Abstract
Corynebacterium glutamicum is currently used for the industrial production of a variety of biological materials. Many available inducible expression systems in this species use lac-derived promoters from Escherichia coli that exhibit much lower levels of inducible expression and leaky basal expression. We developed an arabinose-inducible expression system that contains the l-arabinose regulator AraC, the PBAD promoter from the araBAD operon, and the l-arabinose transporter AraE, all of which are derived from E. coli. The level of inducible PBAD-based expression could be modulated over a wide concentration range from 0.001 to 0.4% l-arabinose. This system tightly controlled the expression of the uracil phosphoribosyltransferase without leaky expression. When the gene encoding green fluorescent protein (GFP) was under the control of PBAD promoter, flow cytometry analysis showed that GFP was expressed in a highly homogeneous profile throughout the cell population. In contrast to the case in E. coli, PBAD induction was not significantly affected in the presence of different carbon sources in C. glutamicum, which makes it useful in fermentation applications. We used this system to regulate the expression of the odhI gene from C. glutamicum, which encodes an inhibitor of α-oxoglutarate dehydrogenase, resulting in high levels of glutamate production (up to 13.7 mM) under biotin nonlimiting conditions. This system provides an efficient tool available for molecular biology and metabolic engineering of C. glutamicum.
INTRODUCTION
Corynebacterium glutamicum is one of the most important microorganisms for producing bulk amino acids and organic acids (18, 44). The development of genetic tools has made it convenient to metabolically engineer specific traits in this bacterium (16, 27). Through expressing exogenous gene clusters to construct new metabolic pathways, C. glutamicum has been engineered to produce a variety of biological materials, such as d-pantothenate, xylitol, trehalose, and polyhydroxybutyrate (2, 15, 19, 32).
As an important tool for molecular biology and metabolic engineering, an efficient inducible expression system should have several characteristics that include sensitivity to a nontoxic and inexpensive inducer, a wide dynamic rang regulation, and little or no leaky basal expression. To date, the Plac-derived promoter systems from Escherichia coli have been the most widely used controllable expression systems in corynebacteria; however, these expression systems exhibit a lower level of inducible expression in C. glutamicum and high basal expression under noninducing conditions (26). Although many attempts have been made to increase the expression and tight regulation of the Ptac promoter, which is a hybrid promoter of Ptrp and PlacUV5 (45, 46), the inducibility of these promoters remains relatively low as a result of the low isopropyl-β-d-thiogalactopyranoside (IPTG) permeability of C. glutamicum strains (30). Moreover, the high cost and potential toxicity of IPTG are not ideal for industrial-scale protein expression or production of biological materials. As an alternative, a heat-inducible expression system and the high constitutive expression promoter (HCE) have been used for protein expression in C. glutamicum (29, 40, 41). Despite the fact that the regulatory mechanisms of many promoters in C. glutamicum are well understood (30, 31, 38), a strong, reliably regulated promoter that is tightly repressed and efficiently induced is still not available for use in corynebacteria (26).
The PBAD promoter from the arabinose operon fulfills all of the criteria of inducible expression systems. This promoter displays tighter control of gene expression, which is attributed to the dual regulatory role of AraC (i.e., AraC functions both as an inducer and as a repressor [20]). Although the level of PBAD-based expression can be modulated over a wide range of l-arabinose concentrations (8), the cell population exposed to subsaturating l-arabinose concentrations is divided into two subpopulations of induced and uninduced cells for the differences between individual cells in the availability of l-arabinose transporter (13, 37). Due to carbon catabolite repression, the araC-PBAD promoter system could provide a broader range of regulation by the addition of glucose (8, 25). This system is now available in many Gram-negative bacteria, such as E. coli, Salmonella enterica serovar Typhimurium, and Xanthomonas (21, 28, 39).
In the present study, we developed an arabinose-inducible expression system that allows for control over a wide range of inducer concentrations, tight regulation, and homogeneous high-level expression in C. glutamicum. This inducible expression system will facilitate the molecular biology and metabolic engineering of C. glutamicum.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in the present study are listed in Table 1. E. coli DH5α was used for vector construction. C. glutamicum strain ATCC 13032 was used for genetic disruption and expression using plasmid pK18mobsacB and pXMJ19 derivatives (10, 35). E. coli was grown aerobically on a rotary shaker (180 rpm) at 37°C in Luria-Bertani (LB) broth or on LB plates with 1.5% (wt/vol) agar. C. glutamicum was routinely grown at 30°C in LB or CGIII medium (23). For the generation of mutants and maintenance of C. glutamicum, brain heart infusion broth with 0.5 M sorbitol was used (43). When needed, antibiotics were used at the following concentrations: ampicillin, 100 μg/ml for E. coli; kanamycin, 50 μg/ml for E. coli and 25 μg/ml for C. glutamicum; and chloramphenicol, 20 μg/ml for E. coli and 10 μg/ml for C. glutamicum.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristicsa | Source or referenceb |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| W3110 | λ− IN(rrnD-rrnE)1 rpb-1 | EGSC |
| C. glutamicum | ||
| ATCC 13032 | Wild type, biotin auxotrophic | ATCC |
| ATCC 13032Δupp | upp gene was deleted, derived from strain ATCC 13032 | This study |
| Plasmids | ||
| pMD19 | T vector; Ampr | TaKaRa |
| pKD46 | pSC101 (Ts−); Ampr araC+ PBAD-Red | 4 |
| pAD123 | Kanr; gfpmut3a | 5 |
| pK18mobsacB | Mobilizable vector, allows for selection of double crossover in C. glutamicum; Kanr | 35 |
| pXMJ19 | Shuttle vector (Cmr; Ptac lacIq pBL1 oriVC. glutamicum pK18 oriVE. coli) | 10 |
| pWYE1067 | pXMJ19 derivative carrying the araC-PBAD | This study |
| pWYE1088 | pXMJ19 derivative carrying the araC-PBAD and Phom-araE | This study |
| pXMJ19-lacZ | pXMJ19 carrying lacZ from E. coli W3110 | This study |
| pWYE1067-lacZ | pWYE1067 derivative carrying the lacZ gene | This study |
| pWYE1088-lacZ | pWYE1088 derivative carrying the lacZ gene | This study |
| pWYE1088-upp | pWYE1088 derivative carrying the upp gene | This study |
| pWYE1067-gfpmut3a | pWYE1067 derivative carrying the gfpmut3a gene | This study |
| pWYE1088-gfpmut3a | pWYE1088 derivative carrying the gfpmut3a gene | This study |
| pWYE1088-odhI | pWYE1088 derivative carrying the odhI gene | This study |
Cmr, chloramphenicol resistance; Ampr, ampicillin resistance; Kanr, kanamycin resistance.
