Abstract
A procedure for the construction of 3'-end labelled yeast tRNAAsp harboring substitutions or additions of any desired nucleotide in T-stem and T-loop (position 57 to 61) has been developed. This was done by in vitro enzymatic manipulations of the yeast tRNAAsp involving specific hydrolysis with RNases, phosphorylation and dephosphorylation with T4 polynucleotide kinase and ligation with T4 RNA ligase. Using this procedure we have replaced conserved or semi-conserved nucleotides located in position 57 to 61 of yeast tRNAAsp. We have also constructed different yeast tRNAAsp with eight bases instead of seven in T-loop. Further use of these tRNAAsp variants will be discussed with the help of the crystallographic three-dimensional structure.
Full text
PDF






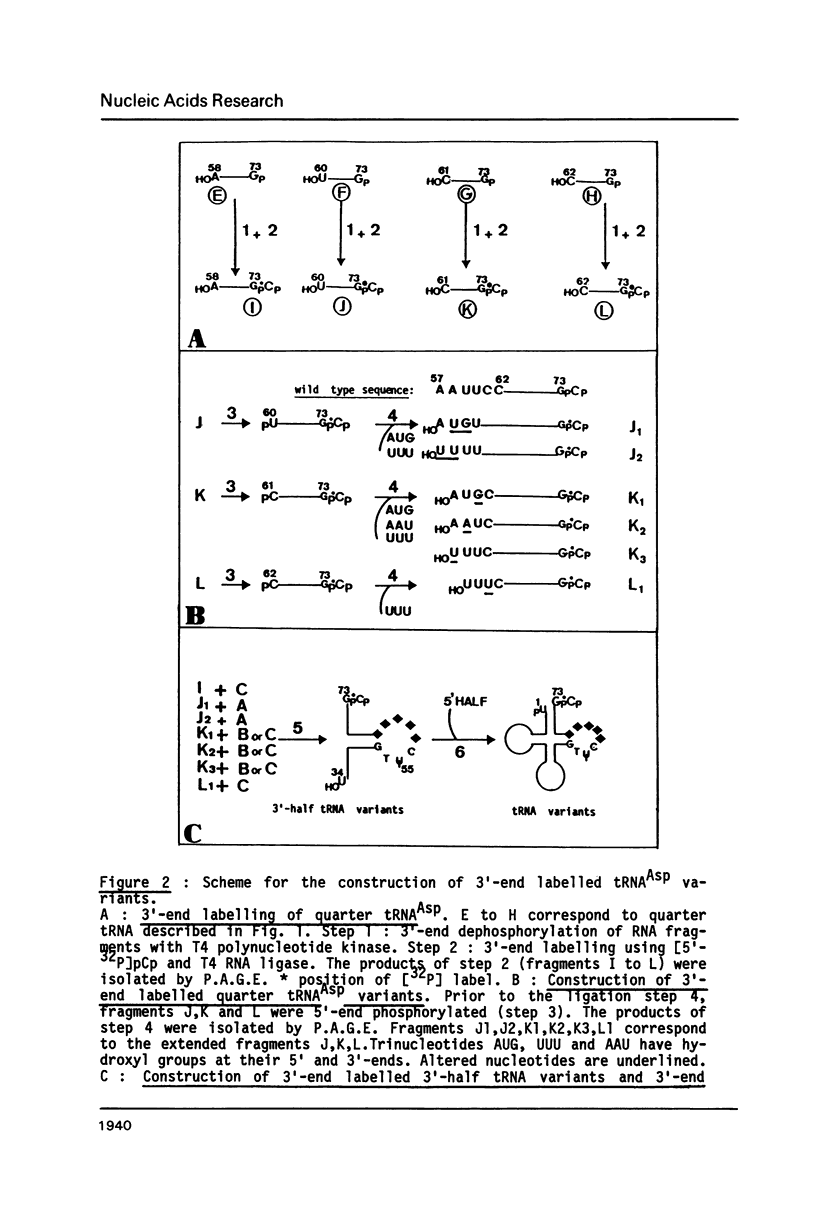










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bare L. A., Uhlenbeck O. C. Specific substitution into the anticodon loop of yeast tyrosine transfer RNA. Biochemistry. 1986 Sep 23;25(19):5825–5830. doi: 10.1021/bi00367a072. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Atkins J. F., Gesteland R. F. tRNA anticodon replacement experiments show that ribosomal frameshifting can be caused by doublet decoding. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5062–5066. doi: 10.1073/pnas.83.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Specific interaction of anticodon loop residues with yeast phenylalanyl-tRNA synthetase. Biochemistry. 1982 Aug 17;21(17):3921–3926. doi: 10.1021/bi00260a003. [DOI] [PubMed] [Google Scholar]
- Cameron V., Soltis D., Uhlenbeck O. C. Polynucleotide kinase from a T4 mutant which lacks the 3' phosphatase activity. Nucleic Acids Res. 1978 Mar;5(3):825–833. doi: 10.1093/nar/5.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Carbon P., Haumont E., De Henau S., Keith G., Grosjean H. Enzymatic replacement in vitro of the first anticodon base of yeast tRNAAsp: application to the study of tRNA maturation in vivo, after microinjection into frog oocytes. Nucleic Acids Res. 1982 Jun 25;10(12):3715–3732. doi: 10.1093/nar/10.12.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Haumont E., Fournier M., de Henau S., Grosjean H. Site-directed in vitro replacement of nucleosides in the anticodon loop of tRNA: application to the study of structural requirements for queuine insertase activity. EMBO J. 1983;2(7):1093–1097. doi: 10.1002/j.1460-2075.1983.tb01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Sprinzl M., Cramer F. Proton nuclear magnetic resonance of minor nucleosides in yeast phenylalanine transfer ribonucleic acid. Conformational changes as a consequence of aminoacylation, removal of the Y base, and codon--anticodon interaction. Biochemistry. 1979 Jul 24;18(15):3189–3199. doi: 10.1021/bi00582a001. [DOI] [PubMed] [Google Scholar]
