Abstract
Two cytolethal distending toxin (Cdt) type V-encoding bacteriophages (Φ62 and Φ125) were induced spontaneously from their wild-type Escherichia coli strains and from the lysogens generated in Shigella sonnei. The stability of Cdt phages was determined at various temperatures and pH values after 1 month of storage by means of infectivity tests using a plaque blot assay and analysis of phage genomes using real-time quantitative PCR (qPCR): both were highly stable. We assessed the inactivation of Cdt phages by thermal treatment, chlorination, UV radiation, and in a mesocosm in both summer and winter. The results for the two Cdt phages showed similar trends and were also similar to the phage SOM23 used for reference, but they showed a much higher persistence than Cdt-producing E. coli. Cdt phages showed maximal inactivation after 1 h at 70°C, 30 min of UV radiation, and 30 min of contact with a 10-ppm chlorine treatment. Inactivation in a mesocosm was higher in summer than in winter, probably because of solar radiation. The treatments reduced the number of infectious phages but did not have a significant effect on the Cdt phage particles detected by qPCR. Cdt phages were quantified by qPCR in 73% of river samples, and these results suggest that Cdt phages are a genetic vehicle and the natural reservoir for cdt in the environment.
INTRODUCTION
Cytolethal distending toxin (Cdt) is produced by different pathogenic microorganisms, including Escherichia coli, Shigella dysenteriae (29), and Campylobacter spp. (18), among others (15, 22, 33). Its cytolethal distending action consists of blocking the G2 and M phases during mitosis, which distends the cells, since cell division ceases but growth continues.
Cdt consists of three subunits (CdtA, CdtB, and CdtC) that are encoded by three adjacent genes. The catalytic subunit CdtB is homologous to DNase I and is the most conserved gene due to its essential role in cellular toxicity. The other subunits act as binding proteins that deliver CdtB into target cells, thus producing the cytotoxic effect (13). Five variants of the toxin have been reported in E. coli. In some of these variants, the cdt genes are flanked by lambda-like and bacteriophage P2 genes (Cdt-I and Cdt-IV) (32). Some variants are present in inducible lambdoid prophages, such as Cdt-I (5) and Cdt-V (2), or are encoded in pVir, a conjugative plasmid (Cdt-III) (30).
Recent genomic sequencing of different E. coli strains indicates that a large part of the genome consists of bacteriophage genes and that they constitute an important mechanism for adaptation to new hosts through the horizontal transfer of virulence genes (7). Cdt genes in E. coli occur independently of the presence of other virulence traits (21), which strongly suggests that they are independently acquired by horizontal gene transfer, probably by means of bacteriophages. cdt can be transduced from a cdt-positive bacteria to a cdt-negative strain by means of bacteriophages (2, 5). This is an important process because this gene exchange can lead to the emergence of virulent strains. Cdt-V phages have been detected in fecally polluted wastewater (2), which suggests a certain prevalence of these phages in the environment and reinforces the hypothesis that they mobilize cdt. Therefore, an important aspect to be considered is the survival and persistence of these phages in different environmental conditions, since this will affect the dissemination of cdt genes.
Bacteriophages show a higher resistance to natural and anthropogenic external stress factors than bacteria (6, 12, 20, 25, 26). Within the different phage groups, it seems that particular morphologies could lead to improved survival of bacteriophages in certain conditions (25, 26). Cdt phages belongs to the Siphoviridae morphology; tailored phages which, according to some authors, are very stable under different conditions (1, 20, 26). In the present study, we evaluated the infectivity and stability of Cdt phages in different external conditions using infectivity assays and a real-time quantitative PCR (qPCR) method, to establish their persistence in the environment.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, and media.
