Abstract
Each Bartonella species appears to be highly adapted to one or a limited number of reservoir hosts, in which it establishes long-lasting intraerythrocytic bacteremia as the hallmark of infection. Recently, we identified Trw as the bacterial system involved in recognition of erythrocytes according to their animal origin. The T4SS Trw is characterized by a multiprotein complex that spans the inner and outer bacterial membranes, and possesses a hypothetical pilus structure. TrwJ, I, H and trwL are present in variable copy numbers in different species and the multiple copies of trwL and trwJ in the Bartonella trw locus are considered to encode variant forms of surface-exposed pilus components. We therefore aimed to identify which of the candidate Trw pilus components were located on the bacterial surface and involved in adhesion to erythrocytes, together with their erythrocytic receptor. Using different technologies (electron microscopy, phage display, invasion inhibition assay, far western blot), we found that only TrwJ1 and TrwJ2 were expressed and localized at the cell surface of B. birtlesii and had the ability to bind to mouse erythrocytes, and that their receptor was band3, one of the major outer-membrane glycoproteins of erythrocytes, (anion exchanger). According to these results, we propose that the interaction between TrwJ1, TrwJ2 and band 3 leads to the critical host-specific adherence of Bartonella to its host cells, erythrocytes.
Introduction
Bartonella species (Bartonella spp.) are small, curved, pleomorphic, fastidious, hemotropic, Gram-negative bacteria, mainly transmitted by arthropod vectors or via direct contact [1]. Until now, 24 species or subspecies, 13 of which being involved in human disease, have been formally validated [2]. Each of them appears to be highly adapted to a limited number of mammalian reservoir hosts, which results in relatively strict host specificity [1], [3].
Bartonella infection can cause many human and animal diseases. For example, B. bacilliformis causes Carrión’s disease, B. quintana causes trench fever and B. henselae causes a variety of clinical manifestations in humans: the main disease in immunocompetent individuals is cat scratch disease (CSD), whereas in immunocompromised patients it causes bacillary angiomatosis (BA) and bacillary peliosis (BP).
Bartonella spp., along with Plasmodium spp., Babesia spp. and Anaplasma marginale, is one of the few infectious agents to infect erythrocytes [4]. The remarkableness, in contrast to other infectious agents infecting erythrocytes, is that all Bartonella spp. described to date, with the exception of the deadly B. bacilliformis, are maintained within the erythrocytes without having a significant effect on their physiology [5].
The dynamics of erythrocyte infection have been monitored in rats infected with fluorescently labelled B. tribocorum. After a primary phase, corresponding to the infection of a still unknown primary niche, potentially vascular endothelial cells [5], [6], [7], [8], [9], [10] or erythrocytic precursors [11]), Bartonella spp. reached the blood stream where they adhered to and invaded mature erythrocytes within 2 days. After infection, intracellular replication started immediately in a membrane-bound compartment, continuing over a period of several days until a steady number of intracellular bacteria was reached, the infected erythrocytes persisting in circulation for several weeks [5].
Bartonellae play an active role during erythrocyte invasion requiring both respiration and proton motive force [12], whereas treatment of erythrocytes with proton-motive force inhibitors has no effect on Bartonella adhesion. This suggests that erythrocytes play a passive role in invasion [13], [14], [15] and that Bartonella spp. are the main active participants in erythrocyte invasion.
The successful infection of a mammalian reservoir host erythrocyte by a Bartonella sp. typically involves a series of intimate host-pathogen interactions. On the molecular level this is reflected by attachment between Bartonella ligands and the erythrocyte receptors. The flagella of B. bacilliformis was identified to mediate initial erythrocyte adhesion [12]. This was supported by the reduction of the erythrocyte-binding ability of B. bacilliformis with anti-flagellin antibodies [16], and the poor adherence of non-motile variants and flagellin-minus mutant [17], [18]. Erythrocyte receptors for attachment to flagella have been partially characterized for B. bacilliformis. Buckles and McGinnis hill [19] demonstrated that B. bacilliformis was able to bind to several erythrocyte proteins: α and β subunits of spectrin, band 3 protein, glycophorin A, and glycophorin B. In addition, Iwaki-Egawa and Ihler [20] demonstrated that spectrin, actin and the other potential erythrocyte membrane proteins from different sources (human, cat, sheep) were able to bind to B. bacilliformis and B. henselae.
However, within the Bartonella genus, 13 Bartonella spp. are represented as a major phylogenic sub-branch of flagella-free Bartonella. All these flagella-free Bartonella possess a Trw Type 4 Secretion System (T4SS). T4SSs are supra-molecular transporters ancestrally related to bacterial conjugation [21]. In Bartonella spp., 2 T4SS, the VirB/D4 and Trw have been described and identified as pathogenicity factors required for bacterial colonization [22], [23]. Interestingly, the distribution of Trw and flagella among Bartonella spp. is mutually exclusive suggesting that, after its acquisition by horizontal transfer, the function of Trw evolved to replace that performed by flagella. In a recent study, using an in vitro model of erythrocyte adherence and invasion we demonstrated the direct role of Trw in erythrocyte recognition [23].
The trw genes of Bartonella species are collinear except for the presence of multiple tandem gene duplications of trwL and trwJIH. The multiple copies of trwL and trwJ are considered to encode variant forms of surface-exposed pilus components which are postulated to have a role in host-interaction with various surface structures of erythrocytes in different species. In contrast, the other duplicated genes, trwI and trwH are considered to encode the components required for pilus elongation and for pilus anchorage to the outer membrane, respectively [24].
Although the Trw locus has been identified as one of the Bartonella spp. factors involved in erythrocytic host-specific recognition, which of the Trw components are associated with the attachment, and the identity of the erythrocytic receptors are still unknown. In this study, combining different technologies and using the B. birtlesii/mouse erythrocytes model, we first identified that among the Trw components, only TrwJ1 and TrwJ2 were expressed at the bacterial surface and could bind to the erythrocyte membrane. Using Far Western blot we identified the major erythrocyte transmembrane glycoprotein Band3 as the receptor of the type IV TrwJ component.
Materials and Methods
Bacterial Strains and Growth Conditions
Bartonella birtlesii (B. birtlesii) (IBS 325T, CIP 106691T) were grown for 5 days on Columbia agar containing 5% defibrinated sheep blood (CBA) in a humidified atmosphere with 5% CO2 at 35°C.
E.coli TOP10 (Invitrogen, USA), BL21 Star (Invitrogen, USA) and BL21 pLysS (Novagen, Germany) were grown overnight in Luria-Bertani (LB) broth or on LB agar plates supplemented when needed with carbenicillin (50 µg/mL) at 37°C.
Animals and Ethics Statement
Animals were handled in strict accordance with good animal practice as defined by the relevant European animal welfare body. Animal work was approved by our institute’s ethics committee. The protocol was approved by the Committee on the Ethics of Animal Experiments of the National Veterinary School of Alfort (Permit Number: 2008-11).
