Abstract
Bacillus amyloliquefaciens NJN-6 produces volatile compounds (VOCs) that inhibit the growth and spore germination of Fusarium oxysporum f. sp. cubense. Among the total of 36 volatile compounds detected, 11 compounds completely inhibited fungal growth. The antifungal activity of these compounds suggested that VOCs can play important roles over short and long distances in the suppression of Fusarium oxysporum.
TEXT
Microbial antagonist strains capable of producing both nonvolatile compounds and volatile compounds (VOCs), which exhibit strong inhibitory activity against plant pathogens, have received much attention (4, 14). These antagonists include bacteria, such as Pseudomonas spp. (5), and nonpathogenic fungi like Trichoderma spp. (11). The release of VOCs by soil microbes has been reported to promote plant growth (13), display nematicidal activity (7), and induce systemic resistance in crops (3). Previous researchers also found that VOCs produced by bacteria could inhibit the growth (6) and the spore germination of pathogenic fungi (10), suggesting that VOCs produced by bacteria could be a mechanism of biocontrol against some soilborne fungal diseases.
Fusarium oxysporum is a well-known soilborne fungus, and some strains of F. oxysporum are pathogenic to plants and are difficult to control; however, biological methods may be a reliable alternative to chemical methods for controlling soilborne fungal growth. For applications in agriculture, the Bacillus species are considered important biological control agents. Bacillus amyloliquefaciens (NJN-6), isolated from the rhizosphere soil of healthy banana plants, acts as an efficient antagonist against F. oxysporum f. sp. cubense by producing several antibiotics (15, 16). In this study, we characterized the volatile organic compounds produced by strain NJN-6. We used solid-phase microextraction (SPME) combined with gas chromatography-mass spectrometry (GC-MS) to extract and identify the VOCs. Finally, we identified antagonistic VOCs as those that reduced the growth and inhibited the spore germination of F. oxysporum.
Microorganisms and culture conditions.
The antagonistic strain NJN-6 was identified as B. amyloliquefaciens (CGMCC [China General Microbiology Culture Collection Center] accession no. 3183) by 16S rRNA sequencing (15). The fungal strain F. oxysporum f. sp. cubense, which exhibited high virulence in banana plants, was used as the target fungus.
Antagonistic assay of VOCs against fungi.
One compartment of the divided plates containing modified MS medium (with 1.5% [wt/vol] agar, 1.5% [wt/vol] sucrose, and 0.4% [wt/vol] TSA [3]) was inoculated with NJN-6, except for control plates. Another compartment containing PDA medium was used for F. oxysporum to test growth inhibition, or 100 μl of spore solution (108 CFU/ml) was spread evenly to test the ability of the VOCs to inhibit the spore germination of fungi, or 10 g of diseased banana field soil from Ledong, Hainan Province, was added to one compartment. The plates were incubated at 28°C for 3 days, and then the diameters of the fungal growth in different plates were measured. Inhibition of fungal spores from soils by VOCs was determined using Komada's salts-carbohydrate (SC) medium (9) after 45 days of incubation.
Collection of VOCs with SPME and analysis by GC-MS.
Three SPME fibers from Supelco (Bellefonte, PA), polydimethylsiloxane (PDMS, 7 μm), stable flex divinylbenzene-carboxene-PDMS (DCP, 50/30 μm), and polydimethylsiloxane-divinylbenzene (PDMS-DVB, 65 μm), were used to collect VOCs. Strain NJN-6 was inoculated into 40 ml of MS medium in a 100-ml vial with the SPME fiber inserted into the headspace. The vial was incubated at 28°C for 3 days and then placed in hot water at 50°C for 30 min. The results indicated that the DCP 50/30 μm SPME fiber was better than the PDMS and PDMS-DVD fibers. GC-MS analysis was performed using Trace DSQ (Finnigan). The SPME fibers were desorbed at 220°C for 1 min with an RTX-5MS column (30 m, 0.25-mm inside diameter, 0.25 μm). Each run was performed for 24 min. The initial oven temperature of 33°C was held for 3 min, ramped up at a rate of 10°C/min to 180°C, further ramped up at a rate of 40°C/min to 240°C, and held for 4 min. The mass spectrometer was operated in the electron ionization mode at 70 eV with a source temperature of 220°C, and a continuous scan from 50 m/z to 500 m/z was used. The mass spectra of VOCs were compared with those in the NIST/EPA/NIH Mass Spectrometry Library with respect to the spectra in the Mainlib and/or Replib databases.
Antifungal activity of bacterial VOCs against F. oxysporum.