EGSC, E. coli Genetic Stock Center; ATCC, American Type Culture Collection.
DNA isolation and manipulation.
The genomic DNA of C. glutamicum was isolated as described by Tauch et al. (42). DNA restriction enzymes, ligase, and DNA polymerase (TaKaRa, Dalian, China) were used as recommended by the manufacturer's instructions. PCR products were separated by agarose gel electrophoresis and purified using a gel extraction kit (Omega Bio-Tek, Norcross, GA). Plasmid DNA from E. coli was prepared using a plasmid isolation kit (Tiangen, Beijing, China). C. glutamicum was transformed by electroporation according to previously described methods (43).
Vector constructions.
All primers are listed in Table 2. To compare the strengths of different constitutive promoters in C. glutamicum, the promoter-less lacZ gene containing the open reading frame from the start codon was amplified from E. coli W3110 chromosome and then ligated into the PstI and SmaI sites of pXMJ19 to generate the E. coli-C. glutamicum shuttle vector pXMJ19-lacZ. Constitutive promoters, including Phom, P45, Pfda, Peno, and PglyA (30, 36), were amplified from C. glutamicum using the different sets of primers listed in Table 2. The Phom and P45 PCR products were ligated into the EcoRV and HindIII sites of pXMJ19-lacZ, and the Pfda, Peno, and PglyA fragments were ligated into the NarI and PstI sites of pXMJ19-lacZ. The resulting vectors were transformed into C. glutamicum cells to measure β-galactosidase activity.
Table 2.
Primers used in this study
| Primer | Sequences (5′–3′)a | Function |
|---|---|---|
| WZ279 | AGTCATGGCGCCCATCGATTTATTATGACAAC (NarI) | araC-PBAD amplification |
| WZ280 | CGAACTGCAGGCATGCAAGCTTTTATAACCTCCTTAG (HindIII, PstI) | |
| WZ291 | CCATCGATCCGTTGAAAACTAAAAAGCTGG (ClaI) | Phom amplification |
| WZ292 | TTTCCTGCCATACTTTGTTTCGGCCACCC | |
| WZ293 | AAACAAAGTATGGCAGGAAAAAATGGT | araE amplification |
| WZ294 | CCATCGATGGCCCGTGAAATCAGA (ClaI) | |
| WZ259 | CCGGATATCCCGTTGAAAACTAAAAAGCTGG (EcoRV) | Phom amplification |
| WZ260 | GATAAGCTTTACTTTGTTTCGGCCACCC (HindIII) | |
| WZ255 | CCGGATATCGTGTTTTTCTGTGATCCTC (EcoRV) | P45 amplification |
| WZ256 | GATAAGCTTGCTTTTAAAACCATGCA (HindIII) | |
| WZ720 | AGTCATGGCGCCCCCCGATAGTGTATGTGC (NarI) | Peno amplification |
| WZ721 | CGACCTGCAG GCATGCAAGCTTAAGGTGTCTCCTCCAAAAG (PstI) | |
| WZ724 | AGTCATGGCGCCCTTAACAAGCGCAACCC (NarI) | Pfba amplification |
| WZ725 | CGACCTGCAGGCATGCAAGCTTGCCTCCTATGCCAACTT (PstI) | |
| WZ421 | AGTCATGGCGCCAGCTACTCCACTAGTGTGATCG (NarI) | PglyA amplification |
| WZ422 | GCCCTGCAGGCGTAAGACCTCACTCGC (PstI) | |
| WZ231 | GCCCTGCAGATGACCATGATTACGGA (PstI) | lacZ amplification |
| WZ232 | GGGATCCCGGGGAAATACGGGCAGACA (BamHI, SmaI) | |
| WZ733 | CGCGGATCCGCTTCGGCAATCATCAGTC (BamHI) | upp deletion |
| WZ734 | CCGCTTTTCCGACCGCCCAGAAGAAGACC | |
| WZ735 | TCTTCTGGGCGGTCGGAAAAGCGGTGGT | upp deletion |
| WZ736 | CCGGAATTCTGGGTATTTTGCGTCCTC (EcoRI) | |
| WZ739 | CCCAAGCTTATGGACATCACCATCGTCAACC (HindIII) | upp amplification expression |
| WZ740 | CCGGAATTCCCGTAATGCCCTTAGAAACT (EcoRI) | |
| WZ741 | CCCAAGCTTTAATGAGCGACAACAACG (HindIII) | odhI amplification expression |
| WZ742 | CCGGAATTCCTGCAAAGAACTTTCCTAG (EcoRI) | |
| WZ743 | CCCAAGCTTATGAGTAAAGGAGAAGAACTT (HindIII) | gfpmut3a amplification |
| WZ746 | CCGGAATTCTTATTTGTATAGTTCAT (EcoRI) |
The sites for the restriction enzymes (indicated in parentheses) are underlined. Complementary sequences are bold.
