence, symptoms, and self-management. Lymphology. 2002;35(Suppl):153–159. [Google Scholar]
- Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. International Journal of Radiation Oncology Biology Physics. 2003;55(5):1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Flickinger JC. Arm edema after lumpectomy and breast irradiation. American Journal of Clinical Oncology. 2003;26(3):229–31. doi: 10.1097/01.COC.0000018177.75673.06. [DOI] [PubMed] [Google Scholar]
- Disa J, Petrek J. Rehabilitation after treatment for cancer of the breast. In: Devita V, Hellman S, Rosenberg S, editors. Cancer-Principles and Practice of Oncology. 6th. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 1717–1725. [Google Scholar]
- Földi M, Földi E, Kubik S. Textbook of Lymphology for Physicians and Lymphedema Therapists. Germany: Elsevier GmbH; 2003. [Google Scholar]
- Geller BM, Vacek PM, O'Brien P, Secker-Walker RH. Factors associated with arm swelling after breast cancer surgery. Journal of Womens' Health. 2003;12(9):921–30. doi: 10.1089/154099903770948159. [DOI] [PubMed] [Google Scholar]
- Lasinski B. The Lymphatic System. In: Goodman Catherine, et al., editors. Pathology: Implications for the physical therapist. 3rd. Saunders; 2007. [Google Scholar]
- Lymphedema in the developing and developed world: contrasts and prospects. Lymphology. 1998;21:242–243. [PubMed] [Google Scholar]
- Machin D, Cheung YB, Parmar MK. Survival analysis: A practical approach. 2nd. England: John Wiley & Sons Ltd; 2006. [Google Scholar]
- National Lymphedema Network. Lymphedema Risk Reduction Practices. 2007 Oct 8; Retrieved. from http://www.lymphnet.org/pdfDocs/nlnriskreduction.pdf.
- Orem DE. Nursing concepts of practice. 2nd. New York: McGraw-Hill; 1980. [Google Scholar]
- Orem DE. Nursing concepts of practice. 6th. St Louis: Mosby; 2001. [Google Scholar]
- Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. American Journal of Surgery. 2004;187(1):69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- Radina ME, Armer JM, Daunt D, Dusold JM, Culbertson SD. Self-reported symptom management of post-breast-cancer lymphedema. Journal of Lymphoedema. 2007 in press. [PMC free article] [PubMed] [Google Scholar]
- Radina ME, Armer JM. Surviving breast cancer and living with lymphedema: Resiliency among women and their families. Journal of Family Nursing. 2004;10(4):485–505. [Google Scholar]
- Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. British Journal of Surgery. 2003;90(1):76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]