Six-week old OF1 or Balb/C female mice were housed in an animal facility (5 mice per cage) for blood sampling or immunization with recombinant proteins. Cats used for blood sampling came from the National Veterinary School of Alfort. White New Zealand male rabbits (16 weeks old) were used to produce polyclonal antibodies against murine band 3 extracted from erythrocytic membrane.
Isolation of Erythrocytes
Erythrocytes from the peripheral blood of mice and cats were isolated and purified by Ficoll gradient centrifugation as previously described [25]. After washing in PBS, erythrocytes were maintained in F12 modified medium (F12 medium supplemented with 10% foetal calf serum, 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM Hepes, 257 mM Histidine (His), 0.1 mg/ml Hematin/His, and non-essential amino acid) (Gibco, France) before being used for further analysis (erythrocyte invasion assay, phage binding assay).
Trw Proteins Expression and Purification
Genomic DNA was isolated from B.birtlesii using the Roche high pure PCR template preparation kit (Roche, Switzerland). Based on the entire trwJ1, trwJ2, trwL1, trwL2,trwL3, trwL4 and trwL5 sequences (F. Biville, unpublished data), DNA inserts corresponding to trw genes were amplified by PCR from genomic B. birtlesii as template and the corresponding specific primers (shown in table 1). PCR consisted of an initial denaturation step at 98°C for 2 min followed by 30 cycles of denaturation at 98°C for 20 s, annealing at 55°C for 50 s and extension at 72°C for 50 s, and a final extension step at 72°C for 10 min. All PCR reactions were performed in a MyCycler™ thermocycler (Biorad, USA) with the Phusion high-fidelity DNA polymerase (New England Biolabs, USA).
PCR products were ligated to the PET-102 expression vector (Invitrogen, USA). This vector allows expression of recombinant protein containing a thioredoxin epitope followed by an enterokinase recognition site at the N-terminal end and a 6-His tag at the C-terminal end. After propagation of the recombinant plasmids in E.coli TOP10, they were then transformed into BL21 Star and BL21 pLysS by electroporation. Expression was obtained for trwJ1 and trwJ2 in BL21 Star incubated with 0.5 mM IPTG (isopropyl β-D-thiogalactoside) for 4 hours at 22°C, and for trwL2, trwL3, trwL4, and trwL5 in BL21 pLysS incubated with 0.5 mM IPTG for 4 h at 37°C.
The recombinant fusion proteins were purified by affinity chromatography using the nickel-nitrilotriacetic acid (Ni-NTA) resin following the manufacturer’s protocol (Qiagen, Germany) under native conditions or denaturing conditions according to their properties. For mice immunization, the thioredoxin parts of the recombinant proteins were cut off by enterokinase (Invitrogen, USA). The digestion reactions were performed in the Ni-NTA-protein mixture overnight at 37°C, under shaking, in 1 ml containing 10X enterokinase buffer and 25U of enterokinase and were followed by 3 washes in PBS. In each case the recombinant proteins were eluted from the resin in 400 µl native elution buffer (300 mM NaCl, 50 mM NaH2PO4, 250 mM imidazole, pH 8.0) or denaturing elution buffer (8 M Urea, 300 mM NaCl, 50 mM NaH2PO4, 250 mM imidazole, pH 8.0).
The purified recombinant proteins were analyzed by a 15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by gel staining with Coomassie brilliant blue R-250 (Sigma, USA). The SigmaMarker™ low range (Sigma; USA) was used as reference for the molecular weights.
Production of Murine Polyclonal Antibodies against Recombinant Trw Proteins
Balb/C mice were injected twice subcutaneously with 10 µg of each recombinant protein mixed in oil Montanide® adjuvant ISA-70 (Seppic, France) at 2-weeks-interval with the same antigen dose. Sera were collected 15 days after the last immunization and stored at -20°C. The titres of polyclonal antibodies were determined by dot-blot analysis using the corresponding recombinant proteins.
Western Blot (WB) Analyses
B. birtlesii (1.108 UFC from 5 days growth on CBA plates) and 0.1 µg of rTrwJ1, rTrwJ2, rTrwL2, rTrwL3, rTrwL4, rTrwL5 recombinant proteins were reduced with 100 mM DTT, resolved by a Tris-Glycine 15% SDS-PAGE gel and blotted onto PVDF membranes (GE Healthcare, UK) at 15 V for 12 min by Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell Instruction (Biorad, USA) in Towbin transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3). The PVDF membranes were blocked in 1X blocking buffer (50 mM Tris, 150 mM NaCl pH 7.4 and 0.05% Tween-20, 5% non-fat dried milk) for 1 h at 37°C and then incubated with 1/1000 dilution of mouse anti-Trw proteins polyclonal antibodies for 1 h at 37°C in blocking buffer. Anti-Trw labelling assays were revealed with an anti-mouse IgG (H+L) alkaline phosphatase (AP)-goat antibody (1∶10,000; Jackson ImmunoResearch Laboratories, USA) for 1 h at 37°C, and a 10 ml solution of NBT (Nitro blue tetrazolium chloride)/BICP (5-Bromo-4-chloro-3-indolyl-phosphate p-toluidine salt) (Sigma, Germany).
Electron Microscopy and Immunolocalization of Trw Components
Pellets of bacteria were fixed for 30 min with 2% paraformaldehyde solution in PBS, then centrifuged and washed in PBS. The bacteria were collected onto 400 mesh formvar-coated nickel grids. Grids were quenched with NH4Cl 50 mM in PBS, blocked with PBS containing 1% BSA, and 0.1% BSA-c™ (BioValley, France). Antibodies (anti-TrwJ1, anti-TrwJ2, anti-TrwL2-L5, naïve mouse serum) were added at a 1/100 dilution in PBS containing 1% BSA, 0.1% BSA-c™ and incubated over night at +4°C. The grids were then washed twice for 3 min in PBS -1% BSA, 0.1% BSA-c™ and goat anti-mouse IgG (1/50 dilution) coupled to 10 nm colloidal gold particles (British Biocell International – TEBU, France) added for 1 hour. The grids were again washed twice with PBS -BSA, twice with PBS, and fixed for 5 min with 2.5% glutaraldehyde in PBS. Finally, the grids were washed three times with distilled water and air dried.
The grids were then examined with a Zeiss EM902 electron microscope operated at 80 kV (Carl Zeiss – France), and images were acquired with a charge-coupled device camera (Megaview III) and analysed with ITEM Software (Eloïse, France) MIMA2 Platform, INRA-CRJ (http//MIMA2@jouy.inra.fr).
Expression of TrwJ1 and TrwJ2 on T7 Phage
The T7 select 10-3b Cloning kit (Novagen, Germany) containing the T7 select 10-3b EcoRI/HindIII vectors and T7 packaging extracts was used to display TrwJ1 and TrwJ2 on T7 phage. Briefly, trwJ1 and trwJ2 genes were amplified by PCR using the specific primers described in table 1. To allow insertion in T7 phage sequences, the forward primer contained an EcoRI restriction enzyme site while the reverse primer contained a HindIII restriction enzyme site.
