Abstract
Cardiac stem cells are promising candidates for use in myocardial regenerative therapy. We test the hypothesis that growing cardiac-derived cells as three-dimensional cardiospheres may recapitulate a stem cell niche-like microenvironment, favoring cell survival and enhancing functional benefit after transplantation into the injured heart. Cardiac stem cells and supporting cells from human endomyocardial biopsies were grown as cardiospheres and compared to cells cultured under traditional monolayer condition or dissociated from cardiospheres. Cardiospheres self-assembled into stem cell niche-like structures in vitro in suspension culture, while exhibiting greater proportions of c-kit+ cells and up-regulated expression of SOX2 and Nanog. Pathway-focused PCR array, quantitative RT-PCR, and immunostaining revealed enhanced expression of stem cell-relevant factors and adhesion/extracellular-matrix molecules (ECM) in cardiospheres, including IGF-1, HDAC2, Tert, integrin-α2, laminin-β1 and MMPs. Implantation of cardiospheres in SCID mouse hearts with acute infarction disproportionately improved cell engraftment and myocardial function, relative to monolayer-cultured cells. Dissociation of cardiospheres into single cells decreased the expression of ECM and adhesion molecules and undermined resistance to oxidative stress, negating the improved cell engraftment and functional benefit in vivo. Growth of cardiac-derived cells as cardiospheres mimics stem cell niche properties, with enhanced “stemness” and expression of ECM and adhesion molecules. These changes underlie an increase in cell survival and more potent augmentation of global function following implantation into the infarcted heart.
Keywords: cardiac stem cells, stem cell niche, extracellular-matrix molecules, adhesion molecule
Stem cells from multiple sources (including bone marrow-derived mononuclear cells, endothelial progenitor cells, cardiac stem cells, and embryonic stem cells) have been used in attempts to regenerate the damaged heart [1-15]. Among these, resident cardiac stem cells are particularly promising, as they inherently mediate cardiogenesis and angiogenesis [5-14], both by direct regeneration [6,10,13] and indirectly via paracrine effects [14]. We have focused upon expanding the very small population of resident cardiac stem cells from minimally-invasive human heart biopsies using ex vivo culture [9,10]. The cells that grow out spontaneously from such biopsies include both cardiac stem cells and supporting cells (collectively termed cardiac-derived cells); millions of cardiac-derived cells can be harvested from minimally-invasive heart biopsies within days to weeks [12]. This technological advance permits the use of autologous cardiac stem cells in the repair of injured hearts, while avoiding many of the complications of other approaches. Clinical application of cells derived using this technology is already under way in the CADUCEUS trial (see clinicaltrials.gov for details).
Direct expansion of resident cardiac stem cells from surgical human biopsies was originally described by Messina et al. [9], who collected cardiac-derived cells and sub-cultured them as three-dimensional (3D) cell aggregates, named cardiospheres after the neurosphere experience [16]. We adapted and miniaturized the cardiosphere methodology for utility with percutaneous endomyocardial biopsies as the tissue of origin, plating cardiospheres in monolayer culture to yield therapeutically-relevant numbers of cardiosphere-derived cells (CDCs) [10]. The advantage of CDC monolayer culture lies in the timely expansion of cells to meaningful intracoronary and intramyocardial doses while avoiding tedious selection or sub-culture techniques. Transplanted CDCs have been shown by us and by others to regenerate myocardium and to improve various functional indices in the injured heart [10,11,15,17].
Although CDCs can be conveniently expanded and delivered via the intracoronary route, several findings give reason to wonder whether cardiospheres might be more potent than dispersed cells such as CDCs. Direct intramyocardial injection of small numbers of cardiospheres effectively doubles left ventricular (LV) fractional shortening following myocardial infarction [9], while large numbers of monolayer-cultured CDCs injected in a similar manner (in a different laboratory) yield only a 64% increase in the LV ejection fraction [10]. Furthermore, cardiosphere culture increases the expression of c-kit, a stem cell marker [9], while subsequent transition from cardiosphere to monolayer culture results in decreased c-kit expression [10]. These results suggest that cardiosphere culture might enhance the “stemness” of cardiac-derived cells, and that the implantation of cardiospheres may disproportionately boost myocardial function relative to monolayer-cultured cells, but a direct, systematic comparison is necessary to reach firm conclusions. Intramyocardial injection is necessary for such a study, as cardiospheres are sufficiently large (>50μm) that they would be expected, from first principles [18], to microembolize the blood vessels if delivered via the intracoronary route.
Here, we tested the hypothesis, first articulated by Anversa et al. [19], that cardiospheres may recapitulate key features of stem cell niches, thereby increasing cell survival as well as functional benefit after implantation into the infarcted heart. The investigation has fundamental as well as practical applications, as emergent insights may point to which cell product (cardiospheres vs. dissociated heart-derived cells) is superior for regenerative applications.
Materials and Methods
Human heart tissue biopsies and ex vivo expansion of cardiac stem cells
Percutaneous endomyocardial heart biopsies were obtained from the right ventricular aspect of the septum in 11 different patients during clinically-indicated procedures after informed consent. Cardiac stem cells and supporting cells were collected and expanded as described [10], but with some modifications (Supporting Information Figure 1). Briefly, biopsies were minced into small fragments. After 30 min digestion with 0.2 mg/ml collagenase, the tissue fragments were cultured as “explants” on dishes coated with 20 μg/ml fibronectin (BD Biosciences). Within 1-2 weeks, stromal-like flat cells, and phase-bright round cells, emerged from the tissue fragments and became confluent. These cardiac-derived cells were harvested using 0.25% trypsin (Gibco), and then cultured as cardiospheres on poly-D-Lysine (20 μg/mL; BD Biosciences) coated plates or as monolayers on fibronectin-coated dishes. CDCs were grown by seeding cardiospheres on fibronectin-coated dishes and passaged twice as described [10]. We also compared cardiospheres primarily formed from cardiac-derived cells of the “explant” culture stage (primary cardiosphere, Pri-CSp) and cardiospheres re-formed from twice-passaged CDCs (termed secondary cardiosphere, 2nd-CSp). All cultures were incubated in 5% CO2 at 37°C, using IMDM basic medium (Gibco) supplemented with 20% FBS (Hyclone), 1% penicillin/streptomycin, and 0.1 mM 2-mercaptoethanol.