- Digweed M., Kumagai I., Pieler T., Erdmann V. A. The effect of a cytidine-to-uridine transition on the stability of Escherichia coli A19 5-S RNA. Eur J Biochem. 1982 Oct;127(3):531–537. doi: 10.1111/j.1432-1033.1982.tb06904.x. [DOI] [PubMed] [Google Scholar]
- Doi T., Yamane A., Matsugi J., Ohtsuka E., Ikehara M. Replacement and insertion of nucleotides at the anticodon loop of E. coli tRNAMetf by ligation of chemically synthesized ribooligonucleotides. Nucleic Acids Res. 1985 May 24;13(10):3685–3697. doi: 10.1093/nar/13.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans L., Haumont E., de Henau S., Grosjean H. Enzymatic 2'-O-methylation of the wobble nucleoside of eukaryotic tRNAPhe: specificity depends on structural elements outside the anticodon loop. EMBO J. 1986 May;5(5):1105–1109. doi: 10.1002/j.1460-2075.1986.tb04329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen H. G. Ligand-induced conformational changes in ribonucleic acids. Prog Nucleic Acid Res Mol Biol. 1980;24:57–86. doi: 10.1016/s0079-6603(08)60671-6. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Cedergren R. J., McKay W. Structure in tRNA data. Biochimie. 1982 Jun;64(6):387–397. doi: 10.1016/s0300-9084(82)80576-2. [DOI] [PubMed] [Google Scholar]
- Hassur S. M., Whitlock H. W., Jr UV shadowing--a new and convenient method for the location of ultraviolet-absorbing species in polyacrylamide gels. Anal Biochem. 1974 May;59(1):162–164. doi: 10.1016/0003-2697(74)90020-7. [DOI] [PubMed] [Google Scholar]
- Haumont E., Fournier M., de Henau S., Grosjean H. Enzymatic conversion of adenosine to inosine in the wobble position of yeast tRNAAsp: the dependence on the anticodon sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2705–2715. doi: 10.1093/nar/12.6.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helk B., Sprinzl M. Interaction of unfolded tRNA with the 3'-terminal region of E. coli 16S ribosomal RNA. Nucleic Acids Res. 1985 Sep 11;13(17):6283–6298. doi: 10.1093/nar/13.17.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook S. R., Kim S. H. Correlation between chemical modification and surface accessibility in yeast phenylalanine transfer RNA. Biopolymers. 1983 Apr;22(4):1145–1166. doi: 10.1002/bip.360220410. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Krug M., Uhlenbeck O. C. Reversal of T4 RNA ligase. Biochemistry. 1982 Apr 13;21(8):1858–1864. doi: 10.1021/bi00537a024. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland G. D., Borer P. N. Separation of oligo-RNA by reverse-phase HPLC. Nucleic Acids Res. 1979 Oct 25;7(4):1067–1080. doi: 10.1093/nar/7.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moras D., Dock A. C., Dumas P., Westhof E., Romby P., Ebel J. P., Giegé R. Anticodon-anticodon interaction induces conformational changes in tRNA: yeast tRNAAsp, a model for tRNA-mRNA recognition. Proc Natl Acad Sci U S A. 1986 Feb;83(4):932–936. doi: 10.1073/pnas.83.4.