After PCR amplification, the PCR products were digested by EcoRI (TaKaRa, Japan) and HindIII (TaKaRa, Japan) and purified by PCR clean-up Gel extraction Kit (MACHEREY-NAGEL, Germany), before being packaged, titered and amplified following the procedures outlined in T7Select system.
Phage Binding Assay with Mouse and Cat Erythrocytes
Mouse and cat erythrocytes were resuspended in PBS at 1×108 cells/ml, and incubated with 1×109 PFU TrwJ1-T7 or TrwJ2-T7 phages with shaking for 4 hours at 35°C. The bound phages were separated as previously described with slight modification [26]. Briefly, 300 µl of the cell-phage mixtures were gently transferred to the top of a non-miscible dibutyl phthalate/cyclohexane (9∶1 [v:v]) organic lower phase (600 µl) and centrifuged at 10,000 g for 10 min. The supernatants were drawn-off. Bound phages were eluted from cells for 10 minutes at room temperature by adding 500 µl of 1% SDS. The titres of the bound phages were then determined by following the procedures outlined in T7Select system.
Effect of Anti-Trw Antibodies on in vitro Infection of Mouse Erythrocytes by B. birtlesii
The effect of the different mouse anti-Trw antibodies on the invasion capacity of erythrocytes by B. birtlesii was measured in vitro as described [23]. Briefly, after culturing B. birtlesii for 5 days on CBA plates, the bacteria were harvested, washed in PBS and suspended in F12 modified medium. Anti-Trw antibodies (1/100 dilution) or serum from a non-immunized mouse (1/100 dilution) were then incubated with bacteria at 35°C for 4 h, while the control was incubated with F12 modified medium. In each case, bacteria were then added to mouse erythrocytes at a multiplicity of infection (MOI, calculation based on 1 OD600 nm = 3×109 bacteria/ml) of 1 and incubated at 35°C. After 48 h of invasion, the erythrocytes were separated from the non-associated bacteria by washing 3 times with PBS and centrifuged at 1500 rpm for 10 min. The erythrocytes were then incubated with 50 µl gentamicin sulfate (125 µg/ml) for 2 h at 35°C to kill any residual extracellular bacteria, washed three times in PBS to remove the antibiotic and then any intracellular bacteria were released by hypotonic lyses of the erythrocytes in 20 µl of sterile water by freezing at -20°C for 15 min. After thawing, serial dilutions of bacteria in PBS were inoculated onto CBA plates and incubated for 5 days before being counted. The impact of anti-Trw antibodies on invasion capacity was then evaluated by comparing the numbers of intra-erythrocytic bacteria with or without antibodies.
Identification of TrwJ2 Erythrocytic Receptor by Far-Western Blot
Far Western blot aims to detect interactions between two proteins, in our case TrwJ2 and receptor. Briefly, this technique consists in (1) performing SDS-PAGE of erythrocytic membrane protein, containing potential receptor (2) fixing receptor to the membrane by transfer (3) Incubating the membrane with the recombinant and purified TrwJ2 and (4) Revealing with antibodies against putative receptor, band 3.
Lysates of erythrocytic membranes were prepared from frozen blood (5×109 erythrocytes) samples, thawed, resuspended in stabilization solution (ID-CellStab, Diamed) and washed in 0.9% NaCl (B. Braun Medical). Membranes were prepared at 0–4°C by hypotonic lysis with 5P8 buffer (5 mM Na2HPO4, pH 8.0 and 350 µM EDTA, pH 8.0), stripped by incubation with 10 mM NaOH and finally solubilized with an equal volume of 4X LDS Sample buffer (Invitrogen). Erythrocytic membrane lysates were reduced with 100 mM DTT, resolved by Tris-Glycine 8% SDS-PAGE and transferred onto PVDF membranes at 20 V for 25 min by Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell. The PVDF membranes were blocked in 1X blocking buffer and incubated for 2 hours at RT with 200µg of rTrwJ2 in 10 ml blocking buffer, immunodetected with 1/1000 dilution of anti-TrwJ2 polyclonal antibodies and 1/10,000 dilution of AP-goat anti-mouse IgG (H+L) (1/10,000), then stained with a solution of NBT/BICP as above. The PVDF membranes were similarly reprobed with a 1/100 dilution of anti-band3 (C-17) monoclonal antibodies (Santa Cruz Biotechnology, USA) and a 1/30,000 dilution of AP-goat anti-donkey IgG (Jackson ImmunoResearch Laboratories, USA), and stained with a solution of NBT/BICP as above.
Inhibition of Bartonella-erythrocytes Interaction using Anti-band 3 Polyclonal Antibodies
As commercially available anti-band3 antibodies did not recognize all the surface part of band3, polyclonal antibodies raised against the entire sequence of murine erythrocytic band3 were produced as follows: lysates of erythrocytic membrane were resolved by Tris-Glycine 8% SDS-PAGE gels, the band corresponding to the size of band3 (90 kDa) was cut from the gels, grinded and resuspended in PBS.
Two rabbits were injected subcutaneously with 200µg of murine erythrocytic band 3 mixed in Montanide® oil adjuvant ISA-70. Second and third injections were given at 2-week-interval with the same antigen dose. Sera were collected 15 days after the final immunization and stored at -20°C. The titres of polyclonal antibodies were determined by dot-blot analysis using the purified erythrocytic band3 protein.
The effect of anti-band3 polyclonal antibodies on the interaction between TrwJ1-T7 or TrwJ2-T7 phages and mouse erythrocyte was assessed by incubating 1×108 mouse erythrocytes with anti-band3 antibodies (1/100 dilution) or serum from a non-immunized rabbit (1/100 dilution) at 35°C for 4 h, while the control was incubated with PBS. Then 1×109 PFU of TrwJ1-T7 or TrwJ2-T7 phages were added and phage binding assays with mouse erythrocytes were performed as described above.
In parallel, the effect of anti-band3 polyclonal antibodies on the invasion capacity of erythrocytes by B. birtlesii was measured by incubating anti-band3 antibodies (1/150 dilution) or serum from a non-immunized rabbit (1/150 dilution) in B. birtlesii-erythrocyte mixture at 35°C for 4 h, while the control was incubated with F12 modified medium. The intracellular bacteria were quantified as described above.
Results
1- Identification of Trw Components that are Expressed at the B. birtlesii Cell Surface
Candidate genes for mediating Trw interaction with erythrocytic receptors encoded surface-exposed components. Among the Trw components, the T4SS pilus components TrwL (L1 to L5) and TrwJ (J1 and J2) were shown to be putative surface proteins [27]. We checked whether they were indeed expressed at the B. birtlesii surface by first producing polyclonal antibodies which reacted specifically with each of the corresponding recombinant proteins.
