Abstract
The fin0 gene of R100 was isolated from the Fin0+ transducing phage VA lambda 57. The limits of the gene were determined by BAL31 digestions and by analysis of deletion mutations derived from an internal restriction site. The DNA sequence contained an open reading frame of 558 nucleotides that would encode a protein of 21,268 daltons. Synthesis of such a protein was observed only when the fragment was cloned in front of the TAC promoter. Deletions entering the large open reading frame from either end were Fin0-, while internal frame shift mutations retained high Fin0 activity. One such strain had a 13 bp internal deletion that would produce a protein of 63 amino acid residues of which 21 were basic. We were consequently unable to rigorously establish that the 558 base orf encoded a fin0 product. The strand opposite the large open reading frame contained several transcription termination signals, and it is possible that the active gene product is one or two small RNAs from this strand.
Full text
PDF





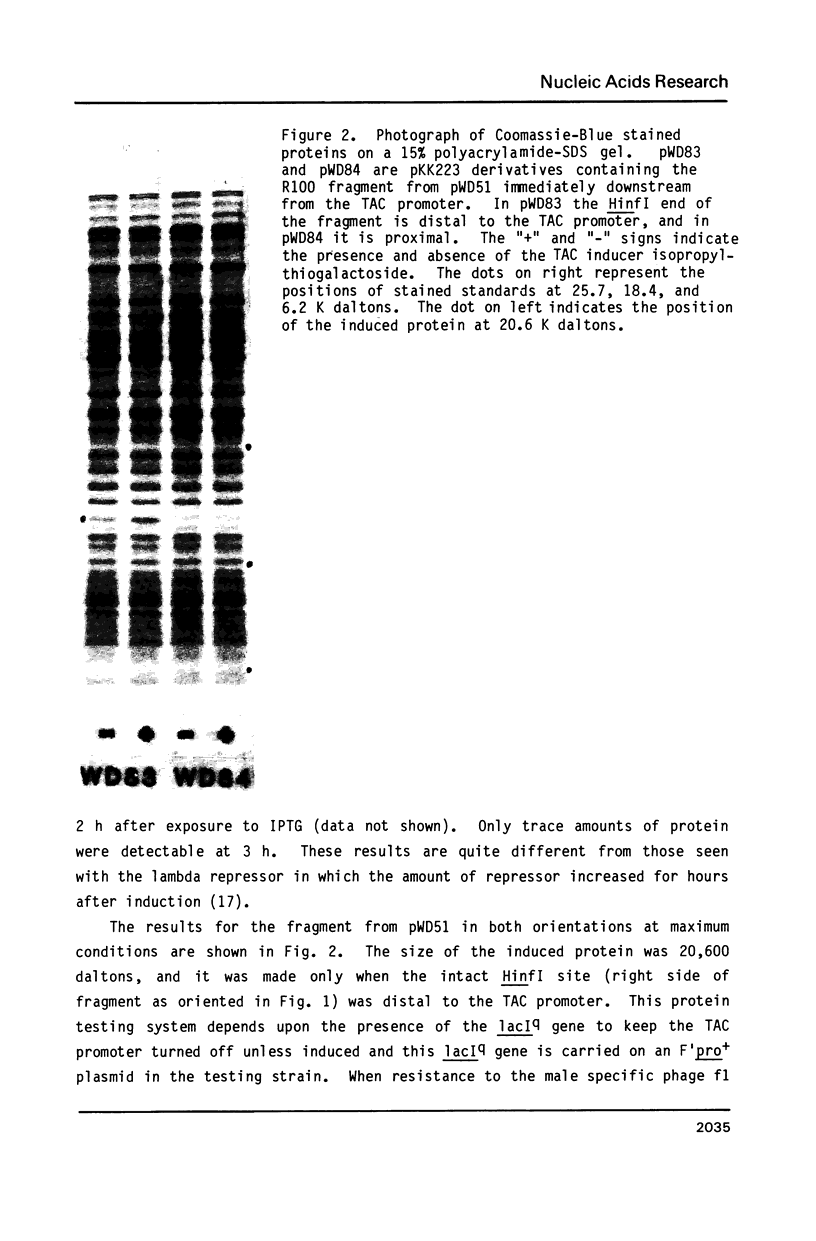

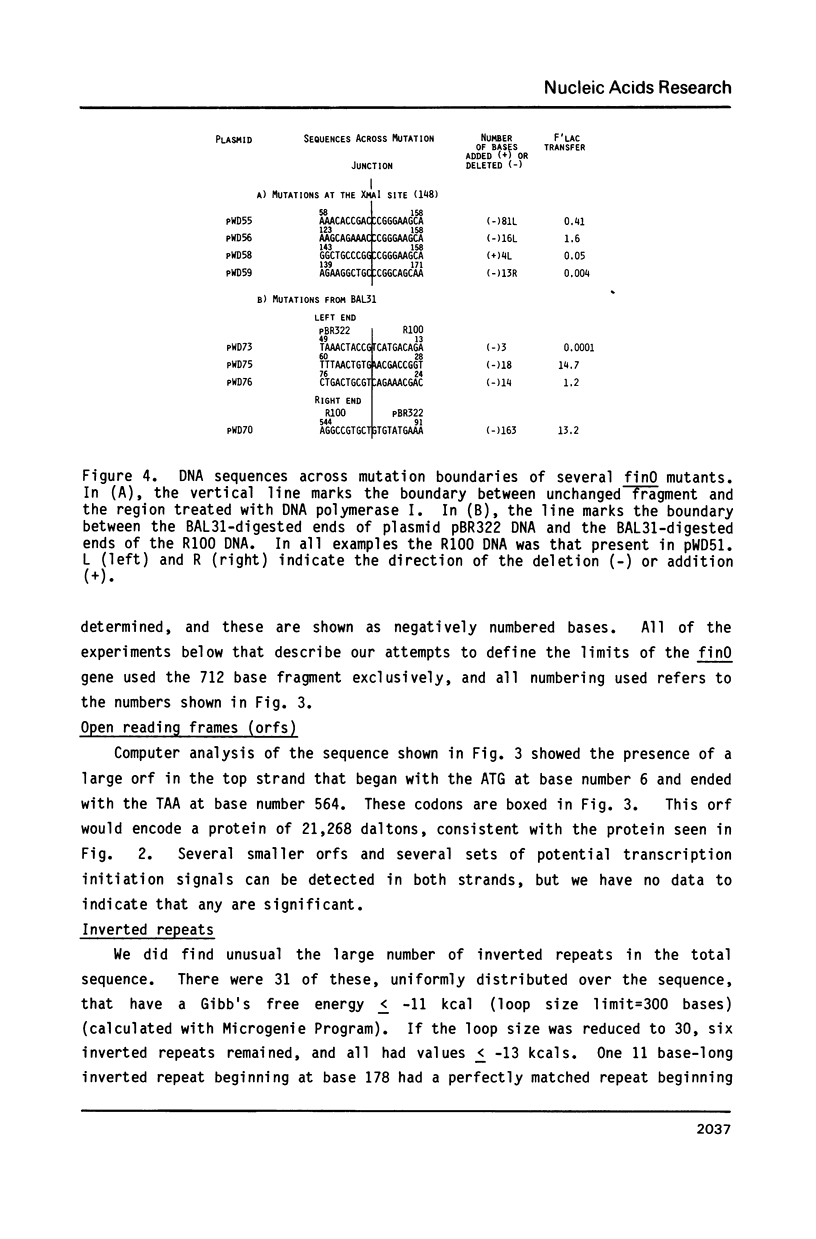





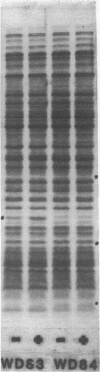
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Cheah K. C., Ray A., Skurray R. Cloning and molecular analysis of the finO region from the antibiotic-resistance plasmid R6-5. Plasmid. 1984 Nov;12(3):222–226. doi: 10.1016/0147-619x(84)90050-7. [DOI] [PubMed] [Google Scholar]
- Cuozzo M., Silverman P. M. Characterization of the F plasmid TraJ protein synthesized in F' and Hfr strains of Escherichia coli K-12. J Biol Chem. 1986 Apr 15;261(11):5175–5179. [PubMed] [Google Scholar]