Estimation of cell proliferative activity
Cardiac-derived cells were collected and cultured as cardiospheres or monolayers for 1, 3 or 7 days. Cardiospheres were collected and dissociated by 30 minutes of digestion with 0.25% trypsin, while monolayer-cultured cells were harvested by 5 minutes of digestion with 0.25% trypsin. The total numbers of cells were counted and the fold increases of seeded cells were calculated to determine the proliferative activity of cells under different culture conditions.
Immunostaining
To observe if cardiospheres architecturally mimic stem cell niches at the cellular and molecular levels [19-21], we harvested cardiospheres after 3 days of culture, fixed and embedded them in OCT compound, and then sectioned them for immunostaining analysis [10]. Briefly, after blocking for 30 min, sections were incubated with FITC-conjugated mouse anti-human c-kit antibody (eBioscience) for 1 hr at room temperature. After washing, sections were incubated with primary antibodies against human collagen IV or CD105 (Lifespan Bioscience), respectively, and then stained with relative PE-conjugated secondary antibodies. Cell nuclei were stained with DAPI.
Flow cytometry
To investigate if cardiosphere culture can maintain and improve the expression of the stem cell antigen c-kit, we grew cells as cardiospheres or monolayers for 3 days. Cells were harvested as single cell suspensions as described above, and then incubated with PE-conjugated mouse anti-human c-kit antibody (eBioscience) for 1 hr. Isotype-identical antibody served as a negative control. Quantitative flow cytometry analysis was performed with FACS Calibur equipment and CellQuest software (BD Biosciences) [10,22].
Quantitative real-time PCR (qRT-PCR)
To determine if cardiosphere culture can maintain and improve “stemness”, we examined the expression of SOX2 and Nanog by qRT-PCR. Briefly, cells were collected by scraping after 3 days of culture as cardiospheres or monolayers, and total RNA was extracted using RNeasy Micro Kit (Qiagen). The specific primers and probes for SOX2 and Nanog were obtained from Applied Biosystems, and RNA expression levels were quantified with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) according to the manufacturer’s protocol. Experiments were performed in triplicate, including no template and reverse transcriptase minus controls. The assays used 50 ng of RNA per sample, and the housekeeping gene GAPDH was used to normalize all samples.
Pathway-focused PCR array
Using the RT2 Profiler™ PCR Array System (SABiosciences Corporation, Frederick, MD), we compared gene expression of stem cell relevant factors, extracellular matrix (ECM) and adhesion molecules after 3 days of culture as cardiospheres or monolayers. Each array consists of more than 80 genes with gene expression quantified using real-time PCR, according to the manufacturer’s instructions. Briefly, total RNA was extracted from cells as described above. cDNA was prepared from the total RNA mixture of six independent cell samples from different patients using the RT2 First Strand Kit (SABiosiences). Experimental cocktail was prepared by adding cDNA to RT2 qPCR Master Mix (SABiosiences) within the 96-well PCR array. Real-time PCR was performed in 7900HT Fast real-time PCR System. Data were analyzed using the 7900HT Sequence Detection System Software v2.3 (Applied Biosystems) and PCR Array Data Analysis Software (SABiosiences).
We also verified the microarray data for representative genes (IGF1, HDAC2, TERT, ITGA2, LAMB1, and MMP3) at the mRNA level by qRT-PCR, as well as at the protein level by immunostaining with the goat anti-human IGF-1 antibody, goat anti-human MMP-3 antibody (R&D Systems, Minneapolis), rabbit anti-human HDAC1 antibody, mouse anti-human Tert antibody, mouse anti-human integrin-α2 antibody, or mouse anti-human laminin-β1 antibody (Lifespan Bioscience), as described above.
Western blot
To examine how enzymatic dissociation affects the expressions of ECM and adhesion molecules, we purified total protein from cells cultured as monolayers with or without 5 min trypsin digestion, from intact cardiospheres, and from cardiospheres dissociated by 30 minutes trypsin digestion. The equivalent total protein was loaded onto SDS-PAGE gels, and then transferred to PVDF membranes. After overnight blocking in 3% milk TBS-T, membranes were incubated with 1:1000 mouse anti-human integrin-α2 antibody, 1:1000 mouse anti-human laminin-β1 antibody, or 1:3000 dilution of rabbit anti-β-actin monoclonal antibody (Lifespan Bioscience), respectively. The appropriate horseradish peroxidase-conjugated secondary antibodies were used, and then the blots were visualized by using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific)) and exposed to Gel Doc™ XR System (Bio-Rad Lab. Inc.). Quantitation for blots was done by Quantity One software, and expressions were normalized by β-actin.
TUNEL staining to estimate the resistance to oxidative stress
To quantify resistance to oxidative stress, cells were cultured as monolayers using fibronectin-coated 4-chamber culture slides or as cardiospheres on poly-D-lysine-coated plates. After 3 days of culture, cells were exposed to 100 μM H2O2 for 24 hrs. Monolayer-cultured cells were fixed while cardiospheres were harvested, fixed, embedded, and sectioned for staining [10]. Apoptotic cells were detected by TUNEL staining using the In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). Cell nuclei were stained with DAPI.
To further determine how the dissociation of cardiospheres into single cells affects the resistance to oxidative stress, we dissociated cardiospheres into single cells and then cultured the cells in fibronectin-coated 4-chamber culture slides with or without 100 μM H2O2. TUNEL staining was done after 24 hrs of culture.