Recombinant soluble proteins rTrwJ2, rTrwL2, rTrwL3, rTrwL4 and rTrwL5 were expressed and recovered from the supernatant of lysated E. coli, while recombinant rTrwJ1 was recovered as an insoluble form in the inclusion body of E. coli. Despite many assays using different E. coli strains and different culture conditions, we failed to express rTrwL1.
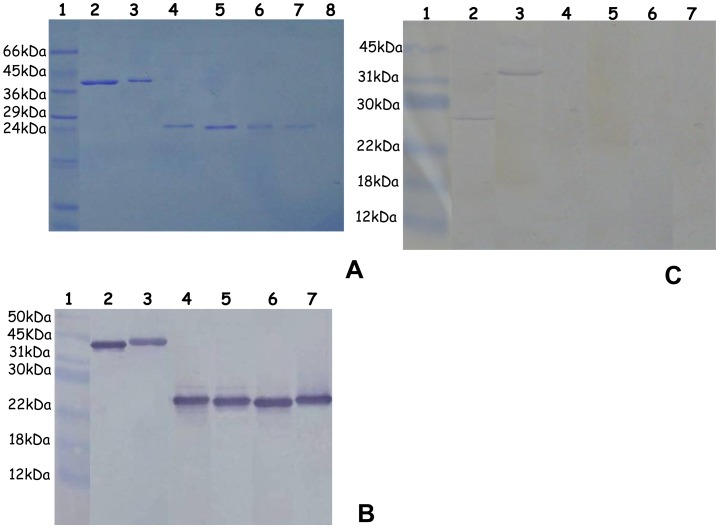
After purification, a single band corresponding to each purified recombinant protein was identified on SDS-PAGE on a Coomassie stained acrylamide gel. The observed molecular mass corresponded to the predicted size of the recombinant protein with the addition of 13 kDa corresponding to the thioredoxin motif and 3 kDa corresponding to the V5 and 6×His-tag motifs, i.e. 43.5 kDa for rTrwJ1, 42 kDa for rTrwJ2, 23.5 kDa for rTrwL2, 23.5 kDa for rTrwL3, 23.5 kDa for rTrwL4 and 23.5 kDa for rTrwL5 (Figure 1A).
Figure 1. Expression and detection of the putative surface Trw components.
A - SDS electrophoresis of the purified recombinant Trw proteins. SDS-PAGE analysis under reduced condition of Trw recombinant proteins expressed in E. coli after purification by affinity chromatography and without elimination of the thioredoxin epitope. Lane 1, Low range molecular weight (Sigma); Lane 2, rTrwJ2; Lane 3, rTrwJ1; Lane 4, rTrwL5; Lane 5, rTrwL4; Lane 6, rTrwL3; lane 7, rTrwL2; Lane 8, rTrwL1. B - Western blot detection of the purified recombinant Trw proteins Immunoblot analysis of Trw recombinant proteins expressed in E. coli after purification by affinity chromatography, without elimination of the thioredoxin epitope, and after separation on SDS-PAGE under reduced conditions, using polyclonal antibodies against rTrwJ2 (lane 2), rTrwJ1 (lane 3), rTrwL5 (lane 4), rTrwL4 (lane 5), rTrwL3 (lane 6), rTrwL2 (lane 7). Lane 1, Prestained molecular weight marker (New England Biolabs). C - Western blot detection of the B. birtlesii Trw proteins Western blot detection of total B.birtlesii proteins separated by SDS-PAGE under reduced conditions, using polyclonal antibodies against rTrwJ1 (lane 2), rTrwJ2 (lane 3), rTrwL2 (lane 4), rTrwL3 (lane 5), rTrwL4 (lane 6) and rTrwL5 (lane 7). Lane 1, Prestained molecular weight marker (New England Biolabs).
The thioredoxin-free recombinant proteins were used to produce polyclonal antibodies from immunized Balb/C mice. The obtained polyclonal antibodies reacted with the corresponding recombinant proteins as shown in Figure 1B.
Recognition of native proteins by antibodies was then evaluated by western blot on proteins extracted from B. birtlesii culture on agar plates and separated on SDS-PAGE. As shown in Figure 1C, only TrwJ1 and TrwJ2 were detected, while Trw L2, L3, L4, L5 were not (Figure 1C). The molecular mass observed for TrwJ1 corresponded approximately to the one calculated from the sequence (27.5 kDa). On the contrary, the molecular mass observed for TrwJ2 was higher than was expected from the sequence (26 kDa) with signal peptides around 32 kDa. This difference would suggest the presence of aggregates or post-translational modifications.
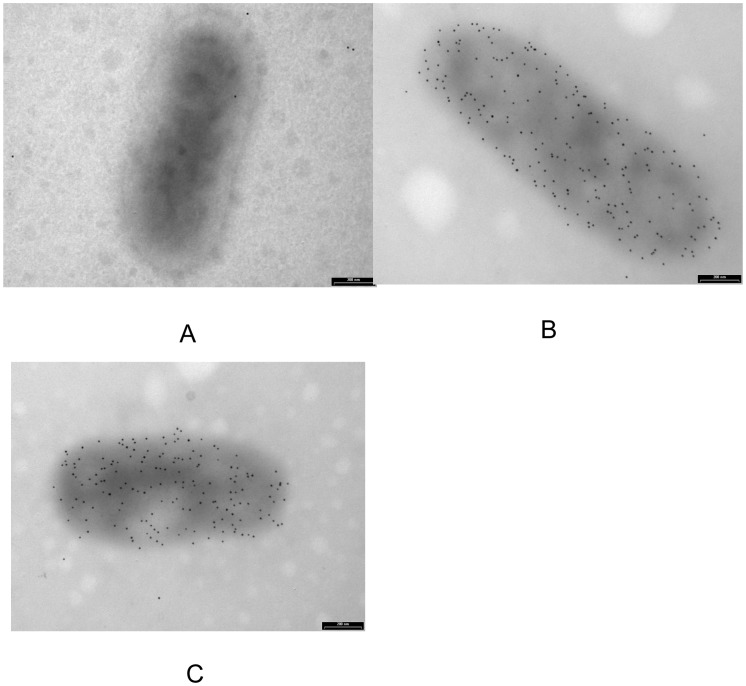
The different putative Trw surface components were localized by immunostaining B. birtlesii whole bacteria with the different anti-Trw polyclonal antibodies and electron microscopy observations. TrwJ1 and TrwJ2 were detected at the B. birtlesii cell surface whereas none of the TrwL proteins was detected at the cell surface (Figure 2).
Figure 2. Immunogold labelling and transmission electron microscopy of Trw components on the B. birtlesii surface.
Electron microscopy detection of TrwJ1 and TrwJ2 on the surface of CBA-cultivated B. birtlesii using polyclonal antibodies against rTrwJ1 (B) and rTrwJ2 (C) recombinant proteins. (A), naïve mouse serum as negative control.
