Abstract
Fermentation of cellulosic and hemicellulosic sugars from biomass could resolve food-versus-fuel conflicts inherent in the bioconversion of grains. However, the inability to coferment glucose and xylose is a major challenge to the economical use of lignocellulose as a feedstock. Simultaneous cofermentation of glucose, xylose, and cellobiose is problematic for most microbes because glucose represses utilization of the other saccharides. Surprisingly, the ascomycetous, beetle-associated yeast Spathaspora passalidarum, which ferments xylose and cellobiose natively, can also coferment these two sugars in the presence of 30 g/liter glucose. S. passalidarum simultaneously assimilates glucose and xylose aerobically, it simultaneously coferments glucose, cellobiose, and xylose with an ethanol yield of 0.42 g/g, and it has a specific ethanol production rate on xylose more than 3 times that of the corresponding rate on glucose. Moreover, an adapted strain of S. passalidarum produced 39 g/liter ethanol with a yield of 0.37 g/g sugars from a hardwood hydrolysate. Metabolome analysis of S. passalidarum before onset and during the fermentations of glucose and xylose showed that the flux of glycolytic intermediates is significantly higher on xylose than on glucose. The high affinity of its xylose reductase activities for NADH and xylose combined with allosteric activation of glycolysis probably accounts in part for its unusual capacities. These features make S. passalidarum very attractive for studying regulatory mechanisms enabling bioconversion of lignocellulosic materials by yeasts.
INTRODUCTION
Cofermentation of glucose (G) and xylose (X) is critical for bioconversion because they are present in virtually all enzymatic hydrolysates of pretreated lignocellulose (4, 47). Their sequential utilization extends fermentation times and can result in incomplete substrate consumption if products reach inhibitory levels before slowly utilized sugars are consumed (14). Moreover, cofermentation of xylose with cellobiose (Cb) could reduce enzyme costs and facilitate simultaneous saccharification and fermentation. Glucose represses utilization of xylose, cellobiose, or other carbon sources in most microbes (11, 12). This is a problem that can be addressed through metabolic engineering or strain selection.
Placing genes for xylose or cellobiose assimilation under the regulation of constitutive promoters or altering transcriptional activators (42) can relieve glucose repression (21). For example, coutilization of glucose and xylose (10, 25) and of cellobiose and xylose (35) by engineered Saccharomyces cerevisiae can produce 60 g/liter ethanol in 72 h from pure sugars (13). However, even when heterologous genes for assimilation are expressed under constitutive promoters, coutilization with glucose is problematic because the affinities of yeast monosaccharide transporters are 8 to 100 times higher for glucose than for xylose (36, 37). This problem can be addressed by introducing heterologous transporters with a higher affinity for xylose. For example, Saccharomyces cerevisiae TMB34006, engineered with the glucose/xylose facilitator Gxf1 from Candida intermedia (34), shows 1.2- to 2-fold higher rates of xylose uptake when the initial glucose concentration is less than 5 g/liter (10). To accommodate higher rates of xylose transport, downstream reactions in the xylose metabolic pathway must be overexpressed, but cofactor imbalances limit xylose assimilation in the presence of glucose (25) and reduce its contribution to metabolic flux (10). Cofermentation of xylose and cellobiose is less difficult because cellobiose, a disaccharide, does not compete with xylose transport (13).
Systematic modeling and metabolic engineering can modify S. cerevisiae to address these problems, but this yeast does not provide a useful model for how overall metabolic flux is natively regulated during cultivation on xylose. In fact, aside from a few studies of genes and enzymes induced during the assimilation of xylose and the induction of pyruvate decarboxylase under oxygen limitation (31), very little is known about how native xylose-fermenting yeasts mediate the simultaneous metabolic demands for cell growth and product formation during ethanol production on xylose. Most native xylose-fermenting yeasts such as Scheffersomyces (Pichia) stipitis (26) do not coutilize glucose and xylose, largely because glucose represses the genes for xylose assimilation (16). However, this does not appear to be the case for all yeasts.
In the present work, we report that an unusual native xylose-fermenting yeast, Spathaspora passalidarum NN245 (15, 30, 46), coassimilates xylose and glucose aerobically, uses xylose faster than glucose when the sugars are presented individually, and coferments glucose, xylose, and cellobiose from mixtures of pure sugars or hydrolysates under oxygen-limiting conditions. These features make S. passalidarum potentially useful for simultaneous saccharification and fermentation (SSF) and for studying enzymatic and regulatory mechanisms enabling simultaneous utilization of cellulosic and hemicellulosic sugars.
MATERIALS AND METHODS
Organism, medium, and culture conditions.
Spathaspora passalidarum NN245 (NRRL Y-27907, CBS 10155, MYA-4345) (30) was used for all experiments and adaptations reported here. Cultures were maintained on YPX agar plates, containing 10 g/liter yeast extract, 10 g/liter peptone, 20 g/liter xylose, and 20 g/liter agar. Inocula were cultivated overnight in 50 ml of YP broth, containing 20 g/liter yeast extract, 10 g/liter peptone, plus 20 to 60 g/liter G, X, or Cb. The cultivation was then transferred to defined minimal medium and grown to an appropriate cell density. All flask cultivations were carried out at 30°C in 125-ml polycarbonate flasks (GeneMate) fitted with membrane filters for aeration. Aerobic and oxygen-limited inoculum preparation conditions were 200 rpm and ≤0.2 g/liter cell dry weight (cdw) and 100 rpm and ≥1 g/liter cdw, respectively. Defined minimal medium (CBS) containing nitrogen, trace metal elements, and vitamins was used in all bioreactor cultivations. It contained 2.4 g/liter urea, 3 g/liter KH2PO4, 0.5 g/liter MgSO4 · 7H2O, 1 ml liter−1 trace element solution, 1 ml liter−1 vitamin solution, and 0.05 ml/liter antifoam 289 (A-8436; Sigma) (modified from the medium described in reference 44). Carbon sources consisted of d-glucose, d-xylose, or a mixture of xylose with glucose and/or cellobiose. Maple hemicellulose hydrolysate (MHH) medium consisted of a 9:1 (vol/vol) mixture of MHH (41) and 10-fold-concentrated CBS basal defined minimal medium. Supplemental glucose was added to the hydrolysate to attain 65 g/liter xylose and 35 g/liter glucose. Control medium contained the same sugars in CBS defined minimal medium. The starting inoculum was 0.8 mg cdw/ml. AFEX hydrolysate medium (27, 47) consisted of AFEX hydrolysate supplemented with 2.4 g/liter urea, 1 g/liter KH2PO4, 1 g/liter K2HPO4, 50 mg/liter FeSO4 · 7H2O, 5.5 mg/liter ZnSO4 · 7H2O, and 8 mg/liter CuSO4 · 5H2O. Modified vitamin solution (40) deleted riboflavin, folic, and thiotic acids. Bioreactor fermentations were performed in duplicate New Brunswick Scientific Bio Flo 110 3-liter reactors with working volumes of 2 liters. The bioreactor temperature was maintained at 25°C (39, 40), and the pH was kept constant at 5.0 ± 0.1 by automatic addition of 5 N KOH. All fermentation kinetics and metabolite levels are reported as averages of biological duplicates. The oxygen transfer rate (OTR) was calculated using the gassing out method based on kinetic oxygen measurements (38). The dissolved oxygen level (dO2) was monitored using an InPro 6800 oxygen sensor. Oxygen transfer rates were measured independently in four bioreactors at two agitation rates using air and a 90:10 mixture of N2 and air (see Table S1 in the supplemental material). The bioreactor was sparged with air at 0.5 liter/min (0.25 volume/volume minute [vvm], 500 rpm). Full air saturation (100% of scale) was set after reaching equilibrium. Under these conditions, the OTR was estimated to be 13.56 ± 2.33 mmol O2/liter · h. The zero level was set following equilibrium when sparging with nitrogen. Fully aerobic cultures (see Fig. 1) were sparged with 1 vvm air and agitated at 700 rpm. For oxygen-limited fermentation of glucose or xylose, the airflow was set to 0.25 vvm, while a dissolved oxygen (dO2) controller (New Brunswick) maintained agitation over the range of from 150 to 300 rpm to attain 10% saturation (∼2.1% dO2). Once the growth phase was complete (Fig. 2, arrow 1), air was replaced with a mixture of 90% N2 and 10% air to attain a mole fraction of O2 in the gas phase. The dO2 controller was set to a range of 300 to 500 rpm, and ethanol production ensued (see Fig. 2). In the glucose, xylose, and cellobiose cofermentation (see Fig. 4) and in the MHH fermentation (see Fig. 5), the bioreactors were equilibrated with 90% N2 and 10% air (2.1% O2) from the start, and the dO2 controller was set to a range of from 400 to 500 rpm.