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T., Nishikawa K., Takemura S. Studies on T. utilis tRNATyr variants with enzymatically altered D-loop sequences. II. Relationship between the tertiary structure and tyrosine acceptance. J Biochem. 1986 Mar;99(3):859–866. doi: 10.1093/oxfordjournals.jbchem.a135546. [DOI] [PubMed] [Google Scholar]
- Pace B., Matthews E. A., Johnson K. D., Cantor C. R., Pace N. R. Conserved 5S rRNA complement to tRNA is not required for protein synthesis. Proc Natl Acad Sci U S A. 1982 Jan;79(1):36–40. doi: 10.1073/pnas.79.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk E., McLaughlin L. W., Neilson T., Romaniuk P. J. The effect of acceptor oligoribonucleotide sequence on the T4 RNA ligase reaction. Eur J Biochem. 1982 Jul;125(3):639–643. doi: 10.1111/j.1432-1033.1982.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Romby P., Moras D., Bergdoll M., Dumas P., Vlassov V. V., Westhof E., Ebel J. P., Giegé R. Yeast tRNAAsp tertiary structure in solution and areas of interaction of the tRNA with aspartyl-tRNA synthetase. A comparative study of the yeast phenylalanine system by phosphate alkylation experiments with ethylnitrosourea. J Mol Biol. 1985 Aug 5;184(3):455–471. doi: 10.1016/0022-2836(85)90294-3. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon loop size and sequence requirements for recognition of formylmethionine tRNA by methionyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6755–6759. doi: 10.1073/pnas.80.22.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Menzel H. M., Gassen H. G. Codon-dependent rearrangement of the three-dimensional structure of phenylalanine tRNA, exposing the T-psi-C-G sequence for binding to the 50S ribosomal subunit. Biochemistry. 1976 Jun 1;15(11):2484–2490. doi: 10.1021/bi00656a035. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1984;12 (Suppl):r1–57. [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Holbrook S. R., Warrant R. W., Church G. M., Kim S. H. Crystal structure of yeast phenylalanine transfer RNA. I. Crystallographic refinement. J Mol Biol. 1978 Aug 25;123(4):607–630. doi: 10.1016/0022-2836(78)90209-7. [DOI] [PubMed] [Google Scholar]
- Uemura H., Imai M., Ohtsuka E., Ikehara M., Söll D. E. coli initiator tRNA analogs with different nucleotides in the discriminator base position. Nucleic Acids Res. 1982 Oct 25;10(20):6531–6539. doi: 10.1093/nar/10.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Giegé R., Ebel J. P. Tertiary structure of tRNAs in solution monitored by phosphodiester modification with ethylnitrosourea. Eur J Biochem. 1981 Sep;119(1):51–59. doi: 10.1111/j.1432-1033.1981.tb05575.x. [DOI] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]