2– TrwJ1 and TrwJ2 Interact with Mouse Erythrocytes
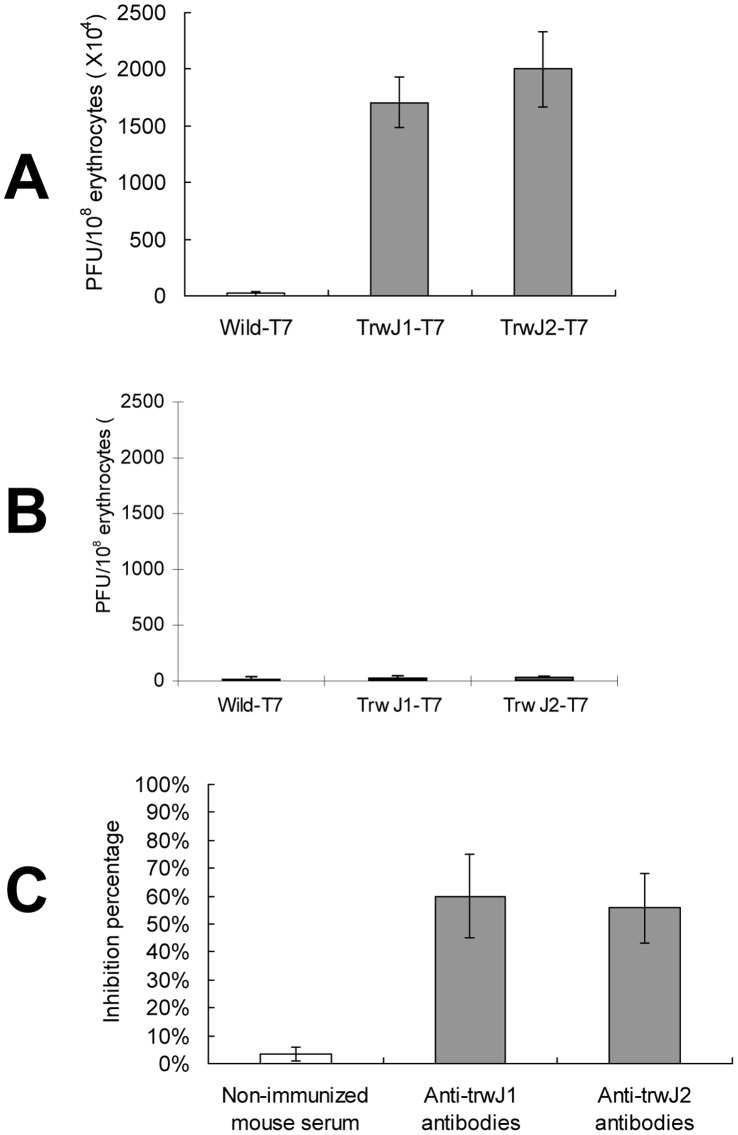
Two complementary analyses were performed to see whether TrwJ1 and TrwJ2 were able to bind to mouse erythrocytes: the first analysis consisted of measuring the capacity of TrwJ1-T7 as well as TrwJ2-T7 phages to bind to mouse or cat erythrocytes; the second consisted of evaluating the capacity of anti-TrwJ1 and anti-TrwJ2 polyclonal antibodies to inhibit mouse erythrocyte invasion by B. birtlesii. As shown in Figures 3A and 3B, T7 phage displaying B. birtlesii TrwJ1 and TrwJ2 were able to bind to mouse erythrocytes but not to cat erythrocyte. The amounts of TrwJ1-T7 and TrwJ2-T7 phages which were able to bind to 1×108 mouse erythrocytes were 1.7×107 PFU and 2×107 PFU respectively, while wild-T7 phages were unable to bind to mouse erythrocytes. Addition of anti-TrwJ1 and anti-TrwJ2 polyclonal antibodies to the B. birtlesii-erythrocytes invasion mixture, significantly reduced the invasion of mouse erythrocytes by B. birtlesii by 60% and 55.7% respectively, while serum from a non-immunized mouse did not reduce invasion (Figure 3C).
Figure 3. Identification of TrwJ1 and TrwJ2 interaction with mouse erythrocytes.
A – Binding assay between mouse erythrocytes and T7 fusion phages Evaluations of the capacity of TrwJ1-T7 and TrwJ2-T7 phages to bind to mouse erythrocytes were evaluated three times (3 replicates each). Wild-T7 phages were used as control and the values are presented as the mean of three independent experiments. B – Binding assay between cats erythrocytes and T7 fusion phages Evaluation of the capacity of TrwJ1-T7 and TrwJ2-T7 phages to bind to cat erythrocytes were evaluated once in 3 replicates each. Wild-T7 phages were used as control and the values are presented as the mean of three experiments. C – Efficiency of in vitro invasion inhibition of mouse erythrocyte by B. birtlesii with anti-Trw polyclonal antibodies Invasion inhibition assays with polyclonal antibodies against rTrwJ1 and rTrwJ2 recombinant proteins and serum from non-immunized mouse were performed three times (3 replicates each). The values are presented as the mean of three independent experiments.
3 Identification of Erythrocytic Receptor of TrwJ2
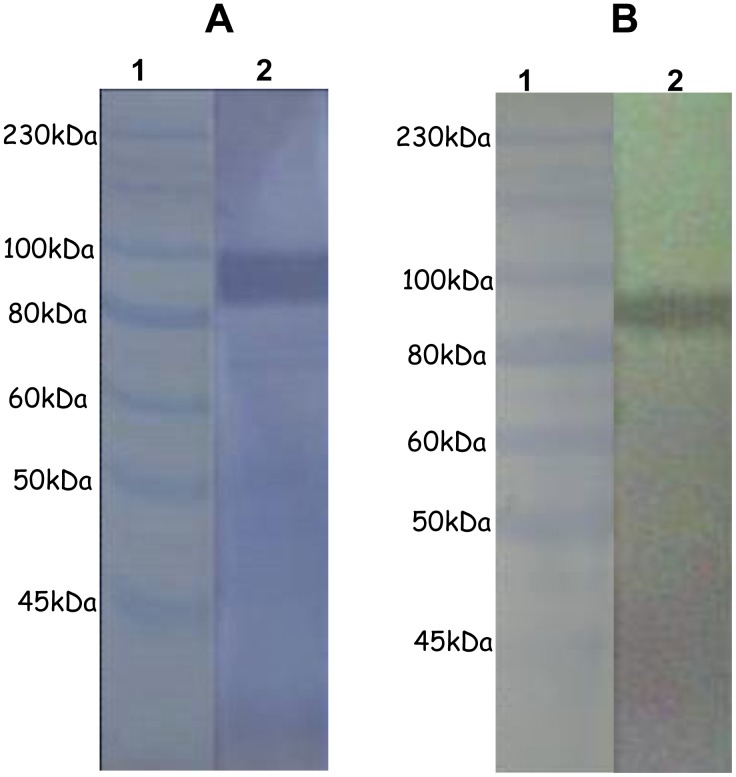
As TrwJ1 was expressed as an insoluble form, identification of the receptor was only conducted with TrwJ2 by Far-Western blotting. When recombinant rTrwJ2 is incubated with mouse erythrocytic membrane proteins, antibodies against TrwJ2 react with a single band which corresponds to a mouse erythrocytic membrane protein with an estimated size of 90 kDa (Figure 4A). This band corresponds to the size of the erythrocytic band3 as validated by immunoblot with goat anti-band3 monoclonal antibody (Figure 4B).