Myocardial infarction model and cell implantation
Acute myocardial infarction was created in adult male SCID-beige mice (10-12 weeks old), as described [10,23]. Briefly, after general anesthesia and tracheal intubation, mice were artificially ventilated with room air. A left thoracotomy was performed through the fourth intercostal space and the left anterior descending artery (LAD) was ligated with 9-0 prolene under direct vision. The mice were then subjected to intramyocardial injections with a 30-gauge needle at four points in the infarct border zone, with one of the following randomly-assigned treatments: 40 μl PBS (Control group, n=10), 1×105 twice-passaged CDCs after 3 days of culture as monolayers (Mono group, n=11), 1×105 twice-passaged CDCs with 3 days of culture as cardiospheres (2nd-CSp group, n=10), or 1×105 cardiac-derived cells from “explant” culture stage that had been cultured for 3 days as cardiospheres (Pri-CSp group, n=7). Two cardiosphere groups were included to test the effect, if any, of the “freshness” of the originating cells. To determine how the enzymatic dissociation affects cell survival and functional benefit of cardiospheres, we dissociated secondary cardiospheres and then injected the dissociated single-cell suspension into the infarcted heart (Diss-CSp group, n=10). All cells used for implantation were virally-transduced to express green fluorescent protein (GFP), as described [10].
To facilitate the comparison of different culture methods without potential confounding effects of inter-patient variability, we used cells from one patient for injection in all studies. Furthermore, we used an identical number of cells to start the culture, either as cardiopheres or monolayers, and then harvested all cells for implantation 3 days after culture. However, the cell numbers in cardiospheres were not counted before implantation. This means that the initial cell numbers for culture as cardiospheres and monolayers are the same, but the final cell numbers for injection were not the same (likely lower in cardiospheres due to their lower proliferation rates [Fig. 1B]).
Figure 1. Growth, proliferation, and recapitulation of stem cell niche-like microenvironment of cells under cardiosphere culture condition.
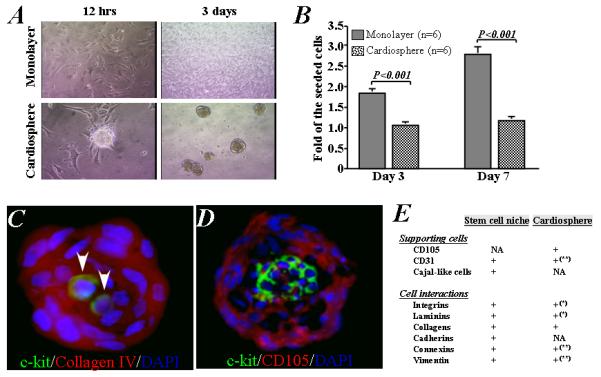
A) Representative images show the growth of cardiac stem cells into cell aggregates and suspended cardiospheres on poly-D-lysine-coated plate (left) or as adherent monolayer cells on a fibronectin-coated plate (right). B) The number of cells increased 2- and 3-fold after 3 and 7 days in monolayer culture, while proliferation was lower in cardiosphere culture. C) Representative images show that two c-kit-positive (green) cardiac stem cells in the central area of a cardiosphere with abundance of collagen IV (red). D) A cluster of c-kit-positive stem cells is localized in the central core of cardiospheres, surrounded by CD105-positive supporting cells. E) Tabular summary of features of cardiac stem cell niches and cardiospheres [NA: not assessed; *based on data presented in Supporting Information Figure 6; ** based on our previous data (Ref #25)].
Echocardiography
Mice underwent echocardiography 3 hrs (baseline), 1 and 3 weeks after surgery using Vevo 770™ Imaging System (VISUALSONICS™, Toronto, Canada) [10,23]. After the induction of light general anesthesia, the hearts were imaged two-dimensionally in long-axis views at the level of the greatest LV diameter. LV end diastolic volume, LV end systolic volume, and LV ejection fraction (LVEF) were measured with VisualSonics V1.3.8 software from 2D long-axis views taken through the infarcted area. The percent changes of LVEF was also calculated as [(LVEF at 3 week - LVEF at baseline) / LVEF at baseline] × 100.
Histology
Mice were sacrificed 3 weeks after treatment. Hearts were sectioned in 5 μm sections and fixed with 4% paraformaldehyde. Masson’s trichrome staining was also performed for quantitative heart morphometry. The infarcted wall (anterior wall) thickness and scar area were measured as described previously [13].
The survival of implanted cells was directly observed as GFP+ cells under fluorescence microscopy. To measure cell survival, 10 images of the infarct and border zones was selected randomly from each animal (three sections/ animal; 1 mm separation between sections; x20 magnification, Eclipse TE2000-U). The area of surviving GFP+ cells was quantified using the Image-Pro Plus software (version 5.1.2, Media Cybernetics Inc., Carlsbad, CA) and the average values from each heart were used for statistical analysis [23]. The differentiation of cardiac stem cells into myocytes and endothelial cells was identified by immunostaining with monoclonal antibodies against human specific α-sarcomeric actin, smooth muscle actin, and VE-cadherin (Sigma), respectively, as described above.
Statistical analysis
All results are presented as mean ± SD. Statistical significance between two groups was determined using the 2-tailed unpaired t test and among groups by ANOVA followed by Tukey’s test (Dr. SPSS II, Chicago, IL). Differences were considered significant when p<0.05.
Results
Growth of cardiac-derived cells into cardiospheres recapitulates stem cell niche-like microenvironment
When cells were seeded on poly-D-lysine coated plates, they spontaneously aggregated into small colonies reminiscent of embryonic stem cell colonies [24]. Within 12-72 hrs, the cells formed a suspension of cardiospheres of 50-80μm in diameter (Figure 1A). Cells grown as cardiospheres proliferated slowly, increasing only 1.1- and 1.2-fold after 3 and 7 days of culture, respectively. On the other hand, when cells were plated on fibronectin-coated dishes and grew as monolayers, they proliferated 2- and 3-fold after 3 and 7 days, respectively (P<0.001 vs. cardiospheres, Figure 1B). We counted all cells, including dead cells, to calculate cell proliferation because the 30 min of enzymatic digestion required to disperse cardiospheres sufficed to increase cell death (Supporting Information Figure 2A). The cell count data led to similar conclusions regarding proliferation as did our measurements of total protein in non-digested cardiospheres and monolayers (Supporting Information Figure 2B).