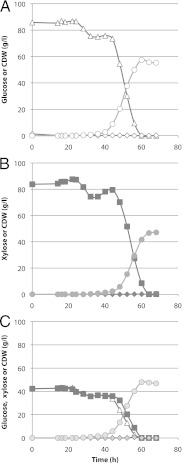
Fig 1.
Spathaspora passalidarum cultivated under fully aerobic conditions. Cells were cultivated at 30°C in 2,000 ml of defined minimal medium (CBS) with glucose (A), xylose (B), or a 50:50 mixture of the two sugars (C). Cultures were aerated with 1 vvm air and an agitation rate of 700 rpm. Symbols: open triangles, glucose; solid squares, xylose; circles, cell mass; diamonds, ethanol.
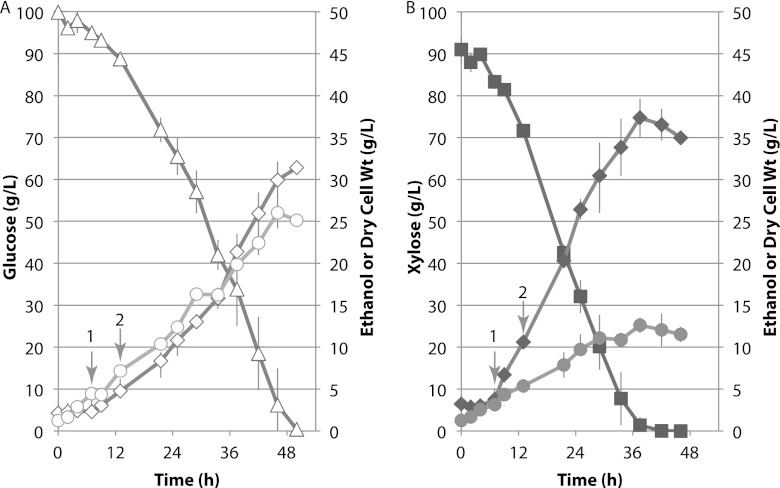
Fig 2.
Spathaspora passalidarum cultivated under oxygen-limited conditions. Cells were cultivated in duplicate 3-liter bioreactors at 25°C in 2,000 ml of defined minimal medium (CBS) with either glucose (open symbols) (A) or xylose (closed symbols) (B). Symbols: triangles, glucose; squares, xylose; diamonds, ethanol; circles, cell dry weight; arrow 1, first metabolomics sample and shift to 2.1% O2; arrow 2, second metabolomics sample. Averages values from duplicate runs are shown. Gray bars depict range of values.
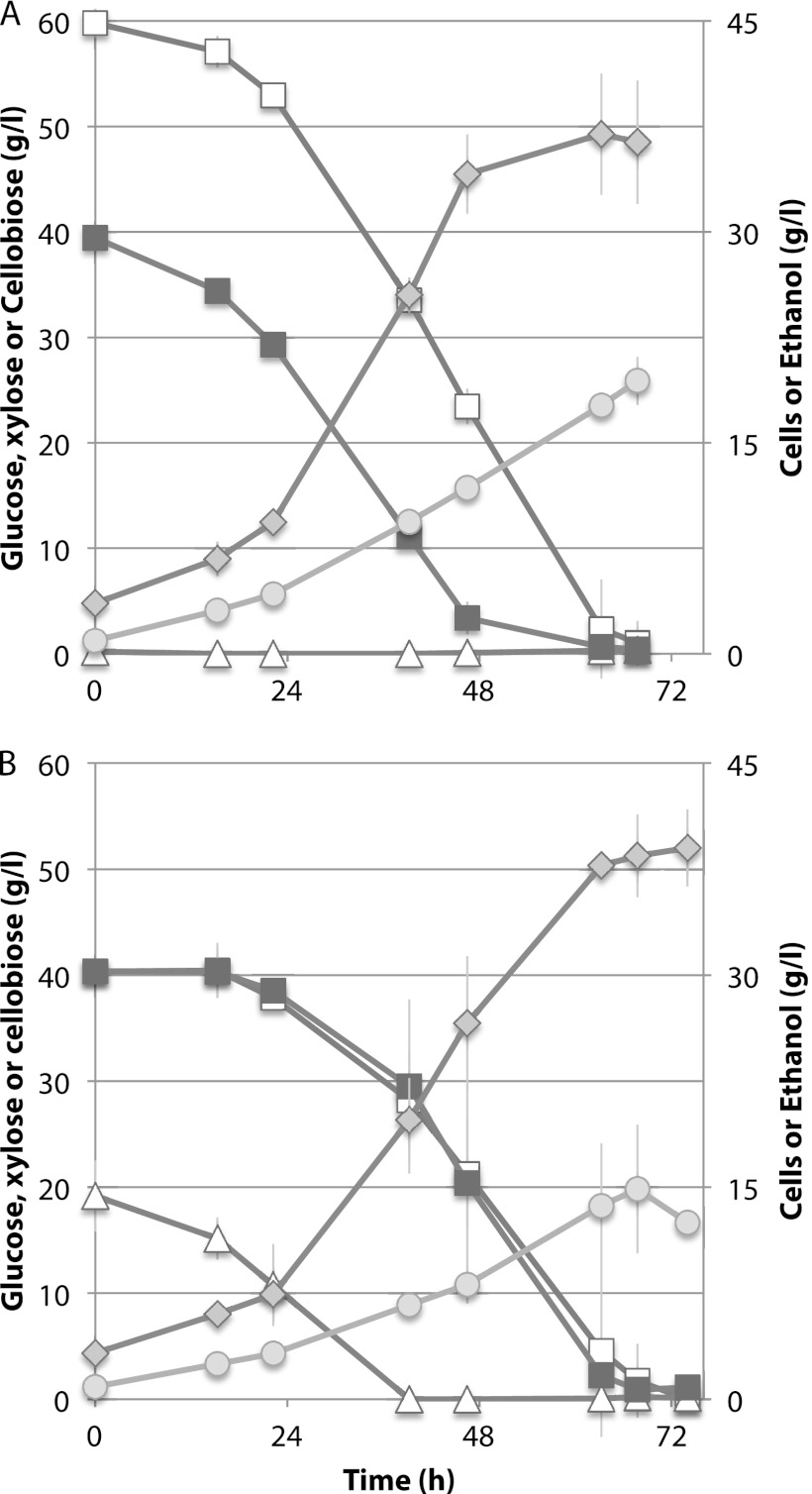
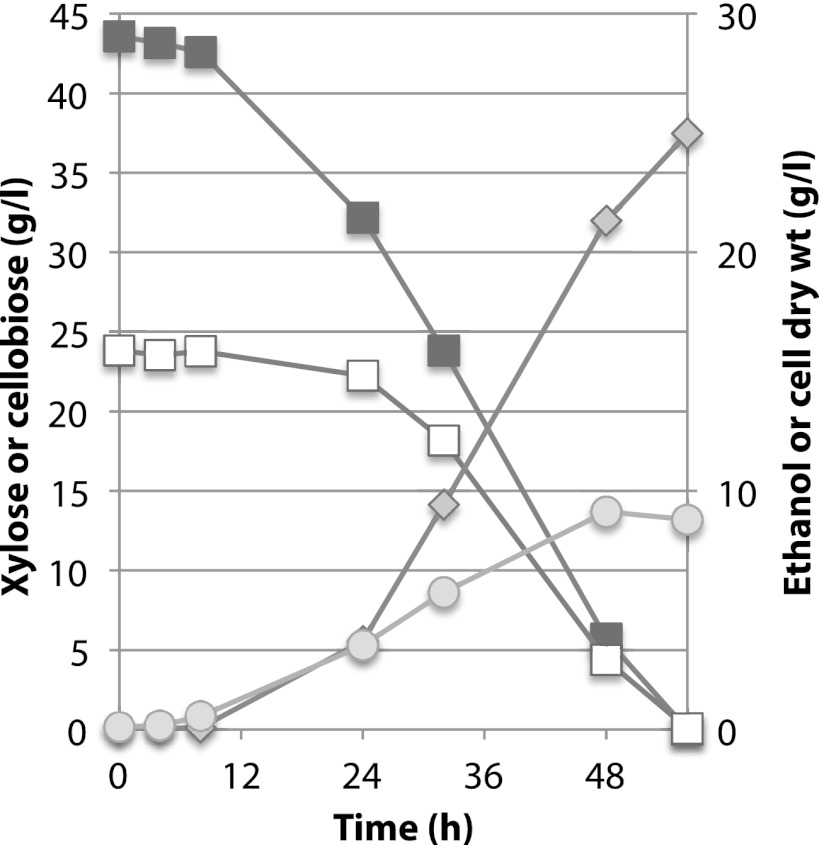
Fig 4.
Coutilization of glucose, xylose, and cellobiose by Spathaspora passalidarum. Cells were cultivated at 25°C. Symbols: open triangles, glucose; closed squares, xylose; open squares, cellobiose; diamonds, ethanol; circles, cell mass.
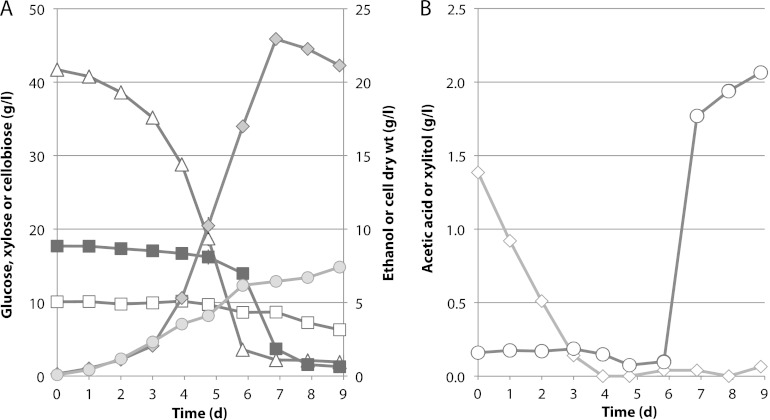
Fig 5.
Fermentation of a maple hemicellulose hydrolysate (A) and a synthetic sugar mixture (B) by Spathaspora passalidarum E7 in defined minimal medium (CBS). Symbols are the same as described in the legend to Fig. 4. Averages of two bioreactors are shown with range of values (gray bars).
Adaptation and fermentation.