Figure 4. Identification of TrwJ2 erythrocytic receptor.
A – Far Western blot analysis of mouse erythrocyte membrane proteins using rTrwJ2 recombinant protein Mouse erythrocyte membrane proteins were separated on SDS-PAGE under reduced conditions, transferred (on? to?) PVDF membrane, probed with rTrwJ2, and analysed by immunoblot using anti-mouse antibodies (lane 2). Lane 1, prestained molecular weight marker (New England Biolabs). The experiment was conducted twice with qualitatively similar results. B –Immunoblot analysis of mouse erythrocytic membrane proteins using Band3 monoclonal antibody Mouse erythrocyte membrane proteins were separated on SDS-PAGE under reduced conditions, transferred (on? to?) a PVDF membrane, and analysed by immunoblot using anti-band3 antibodies (lane 2). Lane 1, Prestained molecular weight marker (New England Biolabs).
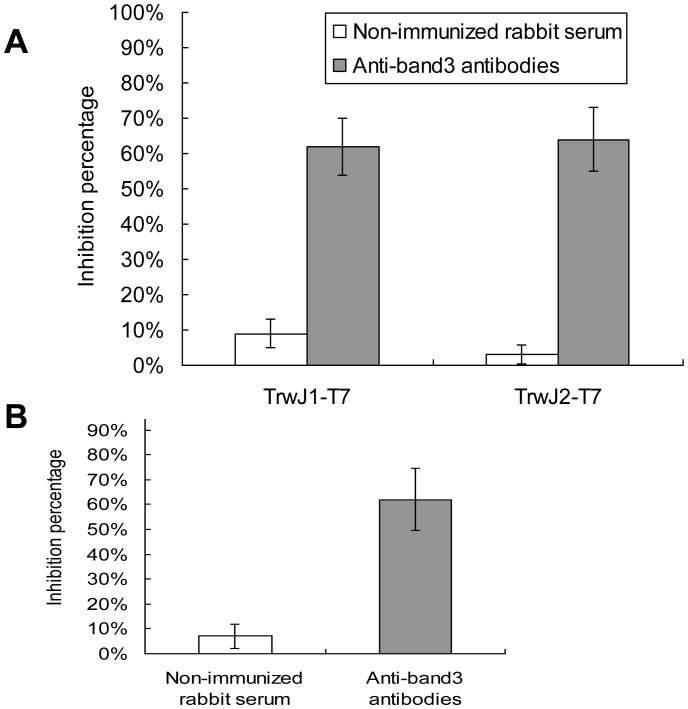
To further demonstrate that band 3 was or was not a receptor of B. birtlesii TrwJ, we then evaluated the capacity of rabbit anti-band3 polyclonal antibodies to inhibit erythrocyte binding with B. birtlesii TrwJ1-T7 or TrwJ2-T7 phages and erythrocyte invasion by B. birtlesii. As shown in Figures 5A and 5B, adding anti-band3 polyclonal antibodies to the TrwJ1-T7 or TrwJ2-T7 phages-erythrocytes binding mixture significantly reduced phage binding capacity by 62% and 64% respectively, and significantly reduced B. birtlesii invasion capacity by 62%, while serum from non-immunized rabbit had only a very slight influence on both adherence and invasion.
Figure 5. Role of erythrocytic band3 in adhesion and invasion between mouse erythrocyte and B. birtlesii.
A – Analysis of the impact of anti-band3 antibodies on the interaction between mouse erythrocytes and TrwJ1-T7 or TrwJ2-T7 phages. Phage binding inhibition assays with anti-band3 polyclonal antibodies and serum from non immunized rabbit were performed three times (3 replicates each).The values are presented as the mean of three independent experiments. B – Analysis of the impact of anti-band3 antibodies on the invasion capacity of mouse erythrocyte by B. birtlesii Invasion inhibition assays with anti-band3 polyclonal antibodies and serum from non immunized rabbit were performed three times (3 replicates each). The values are presented as the mean of three independent experiments.
Discussion
Bacteria-specific adhesion to host cells can be defined as the selective binding between a specific molecular component on the bacterial surface and a substratum-specific receptor in the host cells. We have previously shown that in Bartonella species, the T4SS Trw is involved in erythrocytic recognition [23]. T4SS Trw is characterized by a multiprotein complex that spans the inner and outer bacterial membranes, and possesses a hypothetical pilus structure. TrwJ and trwL are thought to encode minor and major pilus components, which are considered to be potentially responsible for the interaction with erythrocyte [27]. The aim of the present study was therefore to evaluate the role of TrwJ and TrwL proteins in adhesion to erythrocytes.
B.birtlesii trwJ1, trwJ2, trwL2, trwL3, trwL4 and trwL5 were expressed as recombinant proteins and polyclonal antibodies against these proteins were produced, firstly to estimate their expression and secondly to localize them on the cell surface of the bacteria. Immunoblot analysis showed that only TrwJ1 and TrwJ2 could be detected on CBA-cultivated B. birtlesii, and not TrwL2, L3, L4, or L5, suggesting that these latter were not expressed in in vitro culture, or at an undetectable level. Some studies showed that homologues of TrwJ (VirB5) and TrwL (VirB2) were not detected in wild and complemented Agrobacterium tumefaciens, whereas they could be detected in induced complemented cells [28], [29], [30], [31]. Similarly the homologue of VirB5 was not detected in B. henselae cultivated on cell-free laboratory medium but could be detected in bacteria incubated with HMEC-1 cells [32]. The Trw T4SS has also been identified as being upregulated intracellularly during B. henselae interaction with HUVECs or ECs [8]. Finally, Bartonella has the ability to infect different hosts (reservoir or incidental mammalian as well as arthropod hosts), and different host cell types, which suggests the existence of different pathogenicity factors on its surface that are presumably controlled by differential gene expression during the course of infection. These findings suggest that the expression of TrwL, like that of its homologues in other bacteria, might be regulated in response to infection signals, although further work is necessary to unravel the molecular details of this mechanism.
Results obtained by immunoblot analysis were then confirmed by electron microscopic analysis as only Trw J1 and TrwJ2 were detected at the cell surface of B. birtlesii. For these reasons, TrwJ1 and TrwJ2 appeared to us as the best potential candidates for in vitro interaction between B. birtlesii and erythrocytes.
