Abstract
Background
We hypothesize that inferior vena cava-inferior atrial ganglionated plexus nerve activity (IVC-IAGPNA) is responsible for the ventricular rate (VR) control during atrial fibrillation (AF) in ambulatory dogs.
Methods and Results
We recorded bilateral cervical vagal nerve activity (VNA) and IVC-IAGPNA during baseline sinus rhythm and during pacing-induced sustained AF in 6 ambulatory dogs. Integrated nerve activities and average VR were measured every 10-s over 24-hour periods. LVNA was associated with VR reduction during AF in 5 dogs (from 211 bpm, 95% confidence interval [CI], 186 to 233 to 178 bpm [95% CI, 145 to 210], p<0.001) and RVNA in 1 dog (208 bpm [95% CI, 197 to 223] to 181 bpm [95% CI, 163 to 200], p<0.01). There were good correlations between IVC-IAGPNA and LVNA in the former 5 dogs, and between IVC-IAGPNA and RVNA in the latter dog. IVC-IAGPNA was associated with VR reduction in all dogs studied. RVNA was associated with baseline sinus rate reduction from 105 bpm (95% CI, 95 to 116) to 77 bpm (95% CI, 64 to 91, p<0.01) in 4 dogs while LVNA was associated with sinus rate reduction from 111 bpm (95% CI, 90 to 1250) to 81 bpm (95% CI, 67 to 103, p<0.01) in 2 dogs.
Conclusions
IVC-IAGPNA is invariably associated with VR reduction during AF. In comparison, right or left VNA was associated with VR reduction only when it co-activates with the IVC-IAGPNA. The vagus nerve that controls VR during AF may be different than that controls sinus rhythm.
Keywords: atrial fibrillation; atrioventricular node; ECG; nervous system, autonomic; ventricular rate
Randomized clinical trials have shown that in most patients with atrial fibrillation (AF), rate control is not inferior to rhythm control as a management strategy.1 However, the mechanisms of ventricular rate (VR) control during AF remain unclear. Commonly accepted mechanisms are that the complex local atrial wavefronts play an important role in modulating atrioventricular (AV) node conduction through irregularity and randomness of the atrial activity itself,2 and through repetitive anterograde concealment and electrotonic modulation of the AV node conduction.3 In addition, it is also generally accepted that autonomic nervous system inputs, especially the vagal tone, is important in modulating the AV node conduction.4 Based on the studies using anesthetized animals, there are parallel but yet functionally distinct inputs from right and left vagal nerves to AV node.5 Left vagal nerve stimulation has been proposed as a method to control VR during AF.6 In addition to vagal nerves, it is also known that the inferior vena cava-inferior atrial ganglionated plexus (IVC-IAGP) (also known as the inferior right or right inferior GP7) is important in modulating AV node conduction, and that direct electrical stimulation of this GP may slow VR during AF in human patients.8 However, none of these studies was performed in the ambulatory state with direct nerve recording. Therefore, the relative importance of right vagal nerve activity (RVNA), left vagal nerve activity (LVNA) and IVC-IAGP nerve activity (IVC-IAGPNA) in VR control during AF in ambulatory animals remains poorly understood in the ambulatory state. Furthermore, whether or not the VR reduction is achieved by VNA or by IVC-IAGPNA remains unclear. We9 have developed methods to simultaneously record nerve activities from the extrinsic nervous system and GPs in ambulatory dogs. The purpose of the present study is to use these techniques to simultaneously record RVNA, LVNA and IVC-IAGPNA in dogs with pacing-induced sustained AF to test the hypothesis that IVC-IAGPNA is primarily responsible for the VR control during AF in ambulatory dogs.
Methods
The study protocol was approved by the International Animal Care and Use Committee of the Indiana University School of Medicine and Methodist Research Institute, Indianapolis, IN, and conforms to the guideline of the American Heart Association.
Continuous Ambulatory Nerve Recordings
We simultaneously recorded RVNA, LVNA, and IVC-IAGPNA during pacing-induced sustained (≥48 hours) AF in six ambulatory dogs. The dogs were intubated and ventilated artificially using isoflurane inhalation. The chest was opened through a right lateral thoracotomy. A Data Sciences International (DSI; St. Paul, MN) D70-EEE radiotransmitter was implanted to record nerve activity according to methods described in detail elsewhere.9, 10 Figure 1 shows that one pair of electrodes was sutured onto IVC-IAGP beneath its fascia (circle) and an epicardial pacemaker lead was put between the lower right and left atrium (dashed arrow).Two pairs of electrodes were placed in the bilateral cervical vagal nerve through a subcutaneous tunnel. A modified Medtronic EnPulse pacemaker (Medtronic Inc, Minneapolis, MN) was implanted for intermittent high-rate atrial pacing. After 2 weeks of postoperative recovery, the DSI transmitter was turned on to record baseline rhythm and nerve activity for 1 day. The left atrium was paced at 10 Hz (600 bpm, twice the diastolic threshold) for 6 days, followed by 1 day of monitoring during which the pacemaker was turned off. The rhythm was monitored for 24 hours to determine the presence of AF. The alternating pacing-monitoring sequence was repeated until sustained (≥48 hours) AF was documented. The dogs were then monitored for 13±5 days (range, 8 to 21 days) before being euthanized.
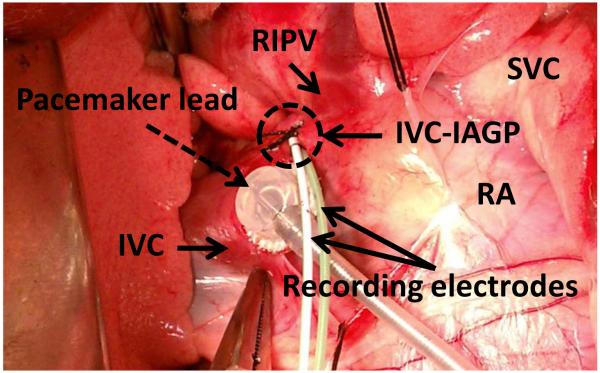
Figure 1.