For cofermentation, S. passalidarum was propagated first in four 50-ml aliquots of YPX-Cb medium (45 g/liter X, 25 g/liter Cb) in 125-ml Erlenmeyer flasks shaken at 200 rpm at 30°C. After 24 h, these cultures were used to inoculate four 200-ml volumes of CBS X-Cb medium in 2-liter Erlenmeyer flasks, which were incubated at 150 rpm and 30°C for 24 h. Each bioreactor was inoculated with 200 ml of S. passalidarum broth into a final volume of 2.0 liter of CBS X-Cb or CBS G-X-Cb medium. S. passalidarum strain AF2 was adapted by two passages (total, ∼11 doublings) of NRRL Y-27907 in 50 ml wood hydrolysate medium under oxygen-limiting conditions (20). It was then adapted to grow in AFEX corn stover hydrolysate under oxygen-limiting conditions for ∼5 doublings. S. passalidarum E7 was derived from NRRL Y-27907 by cultivation in wood hydrolysate corn stover hydrolysates for 2 months (54 doublings) under oxygen-limiting conditions. Spent broth and cells of strain E7 were directly transferred from an adaptation flask containing stover hydrolysate into 500-ml Erlenmeyer flasks containing 120 ml MHH plus CBS medium to create inocula for 2-liter bioreactors. Inocula were cultured at 150 rpm and 30°C, and then cells plus whole broth were added to Bio Flo 110 3-liter bioreactors for a total working volume of 1.2 liters. The inoculum grown in MHH was transferred into bioreactors containing CBS medium plus MHH, and the inoculum grown with synthetic medium was transferred into bioreactors containing CBS synthetic medium plus sugars. The bioreactors were sparged at 0.3 liter/min (0.25 vvm) with a mixture of nitrogen and air at a 9:1 ratio to attain ∼2.1% O2 saturation. Agitation was controlled between 400 and 500 rpm to maintain saturation. After 12 h, 500-rpm agitation could not meet the oxygen demand of the growing culture, and measurable O2 levels declined to nearly zero.