We then investigated whether the surface Trw components TrwJ1 and TrwJ2 were associated with the adherence to erythrocytes, by constructing phage displaying B. birtlesii TrwJ1 and TrwJ2. The results showed that B. birtlesii TrwJ1-T7 and TrwJ2-T7 phages were able to bind to mouse erythrocytes, while wild-T7 phages showed significantly less binding ability. We then confirmed this result by evaluating the capacity of anti-TrwJ1 and anti-TrwJ2 polyclonal antibodies to inhibit mouse erythrocyte invasion by B. birtlesii. We found that incubation with both polyclonal antibodies resulted in inhibition of the invasion of mouse erythrocytes by B. birtlesii. These results clearly suggest that TrwJ1 and TrwJ2 are associated with adherence of the bacteria to erythrocytes. As we have previously shown that the Trw T4SS of Bartonella mediates host-specific adhesion to erythrocytes, and that B. birtlesii is unable to bind and invade cat erythrocytes [23], we performed the same experiment, using cat erythrocytes and showed that B. birtlesii TrwJ1-T7 and TrwJ2-T7 phages were not able to bind to cat erythrocytes. These results enlarged on those obtained in earlier studies of the relationship between Trw and the host erythrocyte, and now suggest that this host-specific adhesion is mediated by TrwJ1 and TrwJ2.
Although TrwL were not detected on the surface of bacteria, this does not exclude an interactive role between the bacteria and their host cells. Indeed, after adherence, bacteria use other virulence factors to become more intimately bound to their host cells via specific and stable interactions that can mediate invasion [33]. Mutagenesis of TrwL is reported to lead to inhibition of intraerythrocytic bacteremia in the reservoirs of for B. tribocorum and B. birtlesii [22], [23], and to loss of the capacity of B. birtlesii to infect mouse erythrocyte in vitro [23] thus demonstrating that TrwL also has an essential role in erythrocyte invasion, both in vivo and in vitro. However, in the absence of direct proof, to suggest that TrwL is involved in intimate adhesion rather than in the initial adhesion occurring during infection of the erythrocytes, remains speculative.
By conducting experiments to determine the receptor of TrwJ1 and TrwJ2, we found that TrwJ2 recombinant protein was able to bind a major glycoprotein present in mouse erythrocyte membrane: band3. We also demonstrated that, in vitro, polyclonal antibodies raised against mouse Band-3 were able to inhibit the adhesion between TrwJ1-T7 and TrwJ2-T7 phages and mouse erythrocytes and reduce the mouse erythrocyte invasion capacity of B. birtlesii. Taken together, all these results clearly suggest an interaction between TrwJ1, TrwJ2 and Band 3 leading to critical adherence of the bacteria to its host cells, the erythrocytes.
Band 3 is a major transmembrane glycoprotein of the erythrocyte membrane and functions in anion transport [34]. It has been suggested to be one of the possible erythrocyte receptors of B. bacilliformis [19]. Erythrocytic band 3 has also been suggested to be involved in the malaria parasite invasion of erythrocytes [35], [36], [37], [38], [39], [40]. In addition, recent studies have revealed that P. falciparum merozoite surface protein 1 (MSP1), an essential parasite protein has a conserved role in the invasion of erythrocytes by P. falciparum and P. chabaudi [41], [42] and this protein interacts with two nonglycosylated exofacial regions of erythrocyte band 3, designated 5ABC (amino acids 720–761) and 6A (amino acids 807–826) [43]. Two regions of merozoite surface protein 9 (MSP9), which is also known as an acidic basic repeat antigen, interact directly with 5ABC during erythrocyte invasion by P. falciparum [44], [45]. Erythrocyte invasion by P. falciparum is thought to proceed via two distinct pathways [46], [47]: a sialic acid-dependent pathway mediated by glycophorin A, B and C [48], [49], [50], [51], [52], and a sialic acid-independent pathway mediated by band3, as described above. Concerning Bartonella, a previous study showed that pre-treatment of feline erythrocytes with neuraminidase and trypsin had no effect on B. henselae invasion, indicating that invasion occurs via a sialic acid-independent pathway [53]. As we have identified band 3 as the erythrocyte receptor of Bartonella, we attempted to determine whether or not the sialic acid-dependent erythrocyte receptors of P. falciparum were also involved in Bartonella infection. Preliminary results demonstrated that the anti-mouse N-terminal extracellular domain of glycophorin A polyclonal antibodies reduced invasion of mouse erythrocytes by B. birtlesii by approximately 50% (data not shown). This result provides additional information which allows us to hypothesize that Bartonella-erythrocyte interactions may also be mediated by two distinct pathways, and expands our understanding of the biology and infection course of Bartonella spp., which is still far from completely understood. Further studies are now conducted to elucidate the complete mechanisms involved in erythrocyte invasion by Bartonella spp.
Acknowledgments
Authors thanks the REID group, Tiques et Maladies à Tiques, for helpful discussion.
Funding Statement
This work was supported by the French National Institute for Agricultural Research (INRA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, et al. (2009) Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 40: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulouis HJ, Haddad N, Vayssier-Taussat M, Maillard R, Chomel B (2007) [Persistent Bartonella infection: epidemiological and clinical implications]. Bull Acad Natl Med 191: 1037–1044; discussion 1047–1039. [PubMed] [Google Scholar]
- 3. Vayssier-Taussat M, Le Rhun D, Bonnet S, Cotte V (2009) Insights in Bartonella host specificity. Ann N Y Acad Sci 1166: 127–132. [DOI] [PubMed] [Google Scholar]
- 4. Barbour AG, Restrepo BI (2000) Antigenic variation in vector-borne pathogens. Emerg Infect Dis 6: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulein R, Seubert A, Gille C, Lanz C, Hansmann Y, et al. (2001) Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med 193: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dehio C, Meyer M, Berger J, Schwarz H, Lanz C (1997) Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci 110 (Pt 18): 2141–2154. [DOI] [PubMed] [Google Scholar]
- 7. Dehio C (1999) Interactions of Bartonella henselae with vascular endothelial cells. Curr Opin Microbiol 2: 78–82. [DOI] [PubMed] [Google Scholar]
- 8. Dehio C (2001) Bartonella interactions with endothelial cells and erythrocytes. Trends Microbiol 9: 279–285. [DOI] [PubMed] [Google Scholar]
- 9. Schmid MC, Schulein R, Dehio M, Denecker G, Carena I, et al. (2004) The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol Microbiol 52: 81–92. [DOI] [PubMed] [Google Scholar]
- 10. Pulliainen AT, Dehio C (2009) Bartonella henselae: subversion of vascular endothelial cell functions by translocated bacterial effector proteins. Int J Biochem Cell Biol 41: 507–510. [DOI] [PubMed] [Google Scholar]
- 11. Mandle T, Einsele H, Schaller M, Neumann D, Vogel W, et al. (2005) Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae. Blood 106: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 12. Walker TS, Winkler HH (1981) Bartonella bacilliformis: colonial types and erythrocyte adherence. Infect Immun 31: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minnick MF (1994) Identification of outer membrane proteins of Bartonella bacilliformis. Infect Immun 62: 2644–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minnick MF, Mitchell SJ, McAllister SJ (1996) Cell entry and the pathogenesis of Bartonella infections. Trends Microbiol 4: 343–347. [DOI] [PubMed] [Google Scholar]