One pair of electrodes under fascia of inferior vena cava – inferior atrial ganglionated plexus (IVC-IAGP, circle) and an epicardial pacemaker lead (dashed arrow) at the junction of right atrium (RA), left atrium and IVC. IVC-IAGP is located at the junction of the inferior vena cava and left atrium. Because IVC-IAGP electrodes are located at the atrioventricular junction, a local ventricular electrogram is always recorded to allow VR analyses. The relative sizes of A, V and T waves vary depending on the location of the electrodes and the filter settings. RIPV; right inferior pulmonary vein, SVC; superior vena cava, IVC; inferior vena cava.
Immunocytochemical Studies
We harvested the left cervical vagus nerve from 4 normal dogs under isoflurane general anesthesia. The tissues were processed routinely, paraffin embedded and cut into 5-μm thick sections and stained for tyrosine hydroxylase (TH) to identify adrenergic nerves and cholineacetyltransferase (ChAT) to identify cholinergic nerves according to methods described elsewhere.10, 11
Data analysis
We analyzed recordings from all channels using custom-written software. Nerve activities were considered present if there was a 3-fold increase in the signal amplitude over baseline noise. We analyzed both the frequency of nerve discharge episodes and the corresponding heart rate change after each discharge. Data from both VNA were high-pass filtered at 100 Hz.11 Spike-triggered averaging was performed to allow removal of the ventricular electrograms from IVC-IAGPNA recordings by subtracting a ventricular electrogram template obtained from averaged ventricular electrograms in the observational window.12 The filtered or transformed signals were then rectified, integrated with a 100-ms time constant, and summed to represent integrated NA of 10-s segments over 24 hours of recordings. We applied bandpass filtering (5 to 100 Hz) on the VNA recording to obtain an ECG for analysis.9, 10
Statistical analysis
All quantitative data are presented as mean ± SD. Differences in the continuous variables were estimated with 95% confidence interval (CI), and the p-values of the comparisons were calculated using paired-T test. Post-hoc analyses of the differences among multiple groups were adjusted for multiple comparisons using Bonferroni test. Pearson correlation coefficients were used to assess correlations between integrated nerve activities. Fisher’s exact test was used to determine the correlation between elevated RVNA, LVNA, IVC-IAGPNA and slow VR. Slopes of the scattered plots were estimated by linear regression. Linear mixed-effects models with random intercepts were used to analyze circadian variation patterns. A two-sided p-value less than or equal to 0.05 was considered statistically significant. The statistical analyses were performed using PASW Statistics (version 18; SPSS Inc, Chicago, IL) and SAS 9.2 (SAS Inc., Cary, NC).
Results
Vagal control of sinus rate at baseline
Sinus rate averaged 82±19 bpm at baseline (prior to rapid atrial pacing). In 8640 10-s segments over a 24-hr period, RVNA and LVNA were detected in 17.7% (1529 segments) and 23.9% (2065 segments), respectively. Intermittent sinus rate reduction or sinus pause (> 2 s) was observed during RVNA discharge in 4 dogs (Figure 2A). In the remaining 2 dogs, LVNA (but not RVNA) discharge was associated with sinus pauses or heart rate reduction (Figure 2B). In the former 4 dogs, RVNA decreased the sinus rate by 27% (from 105 bpm [95% CI 95 to 116] to 77 bpm [95% CI, 64 to 91] at baseline, p<0.01). In these dogs, integrated RVNA during sinus pause was significantly higher than that before pause (11% increase, 45 mV-s [95% CI, 38 to 54] vs. 40 mV-s [95% CI, 38 to 51], p<0.001). However, LVNA was not significantly different before and during sinus pauses (38 mV-s [95% CI, 23 to 54] vs. 41 mV-s [95% CI, 25 to 55], p=0.08). In 2 dogs, LVNA but not RVNA was associated with sinus rate reduction. Figure 2B shows an example in which intermittent LVNA discharge in the absence of RVNA discharge was associated with sinus pauses. In these 2 dogs, LVNA was associated with 26% reduction of heart rate (111 bpm [95% CI, 90 to 125] to 81 bpm [95% CI, 67 to 103], p<0.01). RVNA during sinus pauses was significantly lower than RVNA at baseline (37 mV-s [95% CI, 27 to 48] vs. 45 mV-s [95% CI, 31 to 58], p<0.001). In contrast, LVNA during sinus pauses was significantly higher than at baseline (38 mV-s [95% CI, 27 to 50] vs. 26 mV-s [95% CI, 18 to 33], p<0.001). In all dogs studied, simultaneous RVNA and LVNA reduced spontaneous sinus rate by 11% (from 99 bpm [95% CI, 82 to 107] to 87 bpm [95% CI, 73 to 102], p<0.05).
Figure 2.
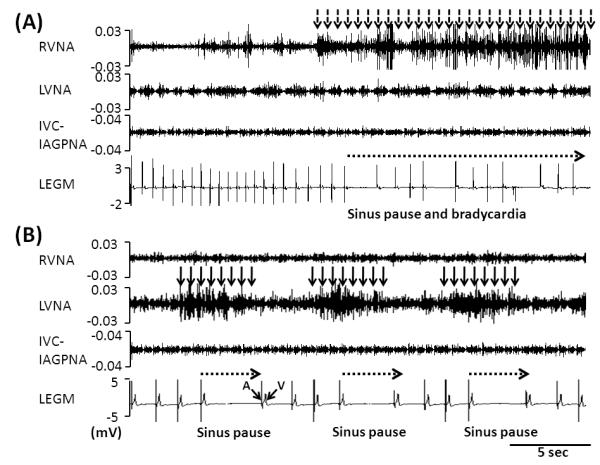
RVNA, LVNA and bipolar local electrogram (LEGM) in sinus rhythm. The LEGM was obtained by filtering the IVC-IAGP electrogram to remove the nerve signals. In this example, the filtered LEGM contains a large and sharp local atrial electrogram (A) and a far field V wave. The T waves are not clearly visualized. A shows that RVNA (dashed arrow) was associated with sinus pause and bradycardia. B shows that LVNA (solid arrows) were associated with sinus pause (dashed arrows).
