Abstract
Memory is one of the most fundamental mental processes. Neuroscientists study this process by using extremely diverse strategies. Two different approaches aimed at understanding learning and memory were introduced in this symposium. The first focuses on the roles played by synaptic plasticity, especially in long-term depression in the cerebellum in motor learning, and its regulatory mechanism. The second approach uses an elegant chick-quail transplantation system on defined brain regions to study how neural populations interact in development to form behaviorally important neural circuits and to elucidate neurobiological correlates of perceptual and motor predispositions.
The brain is the organ that is responsible for what we call the mind. It is the basis for thinking, feeling, wanting, perceiving, learning and memory, curiosity, and behavior. Memory is a fundamental mental process, and without memory we are capable of nothing but simple reflexes and stereotyped behaviors. Thus, learning and memory is one of the most intensively studied subjects in the field of neuroscience. Various approaches have been used to understand the mechanisms underlying this process. In this session, T.H. and E.B. presented their original approaches toward understanding learning and memory.
We define memory as a behavioral change caused by an experience, and define learning as a process for acquiring memory. According to these definitions, there are different kinds of memory. Some memories, such as those concerning events and facts, are available to our consciousness; this type of memory is called “declarative memory.” However, another type of memory, called “procedural memory,” is not available to consciousness. This is the memory that is needed, for example, to use a previously learned skill. We can improve our skills through practice. With training, the ability to play tennis, for example, will improve. Declarative memory and procedural memory are independent. There are patients with impaired declarative memory whose procedural memory is completely spared. Because of this fact, neuroscientists believe that there must be separate mechanisms for each type of memory that probably also require separate brain areas as well. The cerebrum and hippocampus are considered important for declarative memory, and the cerebellum for procedural memory. In any case, neuroscientists think that memory must require alterations to occur in the brain. The most popular candidate site for memory storage is the synapse, where nerve cells (neurons) communicate (1). In other words, a change in the transmission efficacy at the synapse (synaptic plasticity) has been considered to be the cause of memory. A particular pattern of synaptic usage or stimulation, called the conditioning stimulation, is believed to induce synaptic plasticity. Many questions remain to be answered, such as how synaptic plasticity is induced and how synaptic plasticity is implicated in learning and memory. Many studies concerning these issues are now in progress.
In the cerebellum, the combined activation of two different synaptic inputs to a particular neuron (called a Purkinje neuron) depresses the transmission efficacy at a synapse. This depression is persistent and is called long-term depression (LTD) (2, 3). The LTD in the cerebellum has been considered to be the cellular basis of motor learning. Hirano and colleagues succeeded in inducing cerebellar LTD in culture (4). Cellular and molecular analyses of the induction mechanism of LTD have been performed with culture and slice preparations, and a number of molecules implicated in the LTD mechanism have been identified (5). A widely used strategy for identifying molecules implicated in synaptic plasticity has been to inhibit the plasticity by blocking the function of a particular molecule. These studies have relied on tools such as inhibitory drugs or on more specific molecular tools, such as antibodies.
Another frontier in the study of synaptic plasticity is to clarify the role of plasticity in learning and memory. The strategy has been to examine the correlation between synaptic plasticity and learning by inhibiting the plasticity in a living animal. To do this, investigators have used inhibitors for certain molecules that are required for synaptic plasticity. Recently, another set of very useful tools has become available. These tools are genetically engineered mutant mice such as knockout or transgenic mice. A knockout mouse is a mutant mouse that is deficient in a specific native molecule. By using mutant mice, the relationship between synaptic plasticity and learning ability has been examined (6). One model behavior that has been used to analyze the relationship between synaptic plasticity and learning is the vestibulo-ocular reflex (VOR). The VOR is the reflex that moves the eyes in the opposite direction to head motion, allowing the animal to fixate on the visual image (7). The efficacy of the VOR is modifiable and results in adjustments in its gain. For example, the gain of VOR increases if the subject wears magnifying spectacles. It has been proposed that the cerebellar LTD is implicated in such VOR adaptations. The merits of studying the VOR adaptation as a model case for motor learning are as follows. First, the neural circuit for the VOR is simple. Second, both the input (head motion) and the output (eye movement) can be quantified. Efforts are underway to analyze the relationship between the changes in neuronal activity and the VOR adaptation.
Learning does not only involve changes in synaptic efficacy resulting from the convergence of several kinds of concurrent environmental stimulation. We previously described their work on naturalistic models such as song learning in birds, suggesting that brain systems produce unlearned biases that also contribute in important ways to the learning process (8). Such biases can appear in both sensory and motor aspects of learned behaviors. For instance, one taxonomic group of birds, the oscines or true songbirds, all learn to sing by imitation. To produce biologically functional songs, they need to hear examples of species songs during development, which they commit to memory. They subsequently perfect their song performance by ear and are capable of using both memorized material as well as songs of birds they can hear to produce an acceptable species song (9).
Although it is likely that social information is important when a young songbird selects models for learning, experiments by Marler et al. (10) have demonstrated that two closely related species of North American sparrows can choose species-appropriate models in the absence of social information. In work on one of these species, the swamp sparrow Melospiza georgiana, Balaban (11, 12) studied learned intraspecific geographic song variation correlated with population genetic differences and found consistent geographic differences in female sexual responses to songs that appeared to be unaffected by developmental exposure to songs of different types. Such work suggested the existence of brain mechanisms that biased birds' attention and memory toward songs with particular characteristics.