Sampling, extraction, and metabolite analysis.
Each 5-ml culture sample was rapidly withdrawn and injected by syringe into 20 ml of 60:40 methanol-H2O in a 50-ml conical centrifuge tube that had been equilibrated at −80°C. The cell-medium-methanol mixture was immediately vortexed thoroughly, held briefly (<4 min) in an acetone-dry ice bath, and then centrifuged for 12 min at −9°C. The methanol-water supernatant solution was decanted, and the frozen cell pellet was stored at −80°C until extracted. Metabolite analysis followed the method of Rabinowitz and Kimball (33). Nucleotide phosphates, organic acids, sugar phosphates, and phosphorylated intermediates of glycolysis were analyzed by ion chromatography coupled to electrospray ionization-tandem mass spectrometry (ESI–MS/MS) using minor modifications to a previously described method (43). The column (IonPac AS-11HC; Dionex, Inc.) was equilibrated with 0.5 mM NaOH, and metabolites were eluted with 100 mM NaOH. An Agilent 1200 high-performance liquid chromatography system with a gradient pump, micro-vacuum degasser, and chilled autosampler (4°C) was employed. The same equipment was used to analyze the extract in positive-ionization mode to quantify 21 amino acids by the method of Bajad et al. (1). Instrumental precision was estimated by comparing five replicate injections of a mixture of 12 of the reported compounds (pyruvate, malate, succinate, glucose-6-phosphate [G6P], 2-ketoglutarate, fumarate, citrate, isocitrate, fructose-1,6-bisphosphate, AMP, ADP, and ATP). Relative standard deviations ranged from 22.0% to 2.6%, with an average of 8.9% for all 12 compounds.
For routine sugar consumption and end product analysis, 1.0 ml of sample from each bioreactor was used for sugars, xylitol, glycerol, and ethanol determinations by high-performance liquid chromatography using a refractive index detector and Bio-Rad Aminex HPX-87 lead column (300 by 7.8 mm) at 85°C. The mobile phase was distilled deionized H2O at flow rate of 0.6 ml/min.
RESULTS
Aerobic coutilization of glucose and xylose.
The capacity of S. passalidarum for xylose fermentation compared favorably with that of Scheffersomyces stipitis in shake flask studies (9), so we decided to characterize it further. Fully aerobic cultivation in defined minimal medium with a very low initial cell density (0.014 mg cdw/ml) resulted in little growth for the first 20 to 36 h. Subsequently, however, S. passalidarum used glucose or xylose at essentially the same rates when present individually or in a 50:50 mixture (Fig. 1). No ethanol was formed under aerobic conditions. Cell yield was ∼20% higher on glucose (0.67 g cdw/g) than on xylose (0.56 g cdw/g).
Preferential fermentation of xylose.
Prior studies with S. stipitis have shown that native xylose-fermenting yeasts induce ethanol production under oxygen limitation (23). The very long lag time observed with fully aerobic conditions led us to use higher initial cell densities and lower initial aeration rates. We strove to achieve the same initial oxygen-limited fermentative conditions in the bioreactor that were present in the shake flask used for inoculum propagation. We compared sugar consumption and ethanol production rates by S. passalidarum on CBS medium in duplicate bioreactors with either glucose or xylose as the carbon source (Fig. 2A and B). Immediately following inoculation, the cell mass was 1.23 mg/ml, and with air sparging, 150 rpm was sufficient to maintain dO2 at the set point of 2.1% O2 saturation. Initial growth rates during the air-sparging phase were 0.19 and 0.14 h−1 for glucose and xylose, respectively. Specific ethanol production rates were very low (glucose) or undetectable (xylose). After 7 h, cell densities reached 4.45 and 3.15 g/liter for glucose and xylose, respectively. At that point, the sparging gas was switched to 90% N2, 10% air. With agitation capped at 500 rpm and inlet oxygen capped at 2.1%, dO2 decreased to low or undetectable levels as cell densities increased. Following the switch to oxygen limitation, specific growth rates fell by 2.5- to 5-fold, while specific ethanol production rates increased dramatically (Table 1). During the oxygen-limited phase, cell yield on xylose was only about half of that observed with glucose, but the specific ethanol formation rate was three times higher. This resulted in significantly higher ethanol production with xylose, even though cell yield was notably lower (cf. Fig. 2A and B).
Table 1.
Spathaspora passalidarum product yields and specific formation rates on glucose or xylose during the oxygen-limited phasea
| Sugar | Yield (g/g sugar) |
Growth rate for ethanol (g/liter · h) | Qp (g/g cdw · h) |
||
|---|---|---|---|---|---|
| Cells | Ethanol | Cells | Ethanol | ||
| Glucose | 0.22 | 0.31 | 0.72 | 0.05 | 0.05 |
| Xylose | 0.12 | 0.41 | 1.14 | 0.05 | 0.17 |
Ethanol yields, specific growth rates, and ethanol productivities were calculated from time zero to 50 h for glucose and from time zero to 36 h for xylose. Qp, specific product formation rate.
Cofermentation of glucose, xylose, and cellobiose.
During enzymatic saccharification and fermentation of pretreated lignocellulosics, the major sugars present are xylose and glucose, xylan oligosaccharides (from hydrolysis of hemicellulose), and cellobiose (from enzymatic saccharification of pretreated cellulosic solids). We therefore examined the cofermentation of cellobiose and xylose. In duplicate shake flasks, the xylose consumption rate was much greater than that for cellobiose, but after cells attained a density of ≤5 g/liter, oxygen limitation induced ethanol production. Cellobiose and xylose were coutilized at 0.49 and 0.94 g/liter · h, respectively, and the volumetric ethanol production rate was 0.56 g/liter · h (Fig. 3). In bioreactors, cellobiose and xylose were cofermented from the outset at very similar rates in the presence (Fig. 4A) or absence (Fig. 4B) of 20 g/liter glucose. Prior to inoculation, dO2 was 2.1%, but this declined to nearly zero shortly after inoculation. During the fermentation phase, dO2 occasionally rose slowly to ∼0.2 to 0.5% without an apparent effect on ethanol production or growth. In the absence of glucose, xylose and cellobiose were cometabolized at essentially similar rates until xylose was depleted at 48 h (Fig. 4A). The rate of ethanol production declined significantly thereafter, but cell growth continued. In the presence of glucose, coutilization of cellobiose and xylose was delayed slightly in the first 15 h, but all three sugars were coutilized at very similar rates from 15 to 68 h, at which point all sugars were consumed and ethanol production ceased (Fig. 4B). The maximum rate of ethanol production from a mixture of xylose and cellobiose was 1.07 g/liter · h, and from all three sugars it was 0.73 g/liter · h. Ethanol yields during the phase of maximum production rate were 0.43 g/g and 0.42 g/g for xylose-cellobiose and glucose-xylose-cellobiose mixtures, respectively. Overall volumetric productivities and rates (0 to 72 h) were 0.53 g/liter · h for the glucose, xylose, and cellobiose mixture and 0.58 g/liter · h for the xylose and cellobiose mixture.