IVC-IAGPNA and AV conduction in sinus rhythm
IVC-IAGPNA did not change sinus rate or cause sinus pause. The integrated IVC-IAGPNA was 36±13 mV-s in sinus rhythm. PR interval with IVC-IAGPNA discharge was not significantly longer than that without discharge (147 ms [95% CI, 135 to 159] vs. 141 ms [95% CI, 127 to 155], p=0.12). We did not observe any second or third degree atrioventricular block in sinus rhythm.
RVNA, LVNA, IVC-IAGPNA during sustained atrial fibrillation
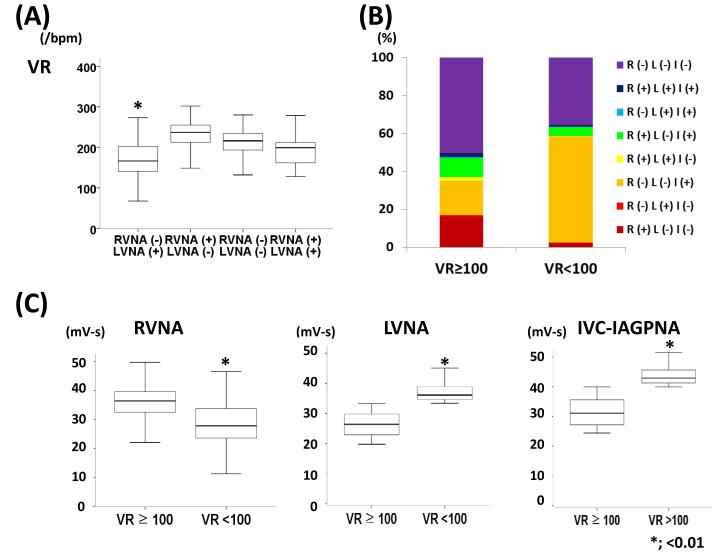
All dogs developed sustained AF after 3.5±1.2 weeks of rapid atrial pacing. Average VR during AF was 184±45 bpm (67 – 269/min) over a 24-hr period. The VR was 215±33 bpm when neither RVNA nor LVNA was active. Ventricular rates associated with LVNA alone, RVNA alone and RVNA-LVNA co-activation were 175±43 bpm, 224±42 bpm and 198±37 bpm, respectively (Table 1, p<0.05). Among all 10-s time segments, 28% of 10-s segments were associated with reduced VR (<100 bpm). Five dogs showed VR reduction with LVNA discharge (Figure 3A, 16.9% reduction of VR, from 219 bpm [95% CI, 185 to 255] vs. 182 bpm [95% CI, 133 to 220], p<0.001). One dog showed VR reduction with RVNA discharge (Figure 3B, 16.6% reduction of VR, 210 bpm [95% CI, 166 to 240] vs. 175 bpm [95% CI, 127 to 213], p<0.001). IVC-IAGPNA discharge was associated with 13.4% VR reduction in all dogs (194 bpm [95% CI, 142 to 232] vs. 168 bpm [95% CI, 126 to 207], p<0.001). Exclusive RVNA discharge was observed in 12.6±3.4%, LVNA discharge in 8.6±2.1%, IVC-IAGPNA discharge in 28.4±4.5%. Coactivation of RVNA and LVNA was seen in 1.5±1.2%, RVNA and IVC-IAGPNA in 0.5±0.4%, LVNA and IVC-IAGPNA in 10.5±3.8%. Discharge of all nerves was observed in 1.6±2.2% and no discharge in 36.6±5.8%. Among 4 dogs with sinus pauses or bradycardia during RVNA activation, one showed VR reduction associated with RVNA discharge during AF. Among two dogs with sinus bradycardia or pause during LVNA discharge, both showed VR reduction with LVNA activation during AF.
Table 1.
RVNA, LVNA and IVC-IAGPNA during sustained AF
| VNA associated with VR reduction in AF |
LVNA | RVNA | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Dog No | Dog#1 | Dog#2 | Dog#3 | Dog#4 | Dog#5 | Dog#6 | |
| Average VR during AF (bpm) | 186±34 | 182±46 | 183±32 | 192±56 | 183±36 | 187±51 | |
|
| |||||||
|
Average VR associated with each
NA (bpm) |
|||||||
| R (−), L (−), I (−) | 214±25 | 217±23 | 227±26 | 221±27 | 217±30 | 226±33 | |
| R (+), L (+),I (+) | 187±42 | 186±44 | 187±59 | 212±48 | 193±52 | 167±39 | |
| R (−), L (+),I (+) | 139±26 | 143±24 | 124±25 | 129±21 | 128±20 | 171±48 | |
| R (+), L (−),I (+) | 197±34 | 185±32 | 182±33 | 180±30 | 168±33 | 126±25 | |
| R (+), L (+),I (−) | 202±33 | 195±44 | 204±38 | 217±38 | 220±33 | 219±44 | |
| R (−), L (−),I (+) | 164±46 | 151±40 | 154±25 | 169±11 | 135±33 | 147±31 | |
| R (−), L (+),I (−) | 181±56 | 180±56 | 176±41 | 165±38 | 166±36 | 212±47 | |
| R (+), L (−),I (−) | 229±46 | 219±45 | 210±47 | 231±39 | 209±47 | 156±35 | |
|
| |||||||
| Integrated NA (mV-s) | |||||||
| RVNA ≥ 100 bpm |
<100 bpm | 46±31 28±26 |
41±27 21±11 |
49±30 36±28 |
44±26 35±20 |
36±28 31±22 |
35±21 47±29 |
| LVNA ≥ 100 bpm |
<100 bpm | 42±16 49±21 |
30±14 41±29 |
34±14 48±21 |
39±13 45±28 |
37±10 46±19 |
41±13 38±11 |
| IVC-IAGPNA ≥ 100 bpm |
<100 bpm | 42±16 55±15 |
36±11 48±10 |
39±14 50±13 |
35±11 56±12 |
36±13 53±14 |
34±11 42±9 |
R; RVNA, L; LVNA, I; IVC-IAGPNA, (+) designates presence of NA, (−) designates absence of NA.