- Dempsey W. B., McIntire S. A. Lambda transducing phages derived from a FinO- R100::lambda cointegrate plasmid: proteins encoded by the R100 replication/incompatibility region and the antibiotic resistance determinant. Mol Gen Genet. 1979 Nov;176(3):319–334. doi: 10.1007/BF00333094. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B., McIntire S. A. The finO gene of antibiotic resistance plasmid R100. Mol Gen Genet. 1983;190(3):444–451. doi: 10.1007/BF00331075. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B., McIntire S. A., Willetts N., Schottel J., Kinscherf T. G., Silver S., Shannon W. A., Jr Properties of lambda transducing bacteriophages carrying R100 plasmid DNA: mercury resistance genes. J Bacteriol. 1978 Dec;136(3):1084–1093. doi: 10.1128/jb.136.3.1084-1093.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Willetts N. S. Plasmid co-integrates of prophage lambda and R factor R100. J Bacteriol. 1976 Apr;126(1):166–176. doi: 10.1128/jb.126.1.166-176.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Easton A. M., Rownd R. H. The incompatibility product of IncFII R plasmid NR1 controls gene expression in the plasmid replication region. J Bacteriol. 1982 Nov;152(2):829–839. doi: 10.1128/jb.152.2.829-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee B. E., Dempsey W. B. Cloning, mapping, and sequencing of plasmid R100 traM and finP genes. J Bacteriol. 1986 Jul;167(1):336–345. doi: 10.1128/jb.167.1.336-345.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Willetts N. S. Nucleotide sequences of five IncF plasmid finP alleles. J Bacteriol. 1986 Aug;167(2):754–757. doi: 10.1128/jb.167.2.754-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The nature of the transfer inhibitor of several F-like plasmids. Mol Gen Genet. 1972;119(1):57–66. doi: 10.1007/BF00270444. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Peterson B. C., Hashimoto H., Rownd R. H. Cointegrate formation between homologous plasmids in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1086–1094. doi: 10.1128/jb.151.3.1086-1094.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. A modified pBR322 vector with improved properties for the cloning, recovery, and sequencing of blunt-ended DNA fragments. Gene. 1982 Feb;17(2):189–196. doi: 10.1016/0378-1119(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riise E., Molin S. Purification and characterization of the CopB replication control protein, and precise mapping of its target site in the R1 plasmid. Plasmid. 1986 May;15(3):163–171. doi: 10.1016/0147-619x(86)90034-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Blakesley R. W., Doran K., Hough C. J., Wells R. D. Purification of nucleic acids by RPC-5 ANALOG chromatography: peristaltic and gravity-flow applications. Methods Enzymol. 1983;100:368–399. doi: 10.1016/0076-6879(83)00068-3. [DOI] [PubMed] [Google Scholar]
- Thompson R., Taylor L. Promoter mapping and DNA sequencing of the F plasmid transfer genes traM and traJ. Mol Gen Genet. 1982;188(3):513–518. doi: 10.1007/BF00330058. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Achtman M. Fertility repression of F-like conjugative plasmids: physical mapping of the R6--5 finO and finP cistrons and identification of the finO protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5836–5840. doi: 10.1073/pnas.75.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae IV. Interactions between resistance transfer factor and F-factor in Escherichia coli K-12. J Bacteriol. 1962 Apr;83:727–735. doi: 10.1128/jb.83.4.727-735.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]