The fragment containing the araC gene under the control of the native ParaC promoter and PBAD promoter was amplified from the E. coli vector pKD46 (4). The PCR product was digested with NarI and PstI and ligated into the vector pXMJ19 to generate the vector pWYE1067. To abolish the l-arabinose-dependent regulation of araE gene encoding l-arabinose transporter under its native promoter, the Phom promoter from C. glutamicum was fused to the araE gene from E. coli by overlap extension PCR. The Phom-araE fragment was ligated into the pMD19 T vector and inserted into the dephosphorylated ClaI site of pWYE1067 to generate the vector pWYE1088.
Genetic disruption and complementation in C. glutamicum.
The pK18mobsacB derivative used for upp gene (encoding uracil phosphoribosyltransferase) disruption and pWYE1088 derivative used for upp gene expression were constructed in the present study (Table 1) and transformed into C. glutamicum cells by electroporation (43). Screening for the first and second recombination events and confirmation of the chromosomal deletion was performed as described previously (35). Expression of the upp gene from pWYE1088 in C. glutamicum was induced by the addition of 0.02% l-arabinose to the culture broth.
β-Galactosidase assay.
For the synthesis of β-galactosidase, the cells were grown to an optical density at 600 nm (OD600) of 0.4, and then l-arabinose or IPTG was added to the indicated final concentrations. The cells were harvested at different cultivation times and resuspended in 1 ml of Z-buffer (40 mM NaH2PO4, 60 mM Na2HPO4, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol [pH 7.0]). The β-galactosidase activity was determined by using a Miller assay based on the degradation of o-nitrophenyl-β-d-galactopyranoside (ONPG) (24). One unit of β-galactosidase activity is defined as the amount that hydrolyzes 1 μmol of ONPG to o-nitrophenol and d-galactose per min per cell.
Flow cytometry analysis.
The gfpmut3a gene encoding the green fluorescent protein (GFP), which has more intense fluorescence, a maximum excitation wavelength at 488 nm, and maximum emission at 511 nm, was used as a reporter gene to investigate the population homogeneity after l-arabinose induction. The gfpmut3a gene was amplified from the vector pAD123 and ligated into the HindIII and EcoRI sites of pWYE1067 and pWYE1088, respectively. The C. glutamicum ATCC 13032 harboring either pWYE1067-gfpmut3a or pWYE1088-gfpmut3a was cultivated in LB medium and harvested after 2 h of induction with different concentrations of l-arabinose.
Flow cytometry was performed on a BD FACSCalibur flow cytometer equipped with an argon laser (emission at 488 nm and 15 mW) and a 525-nm band-pass filter. The cells were diluted to an OD600 of 0.2 using phosphate-buffered saline (PBS) buffer (pH 7.2) and placed on ice prior to analysis. For each sample, 50,000 events were collected at a rate between 1,000 and 2,000 events per s. Cells cultured in the absence of inducer were used as a control to determine background fluorescence.
Shake flask fermentation.
C. glutamicum was cultured in 500-ml shake flasks containing 30 ml of CGIII medium for 16 h. Five percent (vol/vol) inocula were added to shake flasks (500 ml) containing 30 ml of CGX medium (3), and fermentation was performed at 30°C and 200 rpm. After sterilization, glucose and CaCO3 were added to final concentrations of 4 and 2%, respectively. Cell growth was monitored by measuring the absorbance at 600 nm using a UV-visible spectrophotometer.
Analytic methods.
The dry cell weight was estimated based on the correlation 1 OD600 unit is equal to 0.28 g of dry cell weight/liter (17). The glucose concentration was determined using an SBA-40D biosensor automatic analyzer (Shandong, China). The l-glutamate concentration was measured using a high-performance liquid chromatography system equipped with an Eclipse XDB-C18 column (Agilent Technologies, Wilmington, DE) after derivatization with 2,4-dinitrofluorobenzene.
RESULTS AND DISCUSSION
Construction of the l-arabinose-inducible expression vector pWYE1067.
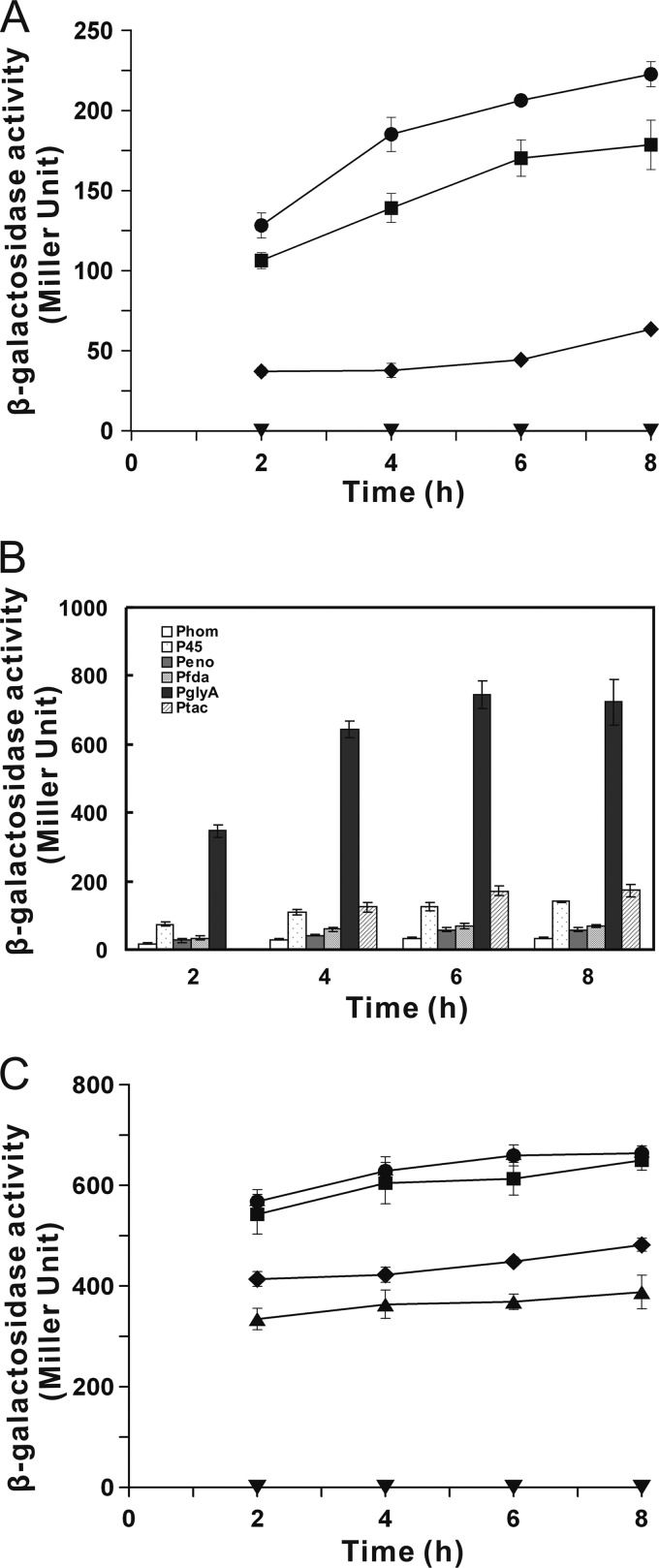