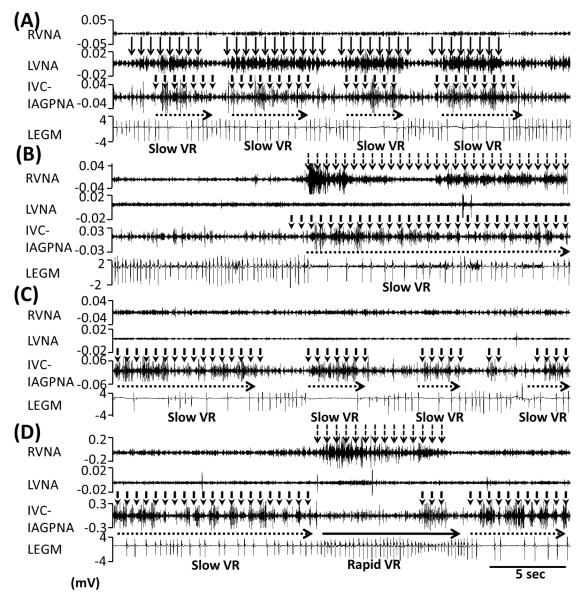
Figure 3.
RVNA, LVNA, IVC-IAGPNA, LEGM during sustained AF. IVC-IAGPNA with LVNA (A) or RVNA (B) co-activation was associated with reduced VR. Panel C shows independent IVC-IAGP was associated with slow VR. RVNA activation after IVC-IAGPNA withdrawal was associated with rapid VR (D). The local electrogram (LEGM) shows ventricular electrograms and T waves.
Figure 3 shows the common patterns of VR control by these 3 nerve structures. Figure 3A shows that simultaneous LVNA and IVC-IAGPNA discharges were associated with reduction of the VR. One dog showed that simultaneous RVNA and IVC-IAGPNA discharge was associated with reduction of the VR, in the absence of LVNA (Figure 3B). IVC-IAGPNA discharge without RVNA or LVNA discharge in the same dog could also reduce VR (Figure 3C). However, RVNA without LVNA or IVC-IAGPNA was associated with rapid VR, not VR reduction (Figure 3D). There was no ventricular tachyarrhythmia during IVC-IAGPNA discharge. These changes are not due to direct electrical stimulation of the IVC-IAGP, as the pacemaker was turned off during these recordings.
Integrated nerve activity and ventricular response in five dogs with LVNA controlling VR
The VR was < 100 bpm in 32±5% of the 10-s time segments, during which the integrated nerve activities were 29±22 mV-s (RVNA), 45±25 mV-s (LVNA) and 52±12 mV-s (IVC-IAGPNA). In comparison, that associated with VR ≥100 bpm were, respectively, 42±29 mV-s (p<0.01), 36±13 mV-s (p<0.01) and 38±13 mV-s vs. (p<0.01). The LVNA reduced VR by 15.3% (from 211 bpm [95% CI, 186 to 233] to 178 bpm [95% CI, 145 to 210], p<0.001). Ventricular rate with and without RVNA discharge (205 ±31 bpm and 198±35 bpm, respectively), was not different (p=0.32). There was a good correlations between IVC-IAGPNA and LVNA in the former 5 dogs (average r of 0.792±0.103, p<0.05 in all).
Integrated nerve activity and ventricular response in one dog with RVNA controlling VR
The VR was < 100 bpm in 26% of the 10-s time segments, during which the integrated nerve activities were 47±29 mV-s (RVNA), 38±11 mV-s (LVNA) and 34±11mV-s (IVC-IAGPNA). In comparison, that associated with VR ≥100 bpm were, respectively, 35±21 mV-s (p<0.01), 41±13 mV-s (p<0.01) and 42±9mV-s (p<0.01).The VR during RVNA discharge reduced from 208 bpm [95% CI, 197 to 223] to 181 bpm [95% CI, 163 to 200], (p<0.01), but VR with and without LVNA discharge was not different (209 bpm [95% CI, 191 to 227] and 197 bpm [95% CI, 179 to 218], respectively, p=0.18). There was also a good correlations between IVC-IAGPNA and RVNA in this dog (r=0.773, p<0.05).
Relationship between LVNA and RVNA
Five dogs showed an ‘L’-shaped relationship between RVNA and LVNA (Figure 4A) during AF. When VR was ≥100 bpm (blue dots), low levels of LVNA and IVC-IAGPNA were observed (Figures 4A and 4B). Significant positive linear correlation was present between LVNA and IVC-IAGPNA (r=0.739, p<0.01, Figure 4C), and negative correlation was present between IVC-IAGPNA and VR (r=-0.406, p<0.05, Figure 4D). However, when VR was <100 bpm (red dots), both LVNA and IVC-IAGPNA were elevated (Figures 4A to C). In the remaining one dog, RVNA and LVNA did not have an L-shaped correlation. Rather, a linear correlation (Figure 5B) is present, indicating that RVNA and LVNA activate together. Coactivation of these two nerves may be associated with rapid VR (Figure 5A). In the same tracing, IVC-IAGPNA alone was associated with a reduction of VR. There was positive linear correlation between RVNA and LVNA in this dog and L shaped relationship between VNA and IVC-IAGPNA during AF (Figure 5B-D). This is the same dog as that shown in Figure 3B, when coactivation of RVNA and IVC-IAGPNA was associated with VR reduction.