Fig 3.
Cofermentation of cellobiose and xylose under oxygen limitation in triplicate shake flasks. Symbols: solid squares, xylose; open squares, cellobiose; diamonds, ethanol; circles, cell mass. Minimal defined medium (CBS) was employed. Flasks were incubated at 30°C and shaken at 200 rpm.
Hydrolysate fermentation.
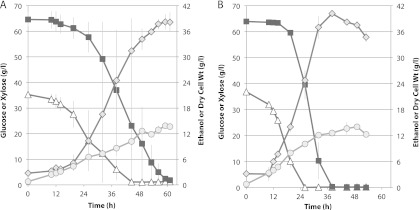
MHH contained 1.8 times more xylose than glucose, and the AFEX hydrolysate contained ∼2.3 times more glucose than xylose. Unlike the AFEX-treated enzymatic hydrolysate of corn stover, MHH did not contain significant amounts of acetic acid or cellobiose, and the glucose/xylose ratio was 35:65 at the start of the fermentation. Fermentation of MHH (Fig. 5A) was compared to that of a sugar mixture (SM) designed to mimic the hydrolysate (Fig. 5B). Almost from the outset, glucose and xylose were cometabolized in the MHH, but utilization of xylose was delayed by ∼12 h in the SM. During the bulk of the fermentation, glucose and xylose were coutilized at similar rates in both the MHH and SM media. Fermentation of the SM proceeded more rapidly to attain a peak of 40 g/liter ethanol in 38 h, whereas fermentation of the MHH required 59 h to attain 38 g/liter ethanol. During the fermentative phase (19.5 to 49 h), cell and ethanol yields were 0.19 and 0.34 g/g sugar consumed from the MHH, respectively, and 0.25 and 0.37 g/g consumed from the SM, respectively. The fermentation time with AFEX was relatively long (Fig. 6A). Its initial acetic acid concentration was about 1.4 g/liter. Cells grew under these conditions but did not exhibit significant ethanol production until acetic acid was depleted at 3 days (Fig. 6B). Xylose utilization was largely delayed while glucose was consumed, but after 6 days, strains adapted to growth in hydrolysate rapidly converted 84% of the xylose into 23 g/liter ethanol with a yield of 0.45 and 16% into xylitol.
Fig 6.
Fermentation of an AFEX hydrolysate by Spathaspora passalidarum AF2 in defined minimal medium (Slininger). Fermentation was conducted in duplicate shake flasks (50 ml/125 shaken at 100 rpm). AF2 was cultured in a 125-ml Erlenmeyer flask containing 50 ml of AFEX hydrolysate medium at 100 rpm and 30°C for 5 days (optical density, above 40) and then transferred directly from the adapted flask into another flask containing the same AFEX hydrolysate medium. The initial targeted optical density at 600 nm was 0.5. Duplicate flask fermentations were conducted at 30°C with rotary shaking at 100 rpm. The starting cell density was 0.1 mg cdw/ml. Symbols: (A) open triangles, glucose; closed squares, xylose; open squares, cellobiose; gray diamonds, ethanol; gray circles, cell mass; (B) open diamonds, acetic acid; gray circles, xylitol. Averages of two shake flasks are shown.
Metabolome analysis.
To better understand the regulatory and metabolic basis for S. passalidarum's preferential utilization and coutilization of xylose, we analyzed samples for relative metabolite abundance in cells cultivated on glucose or xylose at the ends of the aerobic growth phase and during the ethanol production phase (arrows 1 and 2, Fig. 2). At the end of the initial growth phase (arrow 1), levels of glucose 1-phosphate (G1P), glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), fructose 1,6-bisphosphate (F16P), 2-phosphoglycerate (2PG), 3-phosphoglycerate (3PG), phosphoenolpyruvate (PEP), and pyruvate were all significantly higher when cells had been cultivated on glucose than on xylose (Fig. 7). Likewise, 6-phosphogluconate (6PG), which is at the entry to the oxidative pentose phosphate pathway (PPP), and α-ketoglutarate (αKG), which is derived from the tricarboxylic acid cycle, were higher on glucose at that time. Amino acids derived from these intermediates followed essentially the same patterns as their glycolytic or PPP precursors (see Fig. S1 in the supplemental material).
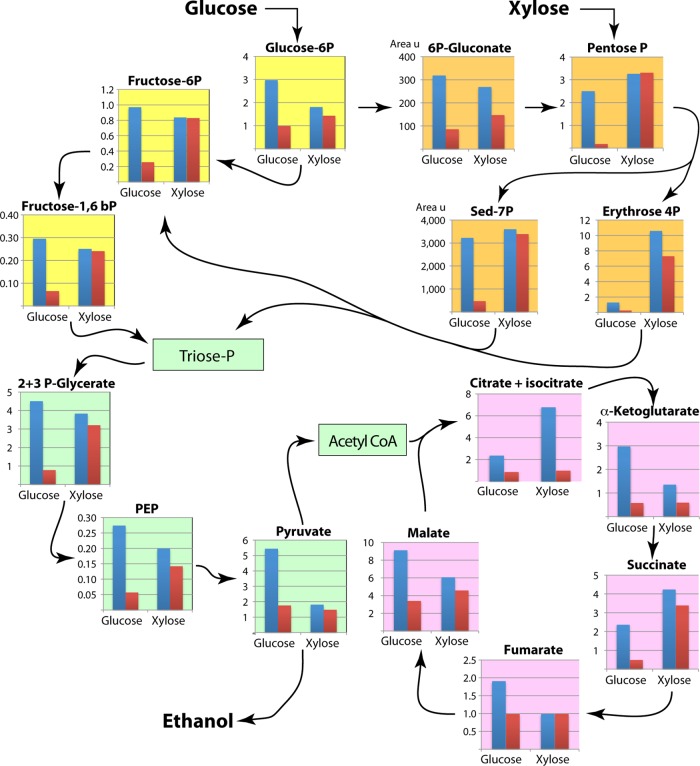
Fig 7.