Immunostaining revealed that cardiospheres consist of c-kit+ cardiac stem cells (CSCs) and supporting cells bound together by extracellular matrix proteins and connexins [6,20]. CSCs were localized and enriched in the central area of cardiospheres (Figure 1C and D). These c-kit+ cells were surrounded by abundant collagen IV (Figure 1C) and CD105+/c-kit− supporting cells (Figure 1D). CD105 is a cell surface marker generally used to identify mesenchymal stem cells, also known as “stromal cells”, but it is also found in endothelial cells of newly-formed tumor vessels. Here, we did not further characterize the CD105+/c-kit− supporting cells. These architectural features of cardiospheres resemble those of in vivo stem-cell niches, where stem cells are surrounded by supporting cells and linked by interactive ECM molecules. Figure 1E compares the features of cardiospheres with those of niches; much commonality is apparent [19-21].
Culturing heart-derived cells as cardiospheres increases the proportion of c-kit+ cells and up-regulates stem cell-related factors
Using flow cytometry (Figure 2A), the proportion of cells expressing c-kit was assessed after 3 days’ culture as cardiospheres or monolayers. Compared with the cells initially plated (baseline), we found that the proportion of c-kit+ cells went up in cardiospheres (16.9±2.1 % vs 9.1±0.7 %, respectively, P<0.01, Figure 2B). In contrast, when cells were cultured as monolayers, the proportion of c-kit+ cells declined (5.2±1.0 %, P<0.01 vs baseline, Figure 2B). The proportion of c-kit+ cells also went up in secondary cardiospheres re-formed from twice-passaged CDCs (Supporting Information Figure 3). Furthermore, qRT-PCR analysis showed that the mRNA expression of Nanog and Sox2, two important stem cell transcription factors, was up-regulated in cardiospheres (P<0.05; Figure 2C and D).
Figure 2. Expression of c-kit, SOX2, and Nanog in cells under cardiosphere and monolayer culture conditions.
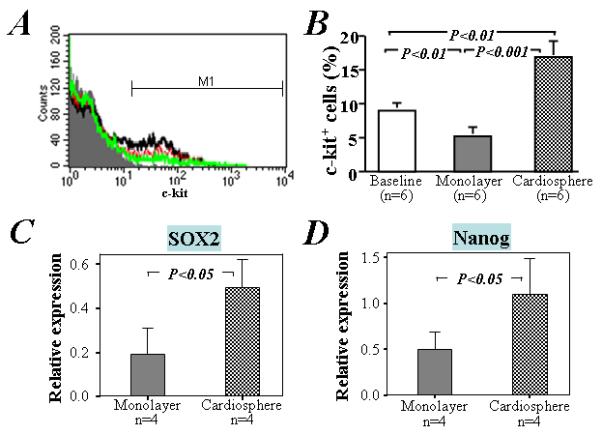
A) Compared to the baseline (red line in top histogram), flow cytometry analysis showed that the expression of c-kit in cardiac outgrowth cells was increased after 3 days culture under cardiosphere condition (black line), but decreased under monolayer condition (green line). B) Quantitative data from 6 separate experiments using different human cardiac outgrowth cells. Quantitative qRT-PCR analysis of 4 separate RNA samples from different human cardiac outgrowth cells showed that the expression of SOX2 (C) and Nanog (D) is also significantly higher in cells cultured under cardiosphere than monolayer conditions.
The changes in c-kit protein, and in Nanog and Sox2 transcripts, hint that “stemness” may be heightened in the cardiospheres. To assess in greater depth the relative expression of stem cell-related genes in cardiospheres versus monolayers, more than 80 relevant transcripts were evaluated by PCR array analysis (Supporting Information Table 1). Among these, 13 transcripts increased more than 4-fold in cells cultured as cardiospheres as compared with monolayers (Figure 3). Some of these up-regulated transcripts, such as IGF-1, encode proteins associated with stem cell growth while others (HDAC2, Dhh, Dll1, Dll3, and Tert) assist stem cells in re-entering the cell cycle and maintaining stemness. In contrast, Myc is among the few genes downregulated in cardiospheres, consistent with their lower proliferative activity compared to monolayers.
Figure 3. Pathway-focused PCR array and qRT-PCR analyses of the expression of stem cell relevant genes in cells cultured as cardiospheres or monolayers.
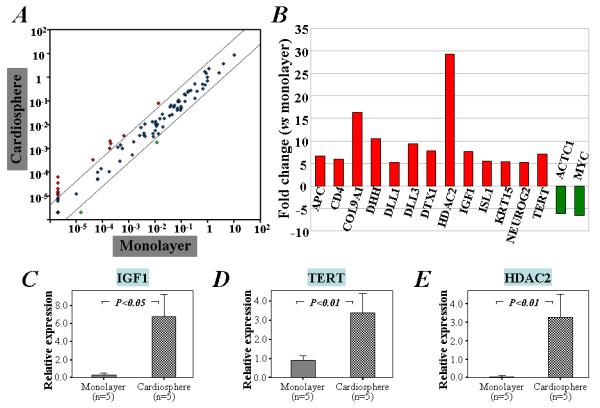
A) Scatter plot demonstrating 13 out of the 84 stem cell genes up-regulated (red circles) in cells under cardiosphere culture condition. B) The names and fold changes of genes that are up- or down-regulated by greater than 4-fold above baseline in cardiosphere culture compared with monolayer culture. Furthermore, qRT-PCR analysis of 5 separate RNA samples from different human cardiac outgrowth cells confirmed that the expression of IGF-1 (C), TERT (D), and HDAC2 (E) is increased in cardiac stem cells cultured under cardiosphere condition when compared with monolayer condition.
Quantitative analysis by qRT-PCR showed that the expression of IGF-1, HDAC2 and Tert mRNA was up-regulated in the cardiospheres (P<0.05; Figure 3C-E). Furthermore, immunostaining confirmed salient results from the PCR array as shown by the marked expression of IGF-1 in cardiospheres, particularly in the center, in contrast to the low expression in monolayer cells (Supporting Information Figure 4). Similarly, HDAC2 and Tert were enhanced in cardiospheres (Supporting Information Figure 4). Taken together, the enhanced c-kit positivity and the upregulation of various stem cell-related genes signify that 3D cardiosphere culture enhances the “stemness” of human cardiac-derived cells. The enhancement of these molecules was confirmed in secondary cardiospheres re-formed from twice-passaged CDCs (Supporting Information Figure 5).