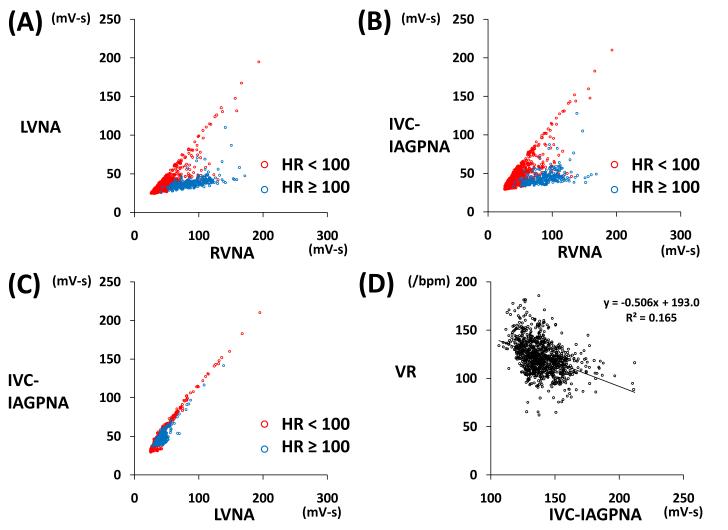
Figure 4.
Example of 10-s integrated NA and VR. Five dogs (83%) showed an ‘L’-shaped relationship between RVNA and LVNA during AF. When VR was ≥ 100 bpm (blue dots), there was little activity of the LVNA and IVC-IAGPNA (A, B). However, when VR was < 100/min (red dots), there was increased LVNA and IVC-IAGPNA activity (A-C). A significantly positive linear correlation was present between IVC-IAGPNA and LVNA (r=0.739, p<0.01, C), and a significant negative correlation between VR and IVC-IAGP (r=-0.406, p<0.05, D)
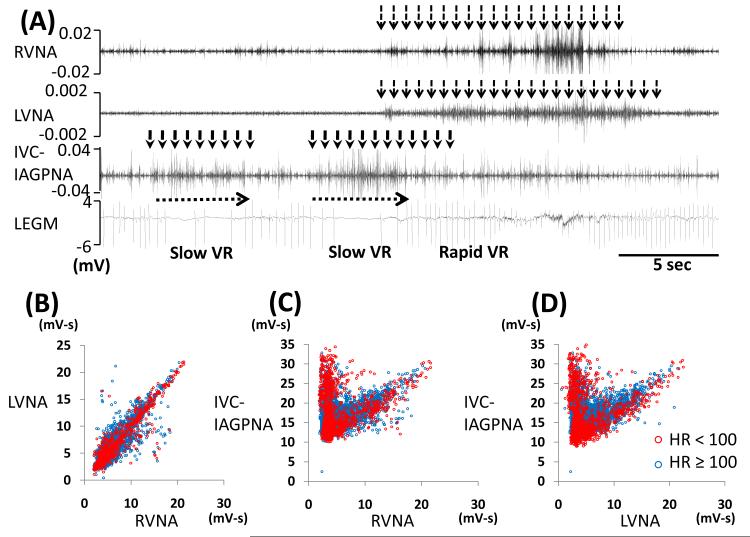
Figure 5.
Slowing of VR associated with IVC-IAGPNA discharge without RVNA or LVNA discharge. Figure A shows independent IVC-IAGPNA resulted in reduction of VR while RVNA and LVNA discharge without IVC-IAGPNA discharge was associated with rapid VR. One dog showed a linear relation between LVNA and RVNA (B) and an ‘L’-shaped relation between RVNA, LVNA with IVC-IAGPNA (C, D). B shows that RVNA and LVNA showed linear relation when VR≥ 100 bpm (blue dots). Unlike that shown in Figure 4, the panels C and D show that blue and red dots are observed at both arms of the L-shaped correlation.
Figure 6A shows the VR and vagal nerve activities in five dogs with LVNA controlling the VR during AF. ANOVA and posthoc test showed the first group (LVNA without RVNA) was associated a lower VR than the second group (RVNA without LVNA). There were no significant differences of VRs when RVNA and LVNA were both absent (third group) or when they were both present (fourth group). Figure 6B shows the distribution of nerve activity patterns with VR ≥ 100 bpm or < 100 bpm in one of the five dogs. The figure shows that when VR was ≥ 100 bpm, the most common nerve activity pattern is no nerve activity (purple). On the other hand, the most common nerve activity pattern associated with VR of < 100 bpm is the lone activation of IVC-IAGP without either RVNA or LVNA (orange). For all dogs studied, the proportion of IVC-IAGPNA alone (without RVNA or LVNA) increased from 18.2% [95% CI, 13.6 to 22.6] at VR ≥100 bpm to 42.2% [95% CI, 29.1 to 55.3] at VR <100 bpm, p=0.024).These findings further confirm the importance of IVC-IAGPNA in controlling the VR during AF. Figure 6C shows that the magnitude of RVNA is higher when VR is ≥100 bpm than when VR is <100 bpm. On the other hand, the magnitudes of LVNA and IVC-IAGPNA were higher when the VR is <100 bpm than when VR is ≥100 bpm.
Figure 6.
Relationship between RVNA, LVNA and VR in a typical dog in which LVNA and IVC-IAGPNA is associated with VR reduction. A shows that the VR was the lowest when LVNA was activated without RVNA. B shows the proportion of various nerve activities in one dog when VR was ≥ 100 bpm or < 100 bpm. In the latter situation, the predominant nerve activity pattern was IVC-IAGP activation without either RVNA or LVNA (orange). C shows the magnitudes of LVNA and IVC-IAGPNA were higher when the VR was <100 bpm than when VR was ≥100 bpm. The magnitude of RVNA was higher when VR was ≥100 bpm than when it was < 100 bpm. R; RVNA, L; LVNA, I; IVC-IAGPNA, (+) designates presence of NA, (−) designates absence of NA.