The entire araC and the PBAD promoter fragment was amplified from pKD46 and ligated into the E. coli-C. glutamicum vector pXMJ19 to create the vector pWYE1067 (Fig. 1). To estimate the inducibility of araC-PBAD promoter system in C. glutamicum, the lacZ gene encoding β-galactosidase from E. coli was used as a reporter gene. In the presence of 0.2% l-arabinose, β-galactosidase activity was maintained at a low level, whereas activity noticeably improved after the addition of 1% l-arabinose (Fig. 2A). In contrast, β-galactosidase activity was barely detectable at each time point during cultivation in the absence of l-arabinose, demonstrating that the PBAD promoter was tightly activated by l-arabinose. However, PBAD-based expression was efficiently induced only at high l-arabinose concentrations in C. glutamicum compared to E. coli, in which 0.03% l-arabinose was sufficient to induce significant PBAD-based expression (8). The sensitivity of the promoter to inducer concentrations depends on the ability of cells to take up the inducer. The inability of some strains to transport IPTG or the deletion of lacY gene encoding lactose permease in E. coli led to the inefficient induction of Plac promoter (6, 9, 22). The results from previous investigations indicated that l-arabinose might enter C. glutamicum through aqueous channels or a low-affinity nonspecific transporter (1, 11); therefore, l-arabinose uptake by C. glutamicum might be a major factor influencing PBAD-based expression.
Fig 1.
Construction of the arabinose-inducible expression vectors pWYE1067 (araC-PBAD) and pWYE1088 (Phom-araE, araC-PBAD). The araC-PBAD fragment was amplified by PCR using the E. coli vector pKD46 as the template. The PCR product was digested with NarI and PstI and ligated into the E. coli-C. glutamicum shuttle vector pXMJ19 to generate the vector pWYE1067 (araC-PBAD). Phom and araE were amplified by PCR using C. glutamicum and E. coli chromosomes, respectively, as the templates. The two fragments were fused by overlap extension PCR and ligated into the ClaI-digested vector pWYE1067 to generate the vector pWYE1088 (Phom-araE, araC-PBAD). rrnB, the transcriptional terminator; cat, chloramphenicol acetyltransferase gene; ori, origin of replication.
Fig 2.
Assessment of different promoter activities in C. glutamicum ATCC 13032. (A) PBAD activities in C. glutamicum carrying pWYE1067-lacZ exposed to different l-arabinose concentrations. The l-arabinose concentrations are represented by different symbols: 0% (▼), 0.2% (◆), 1% (■), and 2% (●). (B) Promoter activities of Phom, P45, PglyA, Pfda, and Peno in C. glutamicum ATCC 13032. To induce the Ptac promoter, IPTG was added at a final concentration of 1 mM after cultivation for 2 h. (C) The PBAD activities in C. glutamicum carrying pWYE1088-lacZ exposed to different l-arabinose concentrations. The l-arabinose concentrations are represented by different symbols: 0% (▼), 0.02% (▲), 0.2% (◆), 1% (■), and 2% (●).
Screening for an appropriate promoter to express the araE gene from pWYE1067.
To improve the sensitivity of the PBAD response to l-arabinose, we further modified the vector pWYE1067 by introducing the araE gene encoding the l-arabinose transporter from E. coli under the control of a constitutive promoter from C. glutamicum. Five candidate promoters derived from C. glutamicum were ligated into the vector pXMJ19-lacZ on the upstream of the promoter-less lacZ gene to evaluate their activities. As shown in Fig. 2B, P45 showed 2-fold-higher lacZ expression compared to Peno and Pfda, both of which had relatively moderate strength, and Phom exhibited the lowest lacZ expression among the promoters tested. Notably, PglyA displayed a high constitutive expression profile with 4-fold-higher lacZ expression compared to Ptac in the presence of 1 mM IPTG (Fig. 2B). In E. coli, the different expression levels of araE gene under the control of constitutive promoters slightly influence the degree of PBAD induction (12). However, the excess expression of the plasmid-based araE gene and araBAD operon did not make the recombinant C. glutamicum grow on l-arabinose (34), indicating that the overexpression of membrane protein (AraE) might interfere with the metabolic process and be unfavorable for the growth of C. glutamicum. Therefore, to appropriately control the araE expression and alleviate the adverse effects on cell growth, the weaker constitutive Phom promoter was used to regulate araE expression from vector pWYE1067, generating the resulting vector pWYE1088 (Fig. 1).
Dose-dependent control of PBAD-based expression by l-arabinose.