Culturing cells as cardiospheres up-regulates the expression of ECM and adhesion molecules
The expression profile of ECM and adhesion molecule genes was analyzed using pathway-focused PCR arrays (Supporting Information Table 2). Twelve of these mRNAs were increased more than 4-fold after 3 days of 3D cardiosphere culture (Figure 4). These included COL14A1, COL7A1, ITGA2, LAMB1, LAMB3, MMP3, MMP10, MMP11, MMP13, SELE, PECAM1, and SPP1. Cadherin type 1 (CDH1) was the sole gene up-regulated in cells cultured as monolayers.
Figure 4. Pathway-focused PCR array analysis of the gene expression of ECM and adhesion molecules in cells cultured as cardiospheres or monolayers.
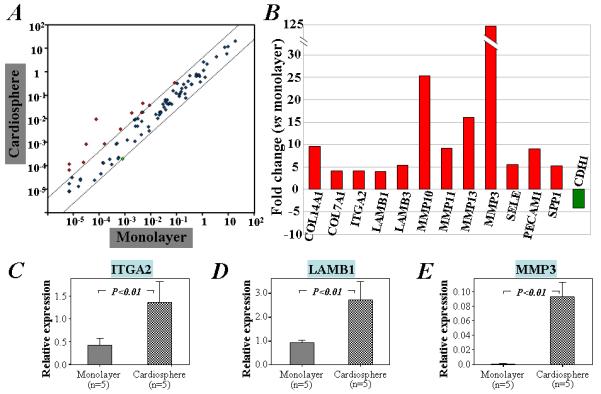
A) The scatter plot demonstrates that 12 of the 84 ECM and adhesion genes are up-regulated (red circles) in cardiospheres relative to monolayer cells. B) The names and fold changes of genes that are up- or down-regulated more than 4-fold in cardiosphere culture compared with monolayer culture. Furthermore, qRT-PCR analysis of 5 separate RNA samples from different human cardiac outgrowth cells confirmed that the mRNA expression of IGTA2 (C), LAMB1 (D), and MMP3 (E) is increased in cells cultured as cardiospheres when compared with monolayer cells.
Confirming the results of the PCR array, qRT-PCR showed that mRNA levels of ITGA2, LAMB1 and MMP3 were up-regulated in cardiospheres (P<0.01; Figure 4C-E). Furthermore, immunostaining demonstrated distinct expression of integrin-α2, laminin-β1, and MMP3 in cardiospheres, particularly in the periphery (Supporting Information Figure 6). In contrast, these molecules were sparse or undetectable in monolayer cells (Supporting Information Figure 6). These molecules were also enhanced in secondary cardiospheres re-formed from twice-passaged CDCs (Supporting Information Figure 7).
Cardiosphere culture improves cell engraftment in the infarcted heart after transplantation
Given the greater expression of ECM and adhesion molecules in cardiospheres, we wondered whether better engraftment might underlie the enhanced functional benefit. We tested this prediction by histological analysis of hearts injected with GFP-labeled human cells. GFP+ cells were infrequently observed in the scar and marginal region of the infarcted mouse heart 3 weeks after the injection of monolayer-cultured cells (Figure 5A), consistent with previous findings [10,14]. In contrast, GFP+ cell survival was much higher in the infarcted hearts of mice implanted with cardiospheres (right, Figure 5A), frequently as distinct clusters of GFP+ cells. Quantitative analysis of GFP+ cell density revealed that cell survival was 5-fold greater in mice implanted with cardiospheres than with monolayer-cultured cells (1.69±0.70 in Pri-CSp group and 1.63±0.62 % in 2nd-CSp group, vs 0.41±0.25 % in Mono group, P<0.001, Figure 5B). As with the functional data, cell survival did not differ between the 2nd-CSp group and the Pri-CSp group (P=0.95; Figure 5B). However, cell engraftment was equally low with cells dissociated from cardiospheres as with monolayer-cultured cells (0.30±0.15 in Diss-CSp group, P=0.95 vs Mono group; Figure 5B).
Figure 5. Engraftment of human cardiac-derived cells 3 weeks after implantation into the infarcted hearts of mice.
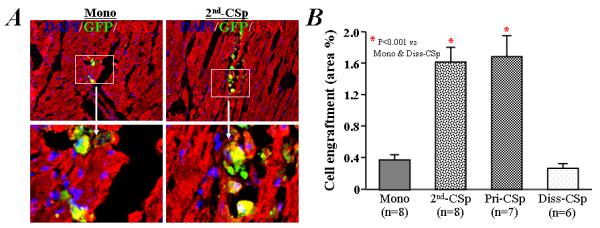
A) Engraftment of GFP+ human cells was infrequently observed in mice receiving the implantation of single cell suspension of CDCs after 3 days culture under monolayer condition (left). However, the survival of GFP+ human cardiac-derived cells was more evident in mice receiving a portion of the same initial CDCs after 3 days of culture under cardiosphere condition (2nd-CSps, right). α-SA: α-sarcomeric actin. B) Quantitative data for cell engraftment (percentage of green (GFP+) area/total area) in the infarcted heart after implantation.
Cardiospheres improve cardiac function more potently than monolayer-cultured cells
LAD ligation and intramyocardial injection were performed using standard techniques [23]. The survival rate of mice is high (~90%) after infarction, and the numbers of mice in each group represent those which survived the full protocol. The baseline LVEF after surgery did not differ among groups, indicating comparable degrees of initial injury. Over the three weeks after infarction, LV end diastolic volume and LV end systolic volume went up in sham-treated mice (Control, P<0.05 vs other groups, Supporting Information Figure 8), but not in mice receiving cell therapy. This indicates that the implantation of human cardiac-derived cells inhibits adverse LV remodeling in mouse heart after infarction, corroborating previous reports [9-12].