Intracellular levels of glycolytic metabolites and amino acids in Spathaspora passalidarum cultivated on glucose or xylose under aerobic (blue bars) and oxygen-limited (red bars) conditions (cf. Fig. 2A and B). All units are μmol/g cdw except for 6-phosphogluconate and sedoheptulose 7-phosphate, which are expressed as area units (area u)/g cdw. Fructose-1,6bP, fructose 1,6-bisphosphate; Fructose-6P, fructose 6-phosphate; Glucose 6P, glucose-6-phosphate; 6P-Gluconate, 6-phosphogluconate; Pentose P, pentose phosphate; Sed-7P, sedoheptulose 7-phosphate; Erythrose 4P, erythrose 4-phosphate; 2+3 P-Glycerate, 2-phosphoglycerate plus 3-phosphoglycerate; Triose-P, triose phosphate; Acetyl CoA, acetyl coenzyme A.
In contrast to these metabolites, levels of pentose phosphate (PP), sedoheptulose 7-phosphate (S7P), and, particularly, erythrose 4-phosphate (E4P) were higher at the end of the growth phase when cells were cultivated on xylose than when they were cultivated on glucose. Interestingly, cellular levels of phenylalanine, tyrosine, and tryptophan, which are formed from E4P and PEP, all followed the profile exhibited by E4P rather than that exhibited by PEP (see Fig. S1 in the supplemental material). In contrast, concentrations of histidine (which is derived from ribose 5-phosphate) followed the profile of 6PG rather than that of PP. Our analytical procedure was not able to distinguish among ribose 5-phosphate (Ri5P), ribulose 5-phosphate (Ru5P), and xylulose 5-phosphate (Xu5P), but although Ri5P is derived directly from 6PG, Xu5P is derived from phosphorylation of xylulose, so the pool of Ri5P might not be directly affected by growth on xylose like the concentrations of S7P and E4P are. Alanine also attained relatively high levels (∼50 μmol/g cdw) that were of the same order of magnitude as those of glutamate, glutamine, and arginine (see Fig. S2 in the supplemental material). The greatest observed differences in metabolite levels occurred during the oxygen-limited fermentative phase (arrow 2, Fig. 2). With the exception of G1P, which is tied closely to growth through synthesis of cell wall glucans, levels of virtually all glycolytic, pentose phosphate, and trichloroacetic acid intermediates (except pyruvate) and the amino acids derived from them were higher on xylose than on glucose. ATP and ADP levels were higher on glucose than on xylose during the aerobic growth phase, but under oxygen limitation, ATP levels were about three times higher on xylose than on glucose (see Fig. S3 in the supplemental material). Ratios of reduced glutathione to oxidized glutathione stayed almost constant for each of the four conditions, but intracellular concentrations increased about 3.5-fold on xylose relative to those on glucose during the oxygen-limited fermentative phase.
DISCUSSION
S. passalidarum was originally isolated from the midgut of a passalid beetle that preferentially inhabits white-rotted hardwoods (30). The midgut of wood-boring beetles is hypothesized to be oxygen limited, so evolution for growth under oxygen-limited conditions on mixtures of cellulosic and hemicellulosic sugars might be expected. S. passalidarum NN245 is the sole known teleomorphic isolate within the species, but other species in the Spathaspora genus and related anamorphic species such as Candida jeffriesii have been isolated and characterized. Spathaspora arborariae, which was isolated from rotting wood in the Brazilian Atlantic Rain Forest, is also capable of fermenting xylose (6, 7), but other members of the Spathaspora clade are not (2, 5). The yeast clade to which Spathaspora belongs is related to but distinct from that of Scheffersomyces (26), which also includes a number of xylose-fermenting yeasts, including S. stipitis (16). The capacity for producing ethanol from cellobiose is infrequent but occurs over a wide taxonomic range within the yeasts. Even so, the traits for xylose and cellobiose fermentation are variable within genera and even species. Although the capacity to ferment both xylose and cellobiose is rare among yeasts, the abilities to ferment xylose faster than glucose and to simultaneously cometabolize and ferment glucose, xylose, and cellobiose are believed to be unprecedented. Fermentation rates and yields for xylose and cellobiose fermentations by native strains of Spathaspora species exceed those by Saccharomyces cerevisiae strains engineered for these traits (see Table S2 in the supplemental material).
Under fully aerobic conditions, S. passalidarum grew poorly at first on glucose or xylose. Once growth started, however, sugar utilization and cell growth rates were similar and aerobic coutilization of glucose and xylose was essentially simultaneous. We inferred that the cells underwent physiological adaptation to achieve full aerobic growth. Under typical low-aeration conditions, cells would grow and ferment rapidly, so the long lag under fully aerobic conditions was atypical. Cell yields on xylose, however, were only about 84% of those on glucose under fully aerobic conditions. The higher efficiency of cell growth on glucose was even more apparent under O2 limitation (Fig. 2A). Cell yield on xylose was only 55% of that on glucose (Fig. 2B); however, ethanol yield was 32% higher on xylose than on glucose, and the specific fermentation rate on xylose was 3.4-fold higher. This was in contrast to the nearly simultaneous coutilization of both sugars in a mixture under aerobic conditions, where no ethanol production was observed.
A lower net ATP yield for cells grown on xylose could account for the higher glycolytic flux under O2 limitation. Hou reported a lower cell yield with S. passalidarum growing on xylose than on glucose (15) and suggested that S. passalidarum might use low-affinity, low-capacity facilitated diffusion for growth under O2 limitation and the high-capacity, high-affinity system during aerobic growth (28). Previous studies have shown that Scheffersomyces stipitis induces respirofermentative growth and pyruvate decarboxylase under oxygen-limiting conditions (23, 31). It seems likely that similar mechanisms for oxygen sensing could regulate transport as well. We observed maximal volumetric fermentation rates of 1.14 and 0.72 g/liter · h for xylose and glucose, respectively, and our maximal rates for xylose and cellobiose consumption in the presence of glucose were 0.55 and 0.57 g/liter · h, respectively, in minimal medium with urea as the nitrogen source. The specific rate of xylose fermentation was more than 3 times greater than the rate of glucose fermentation under conditions that allowed the induction of transcripts and enzymatic activities important for anaerobic or oxygen-limited metabolism.
Metabolite levels measured during oxygen limitation support the observation that glycolytic flux is greater in cells cultivated on xylose than those cultivated on glucose. Levels of glucose-derived metabolites were higher during the initial aerobic growth phase, but they dropped dramatically as cells shifted to fermentative conditions. By comparison, xylose-derived intermediates were significantly higher on xylose under either aerobic or O2-limited conditions. Cells cultivated under O2-limited conditions showed higher levels of ATP on xylose than on glucose. There are several possible reasons: (i) glycolytic flux is greater on xylose, (ii) ATP demand is lower, (iii) net ATP yield is higher, or (iv) regulation of ATP biosynthesis is different on glucose and xylose. Because cell yield was significantly lower on xylose than on glucose, a decreased ATP demand is probably the dominant effector.