As expected, when the araE gene was expressed in C. glutamicum, the l-arabinose concentration to induce PBAD-based expression was significantly decreased, and the level of PBAD-based expression increased by 10-fold in response to 0.2% l-arabinose (Fig. 2C). In addition, C. glutamicum PBAD-based expression increased with increasing incubation time and then remained constant. This effect is attributed to a deficiency in the l-arabinose degradation pathway of C. glutamicum (11) that makes the intracellular pool of l-arabinose invariable during the induction process. This expression system achieved an effective induction of lacZ gene expression in a wider dynamic range from 0.001 to 0.4% l-arabinose (Fig. 3A). In contrast, the Ptac promoter regulated the lacZ expression over a concentration range from 0.01 to 1 mM IPTG (Fig. 3B). Furthermore, the level of PBAD-based expression was ∼2-fold higher than that of Ptac in the presence of the same molar concentration of l-arabinose or IPTG (Fig. 3B). Therefore, this expression system could provide the high-level expression in C. glutamicum compared to the previously available expression system.
Fig 3.
Characterization of the dynamic range of l-arabinose induction. (A) β-Galactosidase activities in C. glutamicum ATCC 13032 carrying pWYE1088-lacZ. Cells were harvested after 4 h of induction at the indicated l-arabinose concentrations for analysis. (B) Comparison of the strength of the PBAD and Ptac promoters in the presence of the same molar concentrations of l-arabinose ( ) and IPTG (□). The mean values from at least three independent cultures are shown with the standard deviations.
) and IPTG (□). The mean values from at least three independent cultures are shown with the standard deviations.
PBAD-based expression is tightly regulated by l-arabinose.
The upp gene encoding uracil phosphoribosyltransferase, which converts 5-fluorouracile (5-FU) to a toxic product for cell growth (7), was chosen as a reporter gene to assess the stringency of l-arabinose induction in C. glutamicum. To inhibit basal levels of upp expression, this gene was deleted from the chromosome of C. glutamicum by homologous recombination. The resulting upp-null mutant was used as the parental strain for the inducible expression of the upp gene from pWYE1088. The mutant strain harboring pWYE1088-upp exhibited normal growth on CGX medium containing 5-FU in the absence of l-arabinose but was unable to grow in the presence of 0.02% l-arabinose (see Fig. S1 in the supplemental material). It indicated that this system tightly controlled the expression of the upp gene by l-arabinose without leaky expression.
Homogeneous expression of the PBAD promoter associated with Phom-araE.
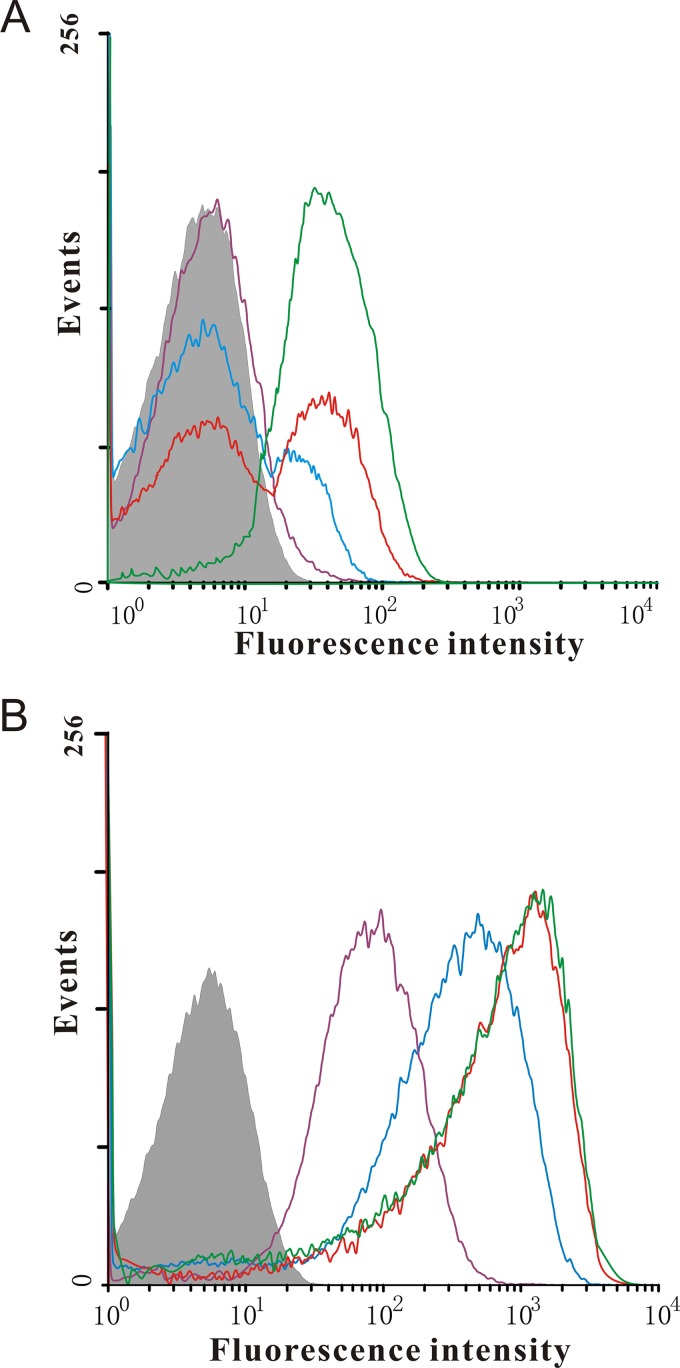
To assess the homogeneity of PBAD-based expression, C. glutamicum harboring either pWYE1067-gfpmut3a (pWYE1067-GFP strain) or pWYE1088-gfpmut3a (pWYE1088-GFP strain) was cultivated in the presence or absence of l-arabinose and harvested for flow cytometry analysis. As shown in Fig. 4A, cultures of the pWYE1067-GFP strain exhibited little fluorescence in the presence of 0.002% l-arabinose. However, two distinct subpopulations were observed in the presence of 0.02 and 0.2% l-arabinose, indicating that the response to l-arabinose induction is heterogeneous. It might be attributed to the differences between individual cells in l-arabinose transport (13). In contrast, the fluorescence of individual cells was reliably detected in all of the cultures of the pWYE1088-GFP strain that were induced with different concentrations of l-arabinose (Fig. 4B). Nearly all of the pWYE1088-GFP population exhibited a positive homogeneous fluorescence signal at 0.02% l-arabinose compared to the pWYE1067-GFP population, demonstrating that the expression of araE under the control of the Phom promoter resulted in a homogeneous population of cells, a finding consistent with a previous report for E. coli (12). In addition, the population-averaged fluorescence intensities of the pWYE1088-GFP strain increased with increasing l-arabinose concentration, indicating that variable promoter control occurs in each cell within the population rather than in a fraction of the population.