The effects on LVEF were even more dramatic and revealed differences not only between cells and control, but also among the various culture conditions. LVEF declined progressively in control mice (Figure 6A, 29.3±3.2 % at baseline vs 23.5±2.6 % at 3 weeks), while LVEF was preserved in mice receiving monolayer-cultured cells (Figure 6A, 29.4±4.0 % at baseline vs 32.1±3.3 % at 3 weeks). These results confirm the finding that implantation of human CDCs prevents functional deterioration [10]. However, primary cardiospheres and 2nd cardiospheres (those re-generated from CDCs rather than directly from cells of “explant” culture stage) actually produced a net increase in LVEF (37.7±4.6 % and 38.2±3.2 % at 3 weeks, respectively), and in so doing were distinctly superior to monolayer-cultured cells (Figure 6A, P<0.01). Figure 6B summarizes the changes in LVEF relative to baseline in each experimental group; these composite treatment effects highlight the disproportionate potency of 3D cardiospheres relative to monolayer cells. The functional superiority of cardiospheres is independent of whether they were formed directly from explant-derived cells or from twice-passaged CDCs [12], hinting that 3D architecture trumps cell “freshness”. This conjecture is consistent with the loss of functional benefit observed with implantation of cells acutely dissociated from 2nd cardiospheres; indeed, the LVEF with dissociated cardiosphere cells was no better than with monolayer-cultured cells (31.2±3.0 % at 3 weeks, P=0.998 vs Mono group, Figure 6).
Figure 6. Cardiac function after treatment and their relationship to cell engraftment.
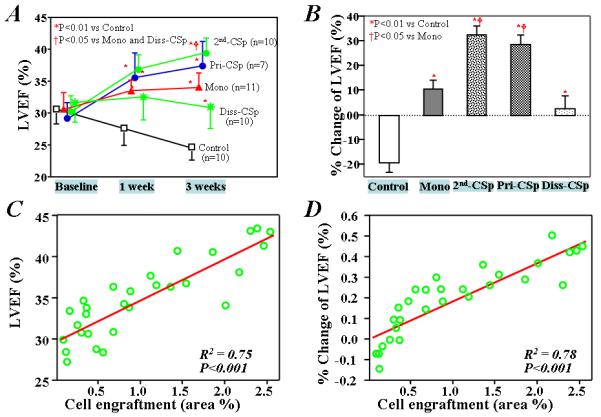
A) LVEF at baseline and 1 and 3 weeks after treatments. B) Percentage change in LVEF from baseline levels 3 weeks after cell injection. (Control group: saline injection only; Mono group: injection with monolayer-cultured CDCs; 2nd-CSp group: injection with cardiospheres reformed from twice-passaged CDCs; Pri-CSp group: injection with cardiospheres primarily formed from cardiac-derived cells; Diss-CSp group: injection with cells dissociated from secondary cardiospheres). The percentage of area of engrafted human cardiac-derived cells within the infarcted heart of mice is strongly correlated with either the absolute values of LVEF (C) or the relative LVEF changes to baseline (D), 3 weeks after treatments.
Masson’s trichrome staining for quantitative analysis of heart morphology (Supporting Information Figure 9) revealed severe LV chamber dilatation and infarct wall thinning in control hearts with PBS injection. In contrast, all cell-treated groups exhibited attenuated LV remodeling. However, the protective effect was greatest in the Pri-CSp and 2nd-CSp group, which had thicker LV walls and smaller infarcts than hearts treated with monolayer-cultured CDCs or dissociated cardiospheres.
Enhanced functional benefit of cardiospheres is related to the increased expression of ECM and adhesion molecules and resistance to oxidative stress
The functional benefit due to direct regeneration versus indirect protection by the implantation of different types of stem cells, including cardiac stem cells, is still unclear. Using human-specific antibodies, we could detect expression of α-sarcomeric actin (Supporting Information Figure 10), smooth muscle actin (Supporting Information Figure 11), and VE-cadherin (Supporting Information Figure 12) in some of the surviving GFP+ cells 3 weeks after implantation, indicating the ability of human heart-derived cells to differentiate into myocytes, smooth muscle cells, and endothelial cells. These results confirm the multilineage potential previously reported for cardiospheres and CDCs [6,9,10-12,14,25]. However, the engraftment of cells in infarcted heart after implantation is critically important for functional benefit [26,27]. In fact, both the absolute values of LVEF and the changes in LVEF relative to baseline strongly correlate with cell engraftment (R2=0.75 and 0.78, respectively, p<0.001, Figure 6C and D), consistent with the notion that improved cell engraftment contributes to the enhanced functional benefit of cardiosphere injection.
To further probe the mechanisms of the enhanced cell engraftment and functional benefit of cardiospheres in vivo, we dissociated 2nd cardiospheres into single cells, and examined the expression of ECM and adhesion molecules. The enhanced expression levels of integrin-α2 and laminin-β1 in cardiospheres disappear in the dissociated cells, falling to levels comparable to those in monolayer-cultured cells (with or without digestion by trypsin; Figure 7A). We also compared the responses of cardiospheres and monolayer-cultured cells to oxidative stress. Apoptosis was blunted in cardiospheres: after 24 hrs exposure to 100 μM H2O2, the number of TUNEL-positive cells was much lower than in monolayer-cultured cells (P<0.01, Figure 7B). However, apoptosis in the cells dissociated from cardiospheres was comparable to that in monolayer-cultured cells. Thus, both the enhanced resistance to oxidative stress and the increased expression of ECM and adhesion molecules in cardiospheres likely contribute to the improved cell engraftment, resulting in greater functional benefit relative to monolayer-cultured cells.
Figure 7. Effect of enzymatic digestion on the expression of ECM and adhesion and the resistance to oxidative stress.