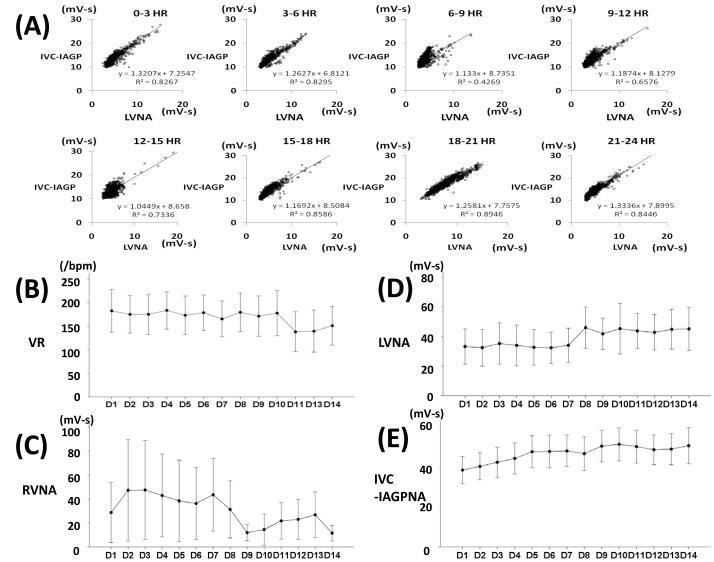
Circadian variation of correlation of LVNA and IVC-IAGPNA and changes of NA during sustained AF
Figure 7A shows typical circadian variation pattern of linear correlation of LVNA and IVC-IAGPNA from 5 dogs that exhibited a linear LVNA-GPNA correlation. These nerve activities were the lowest in the morning and in early afternoon. After sustained AF was induced, VR decreased over 14 days (p<0.01, Figure 7B) of recording. RVNA also decreased (p<0.01, Figure 7C).But average LVNA and IVC-IAGPNA gradually increased over the same period (p<0.01 for both, Figure 7D-E).
Figure 7.
Circadian variation of linear correlation of LVNA and IVC-IAGPNA (A) from a typical dog. LVNA and IVC-IAGPNA were the lowest in the morning and in early afternoon. Ventricular rate decreased over 14 days (B) of recording and average RVNA shows little change (C). However, average LVNA and IVC-IAGPNA gradually increased over the 14 day period (D, E).
Immunocytochemical studies of the cervical vagal nerve
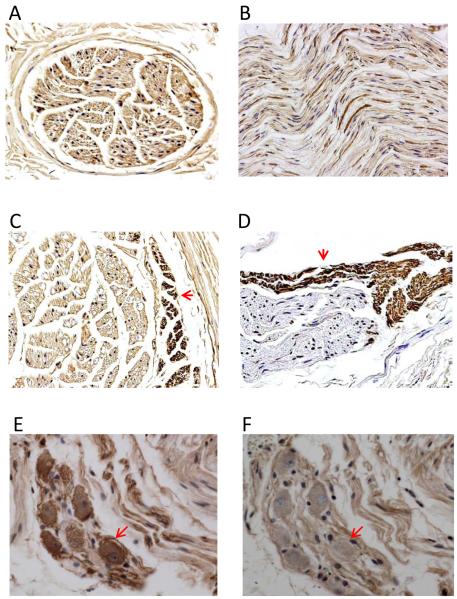
The cervical vagal nerves contain both sympathetic and parasympathetic components. Figure 8 shows examples of TH and ChAT staining of the left cervical vagus nerve. ChAT positive nerve structures formed a majority of the cervical vagus nerve (Panels A and B). However, a small amount of TH-positive nerves were also present at the edge of the nerve bundles (Panels C and D). Most unexpectedly, we identified sympathetic neurons in the vagus nerve (Panel E), indicating that the cervical vagus nerve was a source of sympathetic innervation. The same neurons stained negative for ChAT (Panel F).
Figure 8.
Immunocytochemical staining of the cervical vagus nerve. A and C show examples of the nerves sectioned transversely. Other panels show nerves sectioned longitudinally (craniocaudally). Each cervical vagus nerve contains multiple parallel nerve bundles, most staining positively (brown) for cholineacetyltransferase (ChAT), as shown in Panels A and B. However, a small percentage of the nerve bundles, primarily at the periphery of the nerves, stained positively for tyrosine hydroxylase (TH), as indicated by arrows in C and D. In addition, TH-positive ganglion cells are also present in the nerves (E). These cells were ChAT negative (F). These findings indicate that bundles of sympathetic nerves are present within the cervical vagus nerves. The presence of sympathetic ganglion cells in the vagus nerve suggests that the cervical vagus nerve is also an important source of sympathetic innervation. Original magnification: 100X for Panels A-C, 40X for D-F.
Discussion
In a vast majority of dogs, IVC-IAGPNA and LVNA collaborate to control the VR during AF. However, in 1 of the 6 dogs, IVC-IAGPNA collaborates with RVNA to control VR during AF. IVC-IAGPNA was not entirely slaved to the VNA. Rather, it can act alone to reduce the VR during AF. We also demonstrated that while RVNA reduces sinus rate at baseline in a majority of dogs, some dogs use LVNA (but not RVNA) to reduce sinus rate. The VNA that controls the sinus rate may not be the one that controls the VR during AF.