Fig 4.
Flow cytometry analysis of GFP expression. Histograms showing the numbers of cells and the fluorescence intensity of cultures of C. glutamicum strains harboring the gfpmut3a reporter plasmids. (A) All cultures harbored the gfpmut3a gene on the vector pWYE1067. (B) All cultures harbored the gfpmut3a gene on the vector pWYE1088. The fluorescence intensity of individual cells was measured by flow cytometry 2 h after the addition of l-arabinose at the indicated concentrations (gray-shaded curve, 0%; purple curve, 0.002%; blue curve, 0.02%; red curve, 0.2%; green curve, 2%).
Effects of various carbon sources on the strength of the PBAD promoter.
To investigate the PBAD-based expression in response to different carbon sources, C. glutamicum harboring pWYE1088-lacZ was cultivated in CGX medium using glucose, sucrose, fructose, ribose, gluconate, and acetate as the sole carbon source. The β-galactosidase activity of cells grown on glucose showed a modest decrease compared to that of cells grown on LB medium (Table 3). The strength of PBAD-based expression in cells grown with ribose and gluconate was slightly increased, with as much as 1.5- and 1.6-fold-higher β-galactosidase activities relative to expression in glucose. Moreover, cells grown with sucrose, fructose and acetate showed similar β-galactosidase activity compared to cells grown with glucose (Table 3). In E. coli, the PBAD promoter is subjected to significant catabolic repression in response to glucose (8) because this bacterium preferentially uses glucose and inhibits the uptake rate of secondary carbon sources by phosphotransferase (PTS) systems (33). As for C. glutamicum, the various PTS systems are expressed constitutively (47). In addition, the constitutive expression of araE resulted in an increase in the intracellular pool of l-arabinose for PBAD induction and did not interfere with the uptake of other carbon sources. Therefore, the different carbon sources did not have a strong effect on the strength of PBAD-based expression.
Table 3.
Effects of a variety of carbon sources on PBAD strength in C. glutamicum
| Carbon source | Mean β-galactosidase activity ± SD at an l-arabinose concn ofa: |
|||
|---|---|---|---|---|
| 0.1% |
0.2% |
|||
| Miller unit | Ratio | Miller unit | Ratio | |
| Glucose | 389.7 ± 40.6 | 100 | 409.5 ± 6.2 | 100 |
| Sucrose | 403.4 ± 40.1 | 1.1 ± 0.2 | 531.4 ± 20.3 | 1.3 ± 0.2 |
| Fructose | 353.8 ± 78.8 | 0.9 ± 0.3 | 378.2 ± 26.1 | 0.9 ± 0.1 |
| Gluconate | 640.9 ± 13.2 | 1.6 ± 0.2 | 651.2 ± 33.9 | 1.6 ± 0.1 |
| Ribose | 585.4 ± 14.0 | 1.5 ± 0.1 | 607.7 ± 30.8 | 1.5 ± 0.1 |
| Acetate | 362.4 ± 17.8 | 0.9 ± 0.1 | 400.1 ± 19.4 | 1.0 ± 0.1 |
β-Galactosidase activities, expressed in Miller units, represent means from three independent experiments. Ratios are calculated relative to the glucose culture for each carbon source.
Application of the l-arabinose-inducible system in glutamate fermentation.
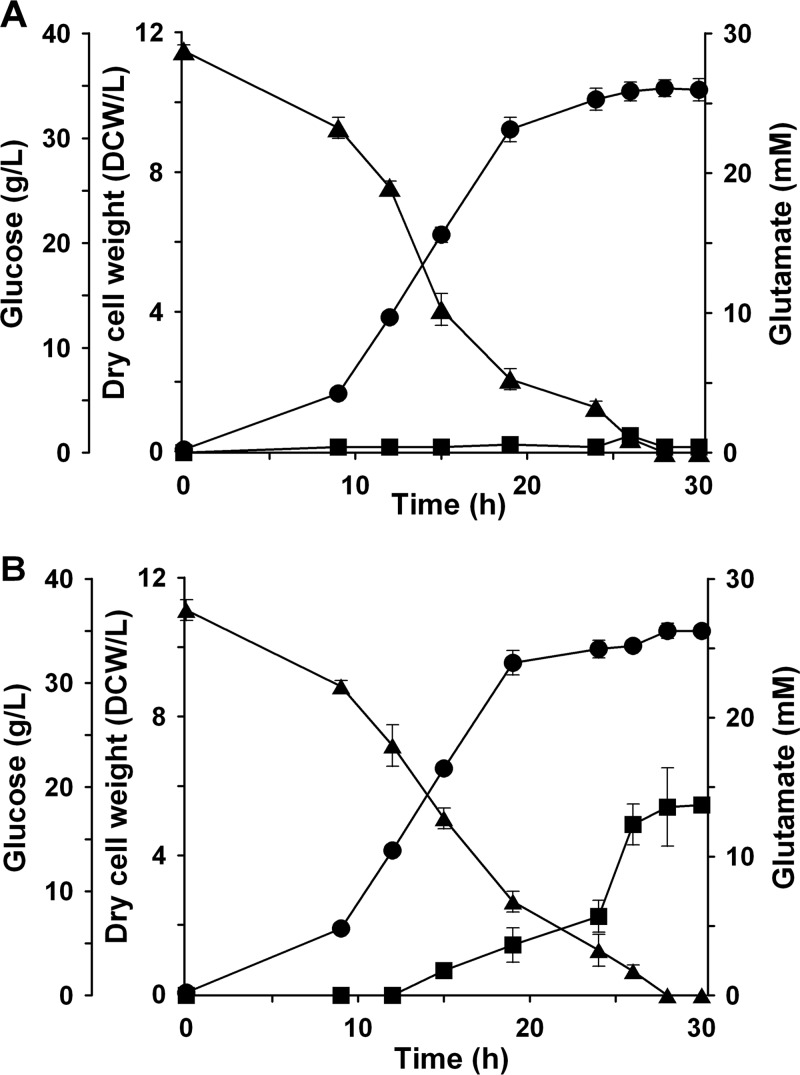
In order to evaluate its effect, the current l-arabinose-inducible system was used to regulate the expression of the odhI gene, which encodes a regulatory protein that inhibits α-oxoglutarate dehydrogenase activity (14). C. glutamicum strains carrying pWYE1088 and pWYE1088-odhI were cultivated in 500-ml shake flasks. l-Arabinose was added at a final concentration of 0.02% to induce odhI expression. As shown in Fig. 5, cellular growth and glucose consumption of the two strains were identical, whereas glutamate did not accumulate in the strain containing pWYE1088 under biotin nonlimiting conditions. In contrast, the odhI-overexpressing strain continuously accumulated glutamate in the late exponential and stationary phases and produced glutamate at levels reaching 13.7 mM after 30 h.