The xylitol reductase (XR) of Scheffersomyces stipitis has about 70% as much activity with NADH as it does with NADPH (45). The capacity to recycle NADH to NAD+ under anaerobic or oxygen-limiting conditions can reduce xylitol production and increase the capacity for xylose assimilation (3, 17, 19, 22, 24, 29, 32). Recently, Hou (15) reported that the crude XR activity of S. passalidarum has a 1.8-fold higher affinity for NADH than for NADPH. By comparison, the XR of S. stipitis shows a 1.6-fold higher affinity for NADPH. Moreover, the affinity of the crude XR activity for xylose is 3-fold higher with NADH than with NADPH. The xylitol dehydrogenase (XDH) activity of S. passalidarum also has a much higher affinity for xylitol than the corresponding enzyme from S. stipitis does (15). These kinetic characteristics are much more favorable than those achieved with the best aldose reductase genes previously engineered for high selectivity and activity with NADH (19, 22, 29), and they could account in large part for the high PPP, F6P, and F16P metabolite levels on xylose. The S. passalidarum genome contains two orthologs each for XYL1 and XYL2 (46), so it is not possible to determine from these data which of these contributes more to the cofactor affinities measured in crude homogenates.
Most glycolytic regulation occurs metabolically, via allosteric mechanisms to determine the overall metabolic flux, rather than hierarchically, through changes in gene expression (8). Thus, the relative affinities of enzymes for cofactors and substrates along with enzyme activities at modulated steps determine the overall flow of metabolites. Pyruvate kinase, which catalyzes the final step in glycolysis, is allosterically activated by F16P and is important for controlling levels of ATP, GTP, and glycolytic intermediates (18). The affinities of NADH-coupled XR and NAD+-coupled XDH would tend to drive both the oxidoreductase pathway for xylose assimilation and the reduction of acetaldehyde to ethanol. The strong xylose assimilation would likewise increase levels of F16P, thereby activating glycolysis via pyruvate kinase. This does not fully explain the higher cell yield and lower glycolytic activity observed with glucose-grown cells, but it does offer a model for further experimentation, and it begins to offer insight into how we might go about engineering rapid coutilization of these sugars.
We describe what we believe to be the first report of an organism capable of cofermentation of xylose and cellobiose in the presence of glucose. This ability makes S. passalidarum a potentially useful organism for SSF and an interesting organism for unraveling the regulatory mechanisms enabling bioconversion of lignocellulosic materials by yeasts.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494) and by a grant from the Heart of Wisconsin (Wisconsin Rapids) to the University of Wisconsin, Madison. T.W.J. is supported by the USDA, Forest Products Laboratory.
We thank Haibo Li and Amanda Prichard for assistance in performing fermentations and analyses; Thomas E. Amidon, State University of New York, Environmental Science and Forestry, Syracuse, NY, for samples of pretreated maple hemicellulose hydrolysate; Wes Marner (GLBRC, Madison, WI) for providing AFEX-pretreated, cellulase-digested corn stover; and Meredith Blackwell, Louisiana State University, for providing the original culture of S. passalidarum.
T.W.J. conceived the project, directed the work, and wrote the manuscript. Y.-K.S., T.M.L., and J.H. performed the fermentation experiments, contributed to experimental designs, and analyzed results. L.B.W. contributed to conceptualization and experimental designs and performed fermentation experiments, data analysis, and editing. A.H. developed and conducted analytical techniques. All coauthors contributed to writing the manuscript.
Footnotes
Published ahead of print 25 May 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Bajad SU, et al. 2006. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. A 1125:76–88 [DOI] [PubMed] [Google Scholar]
- 2. Barbosa AC, Cadete RM, Gomes FCO, Lachance MA, Rosa CA. 2009. Candida materiae sp. nov., a yeast species isolated from rotting wood in the Atlantic Rain Forest. Int. J. Syst. Evol. Microbiol. 59:2104–2106 [DOI] [PubMed] [Google Scholar]
- 3. Bengtsson O, Hahn-Hagerdal B, Gorwa-Grauslund MF. 2009. Xylose reductase from Pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol. Biofuels 2:9 doi:10.1186/1754-6834-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Binder JB, Raines RT. 2010. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl. Acad. Sci. U. S. A. 107:4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boonmak C, et al. 2011. Candida xylanilytica sp. nov., a xylan-degrading yeast species isolated from Thailand. Int. J. Syst. Evol. Microbiol. 61:1230–1234 [DOI] [PubMed] [Google Scholar]
- 6. Cadete RM, et al. 2009. Spathaspora arborariae sp nov., a d-xylose-fermenting yeast species isolated from rotting wood in Brazil. FEMS Yeast Res. 9:1338–1342 [DOI] [PubMed] [Google Scholar]
- 7. da Cunha-Pereira F, et al. 2011. Conversion of sugars present in rice hull hydrolysates into ethanol by Spathaspora arborariae, Saccharomyces cerevisiae, and their co-fermentations. Bioresour. Technol. 102:4218–4225 [DOI] [PubMed] [Google Scholar]
- 8. Daran-Lapujade P, et al. 2007. The fluxes through glycolytic enzymes in Saccharomyces cerevisiae are predominantly regulated at posttranscriptional levels. Proc. Natl. Acad. Sci. U. S. A. 104:15753–15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du Preez JC, Bosch M, Prior BA. 1986. The fermentation of hexose and pentose sugars by Candida shehatae and Pichia stipitis. Appl. Microbiol. Biotechnol. 23:228–233 [Google Scholar]
- 10. Fonseca C, et al. 2011. The glucose/xylose facilitator Gxf1 from Candida intermedia expressed in a xylose-fermenting industrial strain of Saccharomyces cerevisiae increases xylose uptake in SSCF of wheat straw. Enzyme Microb. Technol. 48:518–525 [DOI] [PubMed] [Google Scholar]
- 11. Gancedo JM. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Görke B, Stülke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613–624 [DOI] [PubMed] [Google Scholar]
- 13. Ha SJ, et al. 2011. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc. Natl. Acad. Sci. U. S. A. 108:504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Himmel ME, et al. 1997. Advanced bioethanol production technologies: a perspective, p 2–45 In Saha BC, Woodward J. (ed), Fuels and chemicals from biomass, vol. 666 American Chemical Society, Washington, DC [Google Scholar]
- 15. Hou X. 2012. Anaerobic xylose fermentation by Spathaspora passalidarum. Appl. Microbiol. Biotechnol. 94:205–214 [DOI] [PubMed] [Google Scholar]