Experiments on the songs of birds reared in various degrees of acoustic isolation also suggested that there might be biases in the motor system that produces singing. By using species differences in the normal songs of song sparrows (Melospiza melodia) and the above-mentioned swamp sparrow as a yardstick, Marler et al. (13) found that many of the structural differences in the individual acoustic units of the songs, differences in song temporal characteristics, and differences in large-scale song organization also were found in the songs of birds reared with no exposure to normal species songs. Some differences in song temporal characteristics and song organization also were retained in the highly abnormal songs of birds deafened in early infancy. Both sensory and motor predispositions are just that—biases that can be overridden by experience (14). Their subtlety makes them useful for guiding learning (inflexible biases would be self-defeating in a learning process) but has proven something of an obstacle to neurobiological work.
Balaban et al. (15) described a system for studying neurobiological correlates of perceptual and motor predispositions. Transplants of defined portions of tissue that will later become the central nervous system between the embryos of two bird species (the domestic chicken, Gallus gallus spp., and the Japanese quail, Coturnix coturnix japonica) are performed at early stages of development, and transplanted host embryos are allowed to hatch. The resulting animals, called chimeras, have selected central nervous system regions made up from cells of the donor species. Donor and host regions of the central nervous system can be identified in subsequent histological examination, which allows the identification of regions of the brain that evolution changes to change perceptual and motor behavior. It also provides an experimental system for studying how brain regions interact in development to form behaviorally important neural circuits.
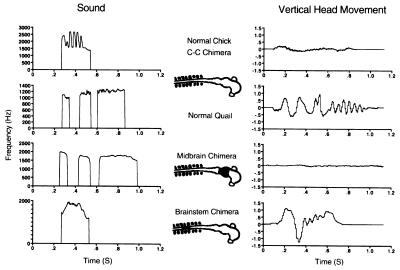
Work on a motor predispositions using species differences in sound production and head movement during the “crowing” vocalization has demonstrated that this difference is caused by changes in several anatomically distinct cell groups in different parts of the brain that have independent effects on the behavioral components of crowing (ref. 16 and Fig. 1). We also described more recent work on auditory perceptual preferences using species differences in an alerting vocalization parents give to young, the “maternal call” (17). These studies suggest that early-developing parts of the brain may influence developmental decisions in later-developing parts to change the operating characteristics of cells involved in complex behavioral circuits in many different parts of the brain. Elucidating these developmental interactions will be a major focus of continuing studies.
Figure 1.
Sound production (Left), schematic of transplant (Center), and vertical component of head movement (Right) in quail-chick chimeras. Transplants are shown on a schematic drawing of a 45-hr embryo neural tube. C-C chimera refers to control transplants between two different chicken embryos. See ref. 16 for further details.
Acknowledgments
E.B.'s work is supported by the National Institute of Mental Health and Neuroscience Research Foundation.
Abbreviations
- LTD
long-term depression
- VOR
vestibulo-ocular reflex
Footnotes
This paper is a summary of a session presented at the second annual Japanese–American Frontiers of Science symposium, held October 1–3, 1999, at the International Conference Center, Tsukuba, Japan.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210381897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210381897
References
- 1.Kandel E R. In: Principles of Neural Science. Kandel E R, Schwartz J H, Jessell T M, editors. New York: Elsevier; 1991. pp. pp.1009–1031. [Google Scholar]
- 2.Ito M, Sakurai M, Tongroach P. J Physiol (London) 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito M. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T. Neurosci Lett. 1990;119:141–144. doi: 10.1016/0304-3940(90)90818-t. [DOI] [PubMed] [Google Scholar]
- 5.Linden D J, Connor J A. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 6.Tsien J Z, Huerta P T, Tonegawa S. Cell. 1996;81:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 7.du Lac S, Raymond J L, Sejinowski T J, Lisberger S G. Annu Rev Neurosci. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- 8.Marler P. In: The Biology of Learning. Marler P, Terrace H, editors. Berlin: Springer; 1984. pp. 289–309. [Google Scholar]
- 9.Marler P. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- 10.Marler P. Dev Psychobiol. 1990;23:557–568. doi: 10.1002/dev.420230703. [DOI] [PubMed] [Google Scholar]
- 11.Balaban E. Proc Natl Acad Sci USA. 1988;85:3657–3660. doi: 10.1073/pnas.85.10.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaban E. Behaviour. 1988;105:250–322. [Google Scholar]
- 13.Marler P, Sherman V. J Neurosci. 1983;3:517–531. doi: 10.1523/JNEUROSCI.03-03-00517.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston T. Behav Brain Sci. 1988;11:617–663. [Google Scholar]
- 15.Balaban E, Teillet M-A, Le Douarin N. Science. 1988;241:1339–1342. doi: 10.1126/science.3413496. [DOI] [PubMed] [Google Scholar]
- 16.Balaban E. Proc Natl Acad Sci USA. 1997;94:2001–2006. doi: 10.1073/pnas.94.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park T, Balaban E. J Comp Psychol. 1991;105:45–54. doi: 10.1037/0735-7036.105.1.45. [DOI] [PubMed] [Google Scholar]