Fig 5.
Shake-flask fermentation profiles of C. glutamicum ATCC 13032 strain carrying the vector pWYE1088 (A) or the vector pWYE1088-odhI (B) under biotin nonlimiting conditions. l-Arabinose (0.02%) was used to induce odhI gene expression. The dry cell weight (●), glucose concentration (▲), and glutamate concentration (■) are indicated. Average measurements with the standard deviations from three independent experiments are shown.
Consequently, the arabinose-inducible expression system generated in the present study provides a novel efficient genetic engineering tool for molecular biology and metabolic engineering in C. glutamicum. Furthermore, the strategy of coexpressing a sugar-regulated promoter and sugar transporter to facilitate the uptake of an inducer provides an effective solution to improve the inducible expression of sugar-responsive promoters in other bacteria that cannot efficiently transport the inducer.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Tong Zhao for the flow cytometry analysis.
This study was supported by the Key Project of the Chinese Academy of Sciences (KSCX2-EW-J-6), the National Natural Science Foundation of China (grant 31100074), the Beijing Natural Science Foundation (grant 5112023), and the Ministry of Science and Technology of China (grant 2010ZX09401-403).
Footnotes
Published ahead of print 8 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Ben-Samoun K, Leblon G, Reyes O. 1999. Positively regulated expression of the Escherichia coli araBAD promoter in Corynebacterium glutamicum. FEMS Microbiol. Lett. 174:125–130 [DOI] [PubMed] [Google Scholar]
- 2. Carpinelli J, Krämer R, Agosin E. 2006. Metabolic engineering of Corynebacterium glutamicum for trehalose overproduction: role of the TreYZ trehalose biosynthetic pathway. Appl. Environ. Microbiol. 72:1949–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cremer J, Eggeling L, Sahm H. 1991. Control of the lysine biosynthesis sequence in Corynebacterium glutamicum as analyzed by overexpression of the individual corresponding genes. Appl. Environ. Microbiol. 57:1746–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunn AK, Handelsman J. 1999. A vector for promoter trapping in Bacillus cereus. Gene 226:297–305 [DOI] [PubMed] [Google Scholar]
- 6. Fukui T, Ohsawa K, Mifune J, Orita I, Nakamura S. 2011. Evaluation of promoters for gene expression in polyhydroxyalkanoate-producing Cupriavidus necator H16. Appl. Microbiol. Biotechnol. 89:1527–1536 [DOI] [PubMed] [Google Scholar]
- 7. Goh YJ, et al. 2009. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 75:3093–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartman AH, Liu H, Melville SB. 2011. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl. Environ. Microbiol. 77:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jakoby M, Ngouoto-Nkili C, Burkovski A. 1999. Construction and application of new Corynebacterium glutamicum vectors. Biotechnol. Tech. 13:437–441 [Google Scholar]
- 11. Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H. 2008. Engineering of an l-arabinose metabolic pathway in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 77:1053–1062 [DOI] [PubMed] [Google Scholar]
- 12. Khlebnikov A, Datsenko KA, Skaug T, Wanner BL, Keasling JD. 2001. Homogeneous expression of the PBAD promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241–3247 [DOI] [PubMed] [Google Scholar]
- 13. Khlebnikov A, Risa O, Skaug T, Carrier TA, Keasling JD. 2000. Regulatable arabinose-inducible gene expression system with consistent control in all cells of a culture. J. Bacteriol. 182:7029–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J, et al. 2010. Requirement of de novo synthesis of the OdhI protein in penicillin-induced glutamate production by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 86:911–920 [DOI] [PubMed] [Google Scholar]
- 15. Kim SH, Yun JY, Kim SG, Seo JH, Park JB. 2010. Production of xylitol from d-xylose and glucose with recombinant Corynebacterium glutamicum. Enzyme Microbiol. Technol. 46:366–371 [Google Scholar]
- 16. Kirchner O, Tauch A. 2003. Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J. Biotechnol. 104:287–299 [DOI] [PubMed] [Google Scholar]
- 17. Koffas MAG, Jung GY, Aon JC, Stephanopoulos G. 2002. Effect of pyruvate carboxylase overexpression on the physiology of Corynebacterium glutamicum. Appl. Environ. Microbiol. 68:5422–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leuchtenberger W, Huthmacher K, Drauz K. 2005. Biotechnological production of amino acids and derivatives: current status and prospects. Appl. Microbiol. Biotechnol. 69:1–8 [DOI] [PubMed] [Google Scholar]
- 19. Liu Q, Ouyang SP, Kim J, Chen GQ. 2007. The impact of PHB accumulation on l-glutamate production by recombinant Corynebacterium glutamicum. J. Biotechnol. 132:273–279 [DOI] [PubMed] [Google Scholar]
- 20. Lobell RB, Schleif RF. 1990. DNA looping and unlooping by AraC protein. Science 250:528–532 [DOI] [PubMed] [Google Scholar]