Relationship between VNA and IVC-IAGPNA
Based on our previous observations in ambulatory animals,9 the intrinsic and extrinsic nervous systems often coordinate their activities to achieve maximal physiological or pathophysiological effects. We reaffirmed in this study that there is extensive collaboration among bilateral vagal nerves and the IVC-IAGP. However, in some dogs, the LVNA and RVNA often activate independently of each other, leading to an L-shaped correlation. In one dog, however, the RVNA and LVNA usually co-activate, leading to a nearly linear correlation. Similar findings also exist between left stellate ganglion nerve activity (SGNA) and LVNA in that 75% of the dogs showed an L-shaped correlation while 25% showed linear correlation.12 In the latter study, the patterns of sympathovagal correlation determine the duration of rapid atrial pacing needed to induce sustained AF. In the present study, the dogs with an L-shaped correlation between LVNA and RVNA primarily use the LVNA and IVC-IAGPNA to slow the VR during AF. On the other hand, the dog with a linear correlation primarily used RVNA and IVC-IAGPNA for VR control during AF. Because VNA that controls the sinoatrial node does not always control the AV node, the functional asymmetry of LVNA and RVNA in controlling one structure does not predict that the same asymmetry is present in controlling the other structure. The relationship between VNA and IVC-IAGPNA could be further studied with unilateral electrical stimulation of either vagal nerve and observe the response of IVC-IAGP during surgery. However, general anesthetic agents may partially suppress nerve activities, making it difficult to interpret the results.
The contribution of IVC-IAGP in VR control during atrial fibrillation
Atrioventricular node specific fat pad or GP is inconsistently named as the AV node fat pad, right inferior fat pad, inferior vena cava-left atrium fat pad, inferior interatrial GP, and inferior right GP.7, 8, 13 The presence and location can be confirmed in vivo with neurostimulation-based exploration. Stimulation of that GP impairs AV node conduction, while leaving sinus node and atrial function largely unaltered. In vivo studies show that both sympathetic and parasympathetic activation can exert direct and indirect effects on AV node conduction.14 Sympathetic activation shortens AV conduction time directly via electrophysiological changes in the nodal tissue. AV conduction is also affected indirectly, with sympathetic stimulation accelerating sinus rate and hence increase the input into the AV node. In contrast, parasympathetic stimulation directly prolongs AV conduction via electrophysiological changes but also has indirect effects on AV conduction by slowing sinus rate.15 During AF, it is also possible that the autonomic nervous system activities may affect the AV node conduction by changing the local wavefront characteristics, which indirectly affects the AV node conduction. Therefore, the overall effects of nerve discharges on AV conduction most likely depend on the balance of the direct and indirect effects. It is possible that combined effects of VNA and IVC-IAGP not only directly affect the AV nodal physiology but also alter the local AF wavefront characteristics and hence the inputs to the AV node. These direct and indirect effects work together to slow the VR during AF. We noted that the IVC-IAGPNA did not cause AV block when the dogs were in the baseline sinus rhythm. However, as AF developed, both the LVNA and IVC-IAGPNA continued to increase over a two-week period, while the RVNA continued to decline. It is possible that significant neural remodeling occurred during this time period, and that the neural remodeling contributes to the importance of IVC-IAGP in VR control. Rapid VR during AF may lead to deterioration of left ventricular function, which may increase both sympathetic and vagal nerve discharges.11 These findings suggest that heart failure induced remodeling involves both branches of the autonomic nervous system. Because GP contains both sympathetic and parasympathetic nerves, the remodeling process may include the IVC-IAGP and increase its discharges. Finally, in addition to interacting with VNA, the IVC-IAGPNA can also activate alone. The ability to activate alone further suggests that the IVC-IAGPNA plays an important and independent role in VR control during AF.
The effects of electrical stimulation
Left vagal nerve stimulation has been proposed as a useful method for controlling VR during AF.6 The present study confirms that in a majority of ambulatory animals, the LVNA is associated with VR reduction while the RVNA is associated with an increased VR. However, in a minority of animals, the RVNA is at least equally important as LVNA in VR control. Patients in whom RVNA controls the VR may not be responsive to this method of treatment. In addition to vagal stimulation, others have proposed to use direct IVC-IAGP stimulation or AV nodal fat pad stimulation to control the VR during AF. These methods have been tested both in animal models and in humans.8, 16Chronic high-frequency electrical stimulation of intrinsic cardiac neurons not only stimulates neurotransmitter release by neuronal cell membrane depolarization but may augment parasympathetic tone by eliciting distinct and diverse neurotrophic effects.17 The continued neurotrophic effects may be important in the mechanisms of VR control during chronic electrical stimulation of the IVC-IAGP.
Sympathetic nerve structures within the vagus nerve
We found that a majority of the nerve structures within the cervical vagus nerve stained positively for ChAT, implying these nerves are cholinergic. However, we also found that a small portion of the nerves stained positively for TH. Furthermore, TH-positive neurons are also present in the cervical vagus nerve, indicating that the vagus nerve is also a source of sympathetic innervation. The vagal nerve activities may include both sympathetic and parasympathetic components. These new findings may prove important in the understanding of autonomic control of cardiac function.
Limitations
We were not able to differentiate afferent and efferent nerve activities or to differentiate parasympathetic and sympathetic nerve activities within the vagosympathetic trunk. However, the primary finding of this study is that neither the RVNA nor LVNA consistently control the baseline sinus rate or ventricular response rate during AF. In contrast, IVC-IAGP nerve activities were consistently associated with the VR reduction during AF, indicating the importance of IVC-IAGP in VR control. Because dogs do not eat well after cervical vagal nerve resection, we were not able to test the hypothesis that IVC-IAGP alone (i.e., without VNA) can control the VR during AF. Due to the limitation of the equipment, we were not able to record additional nerve activities such as the stellate ganglion nerve activity and the activity of other ganglionated plexi within the heart. Therefore, the importance of those nerve activities in VR control remains unclear. Previous studies have shown that the patterns of local atrial activation wavefronts are important in determining whether atrial activation is conducted to the ventricles via an accessory pathway.18 The same may also be true for the AV node. Because we did not map the wavefront characteristics near the AV node, it remains unclear if the nerve activities changed wavefront characteristics and indirectly affected AV nodal conduction. Finally, due to the small number of animals studied, extrapolating the results of these studies to the general population should be done with caution.