- 16. Jeffries TW, Van Vleet JRH. 2009. Pichia stipitis genomics, transcriptomics, and gene clusters. FEMS Yeast Res. 9:793–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeppsson M, et al. 2006. The expression of a Pichia stipitis xylose reductase mutant with higher K(M) for NADPH increases ethanol production from xylose in recombinant Saccharomyces cerevisiae. Biotechnol. Bioeng. 95:665–673 [DOI] [PubMed] [Google Scholar]
- 18. Jurica MS, et al. 1998. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 6:195–210 [DOI] [PubMed] [Google Scholar]
- 19. Khattab SMR, Watanabe S, Saimura M, Kodaki T. 2011. A novel strictly NADPH-dependent Pichia stipitis xylose reductase constructed by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 404:634–637 [DOI] [PubMed] [Google Scholar]
- 20. Kim HY, Lee JW, Jeffries TW, Choi IG. 2011. Response surface optimization of oxalic acid pretreatment of yellow poplar (Liriodendron tulipifera) for production of glucose and xylose monosaccharides. Bioresour. Technol. 102:1440–1446 [DOI] [PubMed] [Google Scholar]
- 21. Kim RS, Ha SJ, Wei N, Oh EJ, Jin YS. 2012. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol. 30:274–282 [DOI] [PubMed] [Google Scholar]
- 22. Klimacek M, Krahulec S, Sauer U, Nidetzky B. 2010. Limitations in xylose-fermenting Saccharomyces cerevisiae, made evident through comprehensive metabolite profiling and thermodynamic analysis. Appl. Environ. Microbiol. 76:7566–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klinner U, Fluthgraf S, Freese S, Passoth V. 2005. Aerobic induction of respiro-fermentative growth by decreasing oxygen tensions in the respiratory yeast Pichia stipitis. Appl. Microbiol. Biotechnol. 67:247–253 [DOI] [PubMed] [Google Scholar]
- 24. Kötter P, Ciriacy M. 1993. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 38:776–783 [Google Scholar]
- 25. Krahulec S, et al. 2010. Fermentation of mixed glucose-xylose substrates by engineered strains of Saccharomyces cerevisiae: role of the coenzyme specificity of xylose reductase, and effect of glucose on xylose utilization. Microb. Cell Fact. 9:16 doi:10.1186/1475-2859-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurtzman CP, Suzuki M. 2010. Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 51:2–14 [Google Scholar]
- 27. Lau MW, Dale BE. 2009. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST). Proc. Natl. Acad. Sci. U. S. A. 106:1368–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leandro MJ, Goncalves P, Spencer-Martins I. 2006. Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose-H+ symporter. Biochem. J. 395:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsushika A, et al. 2008. Expression of protein engineered NADP plus-dependent xylitol dehydrogenase increases ethanol production from xylose in recombinant Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 81:243–255 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen NH, Suh SO, Marshall CJ, Blackwell M. 2006. Morphological and ecological similarities: wood-boring beetles associated with novel xylose-fermenting yeasts, Spathaspora passalidarum gen. sp. nov. and Candida jeffriesii sp. nov. Mycol. Res. 110:1232–1241 [DOI] [PubMed] [Google Scholar]
- 31. Passoth V, Zimmermann M, Klinner U. 1996. Peculiarities of the regulation of fermentation and respiration in the crabtree-negative, xylose-fermenting yeast Pichia stipitis. Appl. Biochem. Biotechnol. 57–58:201–212 [DOI] [PubMed] [Google Scholar]
- 32. Petschacher B, Nidetzky B. 2008. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb. Cell Fact. 7:9 doi:10.1186/1475-2859-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rabinowitz JD, Kimball E. 2007. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal. Chem. 79:6167–6173 [DOI] [PubMed] [Google Scholar]
- 34. Runquist D, Fonseca C, Radstrom P, Spencer-Martins I, Hahn-Hägerdal B. 2009. Expression of the Gxf1 transporter from Candida intermedia improves fermentation performance in recombinant xylose-utilizing Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 82:123–130 [DOI] [PubMed] [Google Scholar]
- 35. Saitoh S, Hasunuma T, Tanaka T, Kondo A. 2010. Co-fermentation of cellobiose and xylose using beta-glucosidase displaying diploid industrial yeast strain OC-2. Appl. Microbiol. Biotechnol. 87:1975–1982 [DOI] [PubMed] [Google Scholar]
- 36. Saloheimo A, et al. 2007. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl. Microbiol. Biotechnol. 74:1041–1052 [DOI] [PubMed] [Google Scholar]
- 37. Salusjärvi L, et al. 2008. Regulation of xylose metabolism in recombinant Saccharomyces cerevisiae. Microb. Cell Fact. 7:18 doi:10.1186/1475-2859-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sikyta B. 1983. Methods in industrial microbiology. Ellis Horwood Limited, Chichester, United Kingdom [Google Scholar]
- 39. Slininger PJ, Bothast RJ, Ladisch MR, Okos MR. 1990. Optimum pH and temperature conditions for xylose fermentation by Pichia stipitis. Biotechnol. Bioeng. 35:727–731 [DOI] [PubMed] [Google Scholar]
- 40. Slininger PJ, Dien BS, Gorsich SW, Liu ZL. 2006. Nitrogen source and mineral optimization enhance d-xylose conversion to ethanol by the yeast Pichia stipitis NRRL Y-7124. Appl. Microbiol. Biotechnol. 72:1285–1296 [DOI] [PubMed] [Google Scholar]
- 41. Stoutenburg RM, Perrotta JA, Amidon TE, Nakas JP. 2008. Ethanol production from a memberane purified hemicellulosic hydrolysate derived from sugar maple by Pichia stipitis NRRL Y-7124. Bioresources 3:1349–1358 [Google Scholar]
- 42. Tyo KE, Alper HS, Stephanopoulos GN. 2007. Expanding the metabolic engineering toolbox: more options to engineer cells. Trends Biotechnol. 25:132–137 [DOI] [PubMed] [Google Scholar]
- 43. van Dam JC, et al. 2002. Analysis of glycolytic intermediates in Saccharomyces cerevisiae using anion exchange chromatography and electrospray ionization with tandem mass spectrometric detection. Anal. Chim. Acta 460:209–218 [Google Scholar]
- 44. Verduyn C, Postma E, Scheffers WA, Vandijken JP. 1992. Effect of benzoic acid on metabolic fluxes in yeasts—a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517 [DOI] [PubMed] [Google Scholar]
- 45. Verduyn C, et al. 1985. Properties of the NAD(P)H-dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis. Biochem. J. 226:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wohlbach DJ, et al. 2011. Comparative genomics of xylose-fermenting fungi for enhanced biofuel production. Proc. Natl. Acad. Sci. U. S. A. 108:13212–13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhong C, Lau MW, Balan V, Dale BE, Yuan YJ. 2009. Optimization of enzymatic hydrolysis and ethanol fermentation from AFEX-treated rice straw. Appl. Microbiol. Biotechnol. 84:667–676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.