- 21. Loessner H, et al. 2007. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of l-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 9:1529–1537 [DOI] [PubMed] [Google Scholar]
- 22. Marbach A, Bettenbrock K. 2012. lac operon induction in Escherichia coli: systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. J. Biotechnol. 157:82–88 [DOI] [PubMed] [Google Scholar]
- 23. Menkel E, Thierbach G, Eggeling L, Sahm H. 1989. Influence of increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl. Environ. Microbiol. 55:684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller JH. 1972. Assay of β-galactosidase activity, p 352–355 In Experiments in molecular genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 25. Miyada CG, Stoltzfus L, Wilcox G. 1984. Regulation of the araC gene of Escherichia coli: catabolite repression, autoregulation, and effect on araBAD expression. Proc. Natl. Acad. Sci. U. S. A. 81:4120–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nešvera J, Pátek M. 2008. Plasmids and promoters in corynebacteria and their applications, p 113–154 In Burkovski A. (ed), Corynebacteria: genomics and molecular biology. Caister, Norfolk, United Kingdom [Google Scholar]
- 27. Nešvera J, Pátek M. 2011. Tools for genetic manipulations in Corynebacterium glutamicum and their applications. Appl. Microbiol. Biotechnol. 90:1641–1654 [DOI] [PubMed] [Google Scholar]
- 28. Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203 [DOI] [PubMed] [Google Scholar]
- 29. Park JU, et al. 2008. Construction of heat-inducible expression vector of Corynebacterium glutamicum and C. ammoniagenes: fusion of lambda operator with promoters isolated from C. ammoniagenes. J. Microbiol. Biotechnol. 18:639–647 [PubMed] [Google Scholar]
- 30. Pátek M, Nešvera J, Guyonvarch A, Reyes O, Leblon G. 2003. Promoters of Corynebacterium glutamicum. J. Biotechnol. 104:311–323 [DOI] [PubMed] [Google Scholar]
- 31. Pátek M, Nešvera J. 2011. Sigma factors and promoters in Corynebacterium glutamicum. J. Biotechnol. 154:101–113 [DOI] [PubMed] [Google Scholar]
- 32. Sahm H, Eggeling L. 1999. d-Pantothenate synthesis in Corynebacterium glutamicum and use of panBC and genes encoding l-valine synthesis for d-pantothenate overproduction. Appl. Environ. Microbiol. 65:1973–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saier MH, Jr, et al. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217–230 [DOI] [PubMed] [Google Scholar]
- 34. Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H. 2009. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl. Microbiol. Biotechnol. 85:105–115 [DOI] [PubMed] [Google Scholar]
- 35. Schäfer A, et al. 1994. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73 [DOI] [PubMed] [Google Scholar]
- 36. Schweitzer JE, Stolz M, Diesveld R, Etterich H, Eggeling L. 2009. The serine hydroxymethyltransferase gene glyA in Corynebacterium glutamicum is controlled by GlyR. J. Biotechnol. 139:214–221 [DOI] [PubMed] [Google Scholar]
- 37. Siegele DA, Hu JC. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. U. S. A. 94:8168–8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srivastava P, Deb JK. 2005. Gene expression systems in corynebacteria. Protein Expr. Purif. 40:221–229 [DOI] [PubMed] [Google Scholar]
- 39. Sukchawalit R, Vattanaviboon P, Sallabhan R, Mongkolsuk S. 1999. Construction and characterization of regulated l-arabinose-inducible broad host range expression vectors in Xanthomonas. FEMS Microbiol. Lett. 181:217–223 [DOI] [PubMed] [Google Scholar]
- 40. Tateno T, Fukuda H, Kondo A. 2007. Production of l-lysine from starch by Corynebacterium glutamicum displaying alpha-amylase on its cell surface. Appl. Microbiol. Biotechnol. 74:1213–1220 [DOI] [PubMed] [Google Scholar]
- 41. Tateno T, et al. 2009. Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing alpha-amylase and lysine decarboxylase. Appl. Microbiol. Biotechnol. 82:115–121 [DOI] [PubMed] [Google Scholar]
- 42. Tauch A, Kassing F, Kalinowski J, Pühler A. 1995. The Corynebacterium xerosis composite transposon Tn5432 consists of two identical insertion sequences, designated IS1249, flanking the erythromycin resistance gene ermCX. Plasmid 34:119–131 [DOI] [PubMed] [Google Scholar]
- 43. Tauch A, et al. 2002. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr. Microbiol. 45:362–367 [DOI] [PubMed] [Google Scholar]
- 44. Wendisch V, Bott M, Eikmanns BJ. 2006. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr. Opin. Microbiol. 9:268–274 [DOI] [PubMed] [Google Scholar]
- 45. Xu D, Tan Y, Huan X, Hu X, Wang X. 2010. Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J. Microbiol. Methods 80:86–92 [DOI] [PubMed] [Google Scholar]
- 46. Xu D, Tan Y, Shi F, Wang X. 2010. An improved shuttle vector constructed for metabolic engineering research in Corynebacterium glutamicum. Plasmid 64:85–91 [DOI] [PubMed] [Google Scholar]
- 47. Yokota A, Lindley ND. 2005. Central metabolism: sugar uptake and conversion, p 215–241 In Eggeling L, Bott M. (ed), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.