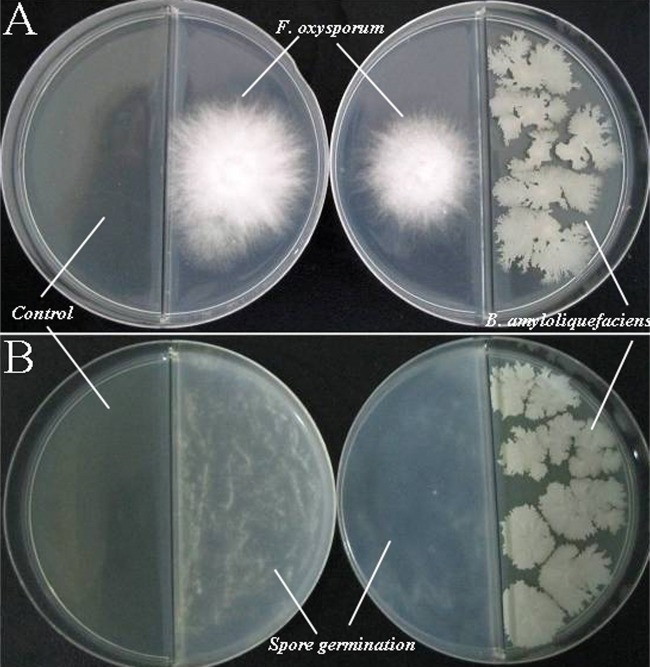
The VOCs produced by NJN-6 reduced the mycelial growth (Fig. 1A) and the germination of spores (Fig. 1B) of F. oxysporum in comparison with the control plates. The VOCs decreased the length of fungal mycelia, and colonies seemed to be significantly reduced. The inhibition of F. oxysporum by VOCs was about 30% to 40% compared with the control after 3 days, suggesting that the bacterial VOCs were unable to kill F. oxysporum but had a significantly inhibitory effect on fungal mycelia (Fig. 2). The VOCs produced by NJN-6 strongly antagonized F. oxysporum in soil and the quantity of F. oxysporum in the treated soil was 102 g−1 after 45 days while it was 104 g−1 in the control soil. Previous research has shown that abundant nutrients in the soil increased the production of bacterial VOCs, suggesting that the amount of organic matter in the soil might affect the ability of NJN-6 to release VOCs into the soil (5, 6). Nonvolatile antibiotics, including lipopeptides, have strong antifungal activities (8, 12). However, these nonvolatile antibiotics cannot spread over long distances, and only when these antagonists directly colonize the plant roots can they prevent pathogenic fungi from infecting plants. In contrast, VOCs can spread over a long distance, and fungistatic microenvironments exist around the antagonist communities. In addition, the antifungal VOCs produced by bacteria can kill surviving spores in the soil and limit both the production and the establishment of the disease (5).
Fig 1.
B. amyloliquefaciens NJN-6 antifungal volatile activity on divided plates. (A) Mycelial plug growth was inhibited in the presence of the bacteria streaked in the different compartments (right), compared to the control (left). (B) Volatile organic compounds produced by bacteria affected the germination of spores (right), compared to the control (left).
Fig 2.
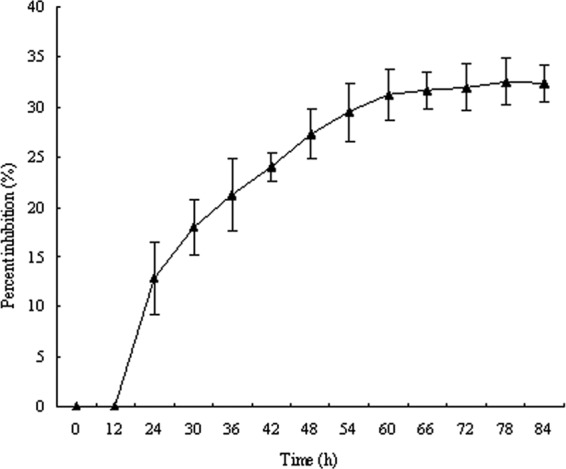
Inhibition of F. oxysporum mycelial growth by the Bacillus amyloliquefaciens NJN-6 antifungal volatile compounds on divided plates.
GC-MS analysis and antifungal activity of VOCs.
The VOCs produced by NJN-6 strain were analyzed by GC-MS, and 36 compounds were detected, including 12 benzenes, seven alkyls, three alcohols, seven ketones, two aldehydes, three naphthyls, one ester, and one ether compound (see Table S1 in the supplemental material). The identified volatile compounds were purchased from Sigma-Aldrich and Aladdin. To check the antagonistic effect of VOCs, sterile filter paper discs containing 200 μl of each VOC were placed in one compartment while the other compartment was inoculated with F. oxysporum on PDA medium, and water was used as a control (5). After 3 days, the diameters of fungal colonies were measured, and percent inhibition was calculated as (control − treatment)/control × 100. All 36 compounds produced by NJN-6 were used for the antifungal activity assays (see Fig. S1 in the supplemental material). The bacterial VOCs had been demonstrated to have antimicrobial activity, could promote plant growth, interacted with the host, and behaved as a signal among the bacterial communities. For example, n-decanal was shown to inhibit Sclerotinia sclerotiorum (5), allyl alcohol was shown to increase the populations of beneficial bacteria (1), and 2,3-butadienol was shown to promote plant growth (13). Phenol has well-established antiseptic uses in clinical applications because of its toxic activity for cells (2).