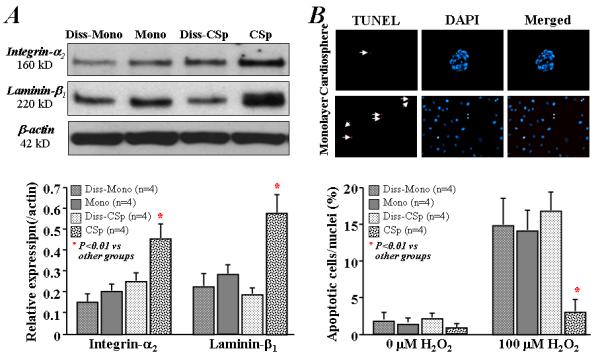
A) Western blotting shows that the dissociation of cardiospheres into single cell suspension decreased the expression of integrin-α2 and laminin-β1. B) Representative images (upper images) show the TUNEL-positive (red) cardiac-derived cells under cardiosphere and monolayer culture conditions with 24 hrs exposure to 100 μM H2O2. Although the number of apoptotic cells (lower bar graph) was lower in cardiospheres than monolayer-cultured cells with 24 hrs exposure to 100 μM H2O2, the resistance to oxidative stress in cardiospheres was negated by dissociating the cardiospheres into single cells. (Diss-Mono: dissociated monolayer-cultured cells by 5 minutes trypsin digestion; Mono: twice-passaged CDCs under monolayer culture; Diss-CSp: dissociated cardiospheres by 30 minutes trypsin digestion; CSp: cardiospheres formed from twice-passaged CDCs.).
Discussion
We find that, compared to traditional monolayer culture, the growth of cardiac-derived cells as cardiospheres recapitulates a stem cell niche-like microenvironment, with an augmented “stemness” profile and enhanced expression of many ECM and adhesion molecules. Although the argument remains at the level of consistency and plausibility, it seems likely that these molecular and cellular alterations in cardiospheres are related to the increase of cell survival and functional benefit of cardiac-derived cells in vivo.
Three-dimensional scaffolds have been frequently used to culture stem cells [28-30]. Compared with monolayer culture, 3D culture has been found to maintain embryonic stem cells in an undifferentiated state [28]. However, this technique has not been well-studied in adult stem cells. Here, the 3D culture of cardiac-derived cells was accomplished by seeding cells on poly-D-lysine-coated plates without a supporting scaffold. Within a few hours, these cardiac-derived cells spontaneously formed aggregates resembling colonies of embryonic stem cells which, after 24 hrs, grew into free-floating 3D cardiospheres. Such scaffoldless 3D structures resemble stem cell niches, with a stem cell-rich core linked with supporting cells via various ECM constituents. We found that cell proliferation was inhibited, while expression of c-kit, SOX2 and Nanog was increased in cardiospheres. Stem cells cultured in 3D conditions are reportedly less proliferative than 2D monolayer-cultured cells [31], in agreement with our own findings (Figure 1B). Similarly, neural stem cells cultured as 3D neurospheres were less proliferative than their 2D counterparts but the expression of stem cell markers was up-regulated [32]. In cardiospheres, PCR array, qRT-PCR, and immunostaining confirmed the up- regulation of many stem cell-relevant factors, such as IGF-1 and Tert, which play an important role in the growth and maintenance of the undifferentiated state. These data further indicate that the growth of cardiac-derived cells as cardiospheres enhances “stemness”. Interestingly, the expression of HDAC2, an important histone deacetylase, was also up-regulated in cardiospheres. The mechanism underlying this culture-acquired enrichment in “stemness” is unclear, but may be related to the recapitulation of a stem cell niche microenvironment and HDAC2-mediated epigenetic modification in cardiospheres [33-35].
Most importantly, cardiac function in infarcted hearts was better with cardiospheres than monolayer cells. Previous studies have documented that both cardiosphere- and monolayer-cultured cells are capable of differentiating into cardiomyocytes after implantation into infarcted heart [9,10]. In this study, we also observed expression of α-sarcomeric actin, smooth muscle actin, and VE-cadherin in some of the engrafted human cells. Our laboratory has recently shown that that CDCs secrete a broad array of cardioprotective and vasculogenic cytokines following implantation into infarcted hearts, further suggesting that supportive paracrine mechanisms play a fundamental role in myocardial repair by transplanted cardiac stem cells [14]. While the mechanism underlying functional improvement is not yet completely understood, the survival/retention of donor cells in the damaged heart after implantation is unquestionably important, whether directly or indirectly [14,36-44]. Our data also show a strong positive correlation between cell engraftment and functional improvement. Therefore, the greater engraftment seen with cardiospheres is likely central to the observed enhancement of cardiac function.
As shown in the 3D culture of embryonic stem cells [45], many ECM and adhesion molecules, including laminin-β1, integrin-α2, and E-selectin were also up-regulated in cardiospheres. As ECM and adhesion molecules are known to be critical for cell survival/retention, up-regulated expression is expected to favor the engraftment of stem cells after implantation into the damaged heart [36-38]. Additionally, methodological differences intrinsic to the different cell processing approaches may affect in vivo cell survival. Specifically, cardiospheres are harvested from poly-D-lysine-coated dishes using manual pipetting without enzymatic digestion, whereas monolayer-cultured cells require trypsinization to form an injectable cell suspension. Enzymatic digestion may damage ECM and adhesion molecules in the monolayer cells, further impeding the capacity of these cells to adhere and engraft. Furthermore, in agreement with a recent report [46], cardiospheres have greater resistance to oxidative stress than monolayer-cultured cells. In fact, the dissociation of cardiospheres into single cells was found to decrease the expression of laminin-β1 and integrin-α2 and resistance to oxidative stress, which negated the enhanced cell engraftment and functional benefit of cardiosphere implantation. Since many features of cardiospheres can account for the enhanced cell engraftment and functional benefit, further experiments are required to identify the precise factor(s) underlying the salutary effects.