- 15. Greub G, Raoult D (2002) Bartonella: new explanations for old diseases. J Med Microbiol 51: 915–923. [DOI] [PubMed] [Google Scholar]
- 16. Scherer DC, DeBuron-Connors I, Minnick MF (1993) Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun 61: 4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benson LA, Kar S, McLaughlin G, Ihler GM (1986) Entry of Bartonella bacilliformis into erythrocytes. Infect Immun 54: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Battisti JM, Minnick MF (1999) Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Environ Microbiol 65: 3441–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buckles EL, McGinnis Hill E (2000) Interaction of Bartonella bacilliformis with human erythrocyte membrane proteins. Microb Pathog 29: 165–174. [DOI] [PubMed] [Google Scholar]
- 20. Iwaki-Egawa S, Ihler GM (1997) Comparison of the abilities of proteins from Bartonella bacilliformis and Bartonella henselae to deform red cell membranes and to bind to red cell ghost proteins. FEMS Microbiol Lett 157: 207–217. [DOI] [PubMed] [Google Scholar]
- 21. Christie PJ, Vogel JP (2000) Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol 8: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saenz HL, Engel P, Stoeckli MC, Lanz C, Raddatz G, et al. (2007) Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet 39: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 23. Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, Cescau S, et al. (2010) The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog 6: e1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dehio C (2008) Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell Microbiol 10: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Rhun D, Malou N, Labed S, Le Naour E, Vayssier-Taussat M (2009) In vitro effect of Bartonella birtlesii on mouse red cell viability. Clin Microbiol Infect 15 Suppl 2 112–113. [DOI] [PubMed] [Google Scholar]
- 26. Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W (2001) Biopanning and rapid analysis of selective interactive ligands. Nat Med 7: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 27. Schroder G, Dehio C (2005) Virulence-associated type IV secretion systems of Bartonella. Trends Microbiol 13: 336–342. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt-Eisenlohr H, Domke N, Angerer C, Wanner G, Zambryski PC, et al. (1999) Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J Bacteriol 181: 7485–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aly KA, Baron C (2007) The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 153: 3766–3775. [DOI] [PubMed] [Google Scholar]
- 30. Lai EM, Kado CI (1998) Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol 180: 2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krall L, Wiedemann U, Unsin G, Weiss S, Domke N, et al. (2002) Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A 99: 11405–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmiederer M, Arcenas R, Widen R, Valkov N, Anderson B (2001) Intracellular induction of the Bartonella henselae virB operon by human endothelial cells. Infect Immun 69: 6495–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirchner M, Heuer D, Meyer TF (2005) CD46-independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect Immun 73: 3072–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cabantchik ZI, Knauf PA, Rothstein A (1978) The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta 515: 239–302. [DOI] [PubMed] [Google Scholar]
- 35. Clough B, Paulitschke M, Nash GB, Bayley PM, Anstee DJ, et al. (1995) Mechanism of regulation of malarial invasion by extraerythrocytic ligands. Mol Biochem Parasitol 69: 19–27. [DOI] [PubMed] [Google Scholar]
- 36. Okoye VC, Bennett V (1985) Plasmodium falciparum malaria: band 3 as a possible receptor during invasion of human erythrocytes. Science 227: 169–171. [DOI] [PubMed] [Google Scholar]
- 37. Jones GL, Edmundson HM (1991) Plasmodium falciparum polypeptides interacting with human red cell membranes show high affinity binding to Band-3. Biochim Biophys Acta 1097: 71–76. [DOI] [PubMed] [Google Scholar]
- 38. Miller LH, Hudson D, Rener J, Taylor D, Hadley TJ, et al. (1983) A monoclonal antibody to rhesus erythrocyte band 3 inhibits invasion by malaria (Plasmodium knowlesi) merozoites. J Clin Invest 72: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roggwiller E, Betoulle ME, Blisnick T, Braun Breton C (1996) A role for erythrocyte band 3 degradation by the parasite gp76 serine protease in the formation of the parasitophorous vacuole during invasion of erythrocytes by Plasmodium falciparum. Mol Biochem Parasitol 82: 13–24. [DOI] [PubMed] [Google Scholar]
- 40. McPherson RA, Donald DR, Sawyer WH, Tilley L (1993) Proteolytic digestion of band 3 at an external site alters the erythrocyte membrane organisation and may facilitate malarial invasion. Mol Biochem Parasitol 62: 233–242. [DOI] [PubMed] [Google Scholar]
- 41. O'Donnell RA, Saul A, Cowman AF, Crabb BS (2000) Functional conservation of the malaria vaccine antigen MSP-119across distantly related Plasmodium species. Nat Med 6: 91–95. [DOI] [PubMed] [Google Scholar]
- 42. O'Donnell RA, de Koning-Ward TF, Burt RA, Bockarie M, Reeder JC, et al. (2001) Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med 193: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goel VK, Li X, Chen H, Liu SC, Chishti AH, et al. (2003) Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc Natl Acad Sci U S A 100: 5164–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kariuki MM, Li X, Yamodo I, Chishti AH, Oh SS (2005) Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Biophys Res Commun 338: 1690–1695. [DOI] [PubMed] [Google Scholar]
- 45. Li X, Chen H, Oo TH, Daly TM, Bergman LW, et al. (2004) A co-ligand complex anchors Plasmodium falciparum merozoites to the erythrocyte invasion receptor band 3. J Biol Chem 279: 5765–5771. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell GH, Hadley TJ, McGinniss MH, Klotz FW, Miller LH (1986) Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood 67: 1519–1521. [PubMed] [Google Scholar]
- 47. Dolan SA, Miller LH, Wellems TE (1990) Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest 86: 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang L, Duriseti S, Sun P, Miller LH (2009) Molecular basis of binding of the Plasmodium falciparum receptor BAEBL to erythrocyte receptor glycophorin C. Mol Biochem Parasitol. 168: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perkins ME (1984) Surface proteins of Plasmodium falciparum merozoites binding to the erythrocyte receptor, glycophorin. J Exp Med 160: 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayer DC, Cofie J, Jiang L, Hartl DL, Tracy E, et al. (2009) Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc Natl Acad Sci U S A 106: 5348–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lobo CA, Rodriguez M, Reid M, Lustigman S (2003) Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 101: 4628–4631. [DOI] [PubMed] [Google Scholar]
- 52. Davidson EA, Perkins ME (1988) Receptor binding domain of glycophorin A for Plasmodium falciparum surface proteins. Indian J Biochem Biophys 25: 90–94. [PubMed] [Google Scholar]
- 53. Mehock JR, Greene CE, Gherardini FC, Hahn TW, Krause DC (1998) Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun 66: 3462–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]