All 12 benzene compounds showed antifungal activity against F. oxysporum. The benzothiazoles phenol and 2,3,6-trimethyl-phenol completely inhibited the growth of F. oxysporum and exhibited high production levels, as indicated by the peak areas of the GCs, so it was likely that the two benzenes played an important role in the antifungal effects of the VOCs. Toluene, ethylbenzene, propylbenzene, and isopropylbenzene were less effective in inhibiting the growth of fungi. The antifungal activities of these compounds appeared to be related to their low benzene contents.
The alkyl group compounds like dodecane and tridecane did not show antifungal activities, while pentadecane and tetradecane exhibited inhibition of 22.5% and 18.4%, respectively. The alcohol, aldehyde, ester, ether, and naphthyl compounds (5 of 10 VOCs) could almost completely antagonize F. oxysporum at a concentration of 200 μl in vitro; however, all of these compounds were produced at very low levels. Consequently, these compounds are not promising candidates as effective antifungal VOCs produced by NJN-6.
In contrast to the other VOCs, ketones were produced at high levels. The antifungal activity of ketones was negatively correlated with the number of carbon atoms in the ketones. The ketones 2-nonanone and 2-decanone exhibited 100% percent inhibition, but their contents were low in VOCs, as indicated by GC. Conversely, 2-tetradecanone and 2-pentadecanone exhibited large peaks areas on the GCs, but they did not show strong antifungal effects against F. oxysporum. Considering both ketone contents and antifungal effects, 2-undecanone, 2-dodecanone, and 2-tridecanone might be considered active ketone compounds.
The results of these experiments suggested a long-distance biocontrol mechanism of action of VOCs produced by B. amyloliquefaciens NJN-6 against F. oxysporum. Antagonistic activities could result from both the reduction of pathogen mycelial growth and the inhibition of spore germination. The results are useful for the better understanding of the biocontrol mechanisms by B. amyloliquefaciens against F. oxysporum.
Supplementary Material
ACKNOWLEDGMENTS
This research was financially supported by Priority Academic Program Development of Jiangsu Higher Education Institutions and by the 111 project (B12009).
Footnotes
Published ahead of print 8 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Altman J, Lawlor S. 1966. The effects of some chlorinated hydrocarbons on certain soil bacteria. J. Appl. Bacteriol. 29:260–265 [DOI] [PubMed] [Google Scholar]
- 2. Breinig S, Schiltz E, Fuchs G. 2000. Genes involved in anaerobic metabolism of phenol in the bacterium Thauera aromatica. J. Bacteriol. 182:5849–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farag MA, Ryu C-M, Sumner LW, Pare PW. 2006. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67:2262–2268 [DOI] [PubMed] [Google Scholar]
- 4. Fernando WGD, Linderman RG. 1995. Inhibition of Phytophthora vignae and root rot of cowpea by soil bacteria. Biol. Agric. Hortic. 12:1–14 [Google Scholar]
- 5. Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC. 2005. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biology & Biochemistry 37:955–964 [Google Scholar]
- 6. Fiddaman PJ, Rossall S. 1994. Effect of substrate on the production of antifungal volatiles from Bacillus subtilis. J. Appl. Bacteriol. 76:395–405 [DOI] [PubMed] [Google Scholar]
- 7. Gu Y-Q, Mo M-H, Zhou J-P, Zou C-S, Zhang K-Q. 2007. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39:2567–2575 [Google Scholar]
- 8. Kim PI, et al. 2004. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 97:942–949 [DOI] [PubMed] [Google Scholar]
- 9. Komada H. 1972. Studies on the method for selective isolation of Fusarium oxysporum from natural soil. Bull. Tokai-Kinki Natl. Agric. Exp. Stn. 23:144–178 [Google Scholar]
- 10. McKee ND, Robinson PM. 1988. Production of volatile inhibitors of germination and hyphal extension by Geotrichum candidum. Trans. Br. Mycol. Soc. 91:157–190 [Google Scholar]
- 11. Minerdi D, Bossi S, Gullino ML, Garibaldi A. 2009. Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ. Microbiol. 11:844–854 [DOI] [PubMed] [Google Scholar]
- 12. Nakano MM, Marahiel MA, Zuber P. 1988. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170:5662–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu CM, et al. 2003. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 100:4927–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu CK, Mo MH, Zhang LM, Zhang KQ. 2004. Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol. Biochem. 36:1997–2004 [Google Scholar]
- 15. Yuan J, et al. 2012. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 60:2976–2981 [DOI] [PubMed] [Google Scholar]
- 16. Yuan J, Raza W, Huang Q, Shen Q. 2011. Quantification of the antifungal lipopeptide iturin A by high performance liquid chromatography coupled with aqueous two-phase extraction. J. Chromatogr. B 879:2746–2750 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.