Cardiac cell-based myocardial repair has been challenged by poor cell retention/survival and marginal benefits in clinical trials to date. This has led multiple investigators to develop methods including hypoxic preconditioning [40,41], genetic modification [42,43], and cytokine stimulation [44] to improve cell survival/retention and enhance therapeutic potency. The present study shows that the retention and potency of stem cells can be enhanced by simple methodological changes in culture conditions. Three-dimensional cell culture represents a straightforward means to improve the “stemness” profile of the plated cells and the expression of ECM and adhesion molecules. The resultant increases in cell survival and regenerative potency represent a compelling option to improve the functional benefit of cardiac-derived cells in myocardial repair.
Supplementary Material
Acknowledgements
This work was supported by NIH (R01HL083109 to E.M.).
Footnotes
Author Contributions:
Tao-Sheng Li: Conception and design, Manuscript writing, Collection and/or assembly of data, Data analysis and interpretation.
Ke Cheng: Collection and/or assembly of data, Data analysis and interpretation.
Shuo-Tsan Lee: Collection and/or assembly of data, Data analysis and interpretation.
Satoshi Matsushita: Collection and/or assembly of data, Data analysis and interpretation.
Darryl Davis: Manuscript writing, Data analysis and interpretation.
Konstantinos Malliaras: Collection and/or assembly of data, Data analysis and interpretation.
Yiqiang Zhang: Collection and/or assembly of data, Data analysis and interpretation.
Noriko Matsushita: Collection and/or assembly of data.
Rachel Ruckdeschel Smith: Data analysis and interpretation.
Eduardo Marbán: Conception and design, Manuscript writing, Financial support, Final approval of manuscript.
Disclosure of Potential Conflicts of Interest
E. Marbán is a founder and equity holder in Capricor, Inc. R.R. Smith is partially employed by Capricor, Inc. Capricor provided no funding for the present study. The remaining authors report no conflicts.
References
- 1.Li TS, Murakami M, Kobayashi T, et al. Long-term efficacy and safety of the intramyocardial implantation of autologous bone marrow cells for the treatment of ischemic heart disease. J Thorac Cardiovasc Surg. 2007;134:1347–1349. doi: 10.1016/j.jtcvs.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 3.Yamada S, Nelson TJ, Crespo-Diaz RJ, et al. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells. 2008;26:2644–2653. doi: 10.1634/stemcells.2008-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 7.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Wilson RM, Kubo H, et al. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 9.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 10.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 11.Johnston P, Sasano T, Mills K, et al. Engraftment, differentiation and functional benefit of autologous cardiosphere-derived cells in a porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DR, Kizana E, Terrovitis J, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol. 2010 Mar 4; doi: 10.1016/j.yjmcc.2010.02.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis DR, Smith RR, Marbán E. Human Cardiospheres are a Source of Stem Cells with Cardiomyogenic Potential. Stem Cells. 2010 Mar 22; doi: 10.1002/stem.413. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, Gaballa MA. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010 Mar 7; doi: 10.1093/cvr/cvq027. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 17.Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 18.Koretsune Y, Corretti MC, Kusuoka H, Marban E. Mechanism of early ischemic contractile failure. Inexcitability, metabolite accumulation, or vascular collapse? Circ Res. 1991;68:255–262. doi: 10.1161/01.res.68.1.255. [DOI] [PubMed] [Google Scholar]
- 19.Anversa P, Kajstura J, Leri A. If I can stop one heart from breaking. Circulation. 2007;115:829–832. doi: 10.1161/CIRCULATIONAHA.106.682195. [DOI] [PubMed] [Google Scholar]
- 20.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popescu LM, Gherghiceanu M, Manole CG, Faussone-Pellegrini MS. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–886. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li TS, Suzuki R, Ueda K, Murata T, Hamano K. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells. 2007;25:911–917. doi: 10.1634/stemcells.2006-0497. [DOI] [PubMed] [Google Scholar]
- 23.Li TS, Hayashi M, Ito H, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 24.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 25.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng K, Li TS, Malliaras K, Davis D, Zhang Y, Marbán E. Magnetic Targeting Enhances Engraftment and Functional Benefit of Iron-Labeled Cardiosphere-Derived Cells in Myocardial Infarction. Circ Res. 2010;106:1570–81. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerecht S, Burdick JA, Ferreira LS, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang A, Ng R, Yang ST. Long-term culturing of undifferentiated embryonic stem cells in conditioned media and three-dimensional fibrous matrices without extracellular matrix coating. Stem Cells. 2007;25:447–454. doi: 10.1634/stemcells.2006-0322. [DOI] [PubMed] [Google Scholar]
- 30.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 31.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 32.Cheng K, Lai Y, Kisaalita WS. Three-dimensional polymer scaffolds for high throughput cell-based assay systems. Biomaterials. 2008;29:2802–12. doi: 10.1016/j.biomaterials.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong L, Lako M, Dean W, Stojkovic M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells. 2006;24:805–814. doi: 10.1634/stemcells.2005-0350. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey GW, Wang YH, Hirai T, et al. Complementary roles for histone deacetylases 1, 2, and 3 in differentiation of pluripotent stem cells. Differentiation. 2008;76:348–356. doi: 10.1111/j.1432-0436.2007.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azuara V, Perry P, Sauer S, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 36.Li TS, Hayashi M, Liu ZL, et al. Low angiogenic potency induced by the implantation of ex vivo expanded CD117-positive stem cells. Am J Physiol Heart Circ Physiol. 2004;286:H1236–1241. doi: 10.1152/ajpheart.00950.2003. [DOI] [PubMed] [Google Scholar]
- 37.Li TS, Ito H, Hayashi M, et al. Cellular expression of integrin-beta 1 is of critical importance for inducing therapeutic angiogenesis by cell implantation. Cardiovasc Res. 2005;65:64–72. doi: 10.1016/j.cardiores.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 40.Li TS, Hamano K, Suzuki K, et al. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–473. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- 41.Kubo M, Li TS, Suzuki R, et al. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2008;294:H590–595. doi: 10.1152/ajpheart.00856.2007. [DOI] [PubMed] [Google Scholar]
- 42.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 43.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 45.Bartosh TJ, Wang Z, Rosales AA, Dimitrijevich SD, Roque RS. 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem. 2008;105:612–623. doi: 10.1002/jcb.21862. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978–5989. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