Ventricular rate (VR) control is important in managing patients with atrial fibrillation (AF). However, the mechanisms of VR control during AF remain unclear. We simultaneously and continuously recorded right vagus nerve activity (RVNA), left vagus nerve activity (LVNA) and inferior vena cava-inferior atrial ganglionated plexus (IVC-IAGP) nerve activity (IVC-IAGPNA) in ambulatory dogs with AF. We then compared the nerve discharges with the VR. Immunohistochemical staining of the cervical vagus nerves were performed. There are several unexpected findings. First of all, cervical vagus nerves contain both sympathetic and parasympathetic nerve fibers. The cervical vagus nerves also contain sympathetic ganglion cells. Therefore, vagus nerves can be a source of sympathetic tone. A second unexpected finding is that IVC-IAGPNA is invariably associated with VR reduction during AF. In comparison, RVNA or LVNA is associated with VR reduction only when it co-activates with the IVC-IAGPNA. Sometimes IVC-IAGPNA alone, without either RVNA or LVNA, can slow down the VR. These findings suggest that IVC-IAGPNA is primarily responsible for the VR control during AF in ambulatory dogs. The clinical implications of these findings are as follows: (1) Clinicians should not use the term “vagal tone” to describe the increased activity of the parasympathetic arm of the autonomic nervous system. Vagal tone could be either sympathetic or parasympathetic. (2) IVC-IAGPNA, not the RVNA or LVNA, controls the VR during AF. Therefore, IVC-IAGP is a better therapeutic target than either right or left vagus nerves in VR control during AF.
Acknowledgments
We thank Lei Lin, Nicole Courtney, Jessica Warfel and Janet Hutcheson for their assistance; Dr Xiaohong Zhou of Medtronic Inc and Mr Michael Bova of St Jude Medical Inc for donating the pacemaker equipment used in the study.
Funding Sources: This study was supported in part by NIH Grants P01HL78931, R01HL78932, R01HL71140, R21HL106554, a Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology (M.J.S.), a Nihon Kohden/St Jude Medical electrophysiology fellowship (M.M.), a Piansky Endowment (M.C.F.) and a Medtronic-Zipes Endowment (P.-S.C.).
Footnotes
Conflicts of Interest Disclosures: Medtronic Inc, St Jude Inc, Cyberonics and Cryocath Inc donated research equipments used in our laboratory.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 2.Zankov DP, Omatsu-Kanbe M, Isono T, Toyoda F, Ding WG, Matsuura H, Horie M. Angiotensin ii potentiates the slow component of delayed rectifier k+ current via the at1 receptor in guinea pig atrial myocytes. Circulation. 2006;113:1278–1286. doi: 10.1161/CIRCULATIONAHA.104.530592. [DOI] [PubMed] [Google Scholar]
- 3.Akao M, Sakurai T, Horie M, Otani H, Takano M, Kouchi I, Murakami T, Sasayama S. Angiotensin ii type 1 receptor blockade abolishes specific k(atp)channel gene expression in rats with myocardial ischemia. J Mol Cell Cardiol. 2000;32:2239–2247. doi: 10.1006/jmcc.2000.1251. [DOI] [PubMed] [Google Scholar]
- 4.Ai T, Horie M, Obayashi K, Sasayama S. Accentuated antagonism by angiotensin ii on guinea-pig cardiac l-type ca-currents enhanced by beta-adrenergic stimulation. Pflugers Arch. 1998;436:168–174. doi: 10.1007/s004240050619. [DOI] [PubMed] [Google Scholar]
- 5.Gassanov N, Brandt MC, Michels G, Lindner M, Er F, Hoppe UC. Angiotensin ii-induced changes of calcium sparks and ionic currents in human atrial myocytes: Potential role for early remodeling in atrial fibrillation. Cell Calcium. 2006;39:175–186. doi: 10.1016/j.ceca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Geddes LA, Elabbady T, Schoenlein WE, Waninger MS, J.D. B. Method and apparatus using vagal stimulation for control of ventricular rate during atrial fibrillation. 5916239 U.S. Patent. 1999
- 7.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 8.Rossi P, Bianchi S, Barretta A, Della Scala A, Kornet L, De Paulis R, Bellisario A, D’Addio V, Pavaci H, Miraldi F. Post-operative atrial fibrillation management by selective epicardial vagal fat pad stimulation. J Interv Card Electrophysiol. 2009;24:37–45. doi: 10.1007/s10840-008-9286-2. [DOI] [PubMed] [Google Scholar]
- 9.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 12.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 14.Loeb JM, deTarnowsky JM. Integration of heart rate and sympathetic neural effects on av conduction. Am J Physiol. 1988;254:H651–657. doi: 10.1152/ajpheart.1988.254.4.H651. [DOI] [PubMed] [Google Scholar]
- 15.Lazzara R, Scherlag BJ, Robinson MJ, Samet P. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ Res. 1973;32:393–401. doi: 10.1161/01.res.32.3.393. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang S, Zhang Y, Mowrey KA, Li J, Tabata T, Wallick DW, Popovic ZB, Grimm RA, Natale A, Mazgalev TN. Ventricular rate control by selective vagal stimulation is superior to rhythm regularization by atrioventricular nodal ablation and pacing during atrial fibrillation. Circulation. 2002;106:1853–1858. doi: 10.1161/01.cir.0000031802.58532.04. [DOI] [PubMed] [Google Scholar]
- 17.Rana OR, Saygili E, Gemein C, Zink MD, Buhr A, Mischke K, Nolte KW, Weis J, Weber C, Marx N, Schauerte P. Chronic electrical neuronal stimulation increases cardiac parasympathetic tone by eliciting neurotrophic effects. Circ Res. 2011;108:1209–1219. doi: 10.1161/CIRCRESAHA.110.234518. [DOI] [PubMed] [Google Scholar]
- 18.Ong JJC, Cha YM, Kriett JM, Boyce K, Feld GK, Chen PS. The relation between atrial fibrillation wavefront characteristics and accessory pathway conduction. J Clin Invest. 1995;96:2284–2296. doi: 10.1172/JCI118284. [DOI] [PMC free article] [PubMed] [Google Scholar]