Lysates of Cdt-V-encoding bacteriophages Φ62 and Φ125, initially isolated from wild-type E. coli strain 62 (serotype O22:H8) and E. coli strain 125 (serotype O157:H7) were obtained from S. sonnei lysogens as described elsewhere (2). The bacteriophage SOM23, an environmental isolate belonging to the Siphoviridae morphology (27), was used as an E. coli phage control. E. coli strain 125 (cdt+) (2) was used as a bacterial control. S. sonnei strain 866 was used as the host strain. Luria-Bertani (LB) broth, LB agar, Trypticase soy agar (TSA), and ChromoCult Coliform Agar (Merck, Darmstadt, Germany) were used to culture the bacteria. Mitomycin C (0.5, 2, and 5 μg/ml) and EDTA (20 mM) were added to the lysogens to induce Cdt phages.
Isolation of phage DNA, PCR studies, and sequencing.
Phage DNA was isolated from the phage lysates and from 100-ml samples of river water as previously described (2). For phage DNA isolation from lysis plaques, each plaque was recovered from the agar layer using a sterile loop, suspended in 100 μl of phosphate-buffered saline, and treated with DNase, and, after heat inactivation of DNase (15 min at 95°C), the sample was processed for DNA extraction. Conventional PCRs, real-time qPCR assays for Cdt-V detection, and sequencing of PCR products were performed using protocols and primers previously described (2).
Infectivity of Cdt phages and plaque blot hybridization.
Cdt phages were enumerated by the double agar layer method using S. sonnei strain 866 as the host strain (2). Those plates of Cdt phages containing approximately 100 to 300 PFU were transferred to a nylon membrane and hybridized with the digoxigenin-labeled cdt-B-V specific probe as previously described (2).
Stability at different temperatures and at different pH.
Aliquots of phage lysates containing 103 to 104 PFU of Cdt phages/ml and 106 PFU of SOM23/ml were placed in 50-ml tubes and incubated at 4, 22, and 37°C. For pH assays, the medium was adjusted to pH 3, 7, and 9 and confirmed before each sampling interval. Tubes were incubated for 1, 3, 7, 14, 21, and 28 days. Infectious Cdt phages and Cdt phage particles were determined at each interval.
Inactivation experiments.
Aliquots of phage lysates containing approximately 104 to 105 PFU of Cdt phages/ml, 104 to 106 PFU of SOM23/ml, and 105 to 106 CFU of Cdt-STEC strain 125/ml were used for the inactivation experiments. For thermal treatment, the phages and bacteria were placed into a water bath at 60 and 70°C. The samples were removed after 30 and 60 min. As a source of UV radiation, an 8-W germicidal lamp that emitted monochromatic UV radiation at 253.7 nm (G8T5.2N; Sankyo Denk, Tokyo, Japan) was used (31). Samples (5 ml) were placed 5 cm from the lamp in sterile petri dishes (90 mm in diameter) and irradiated for 1, 5, 10, and 30 min.
For chlorine inactivation, phage lysates and bacteria were diluted 1:20 in double-distilled water, treated with 10-ppm chlorine supplied as sodium hypochlorite, and incubated for 1, 3, 5, 10, 20, and 30 min at room temperature (21°C). Residual chlorine was neutralized by adding 3% (wt/vol) sodium thiosulfate (12).
To assay inactivation in a mesocosm, phage lysates and bacterial cultures were diluted 1:10 in the well water used for these experiments. Suspensions containing phages or bacteria were placed into dialysis tubes (cutoff, 14 kDa), which were sealed and placed in an outdoor pond with a water volume of 60 m3, protected by a cage, at a depth of 20 cm. The pond was supplied with nonchlorinated water from a well and provided a habitat for some species of goldfish. The experiments were performed in July (temperature, 18.5 to 24°C; solar radiation, 23 MJ m−2) and January (temperature, 7 to 14°C; solar radiation, 8.5 MJ m−2). The pH (6.8) and turbidity (4.1 to 6.8 nephelometric turbidity units [NTUs]) were stable throughout the year. The tubes containing the phage lysates were sampled at various intervals and analyzed.
Analysis of water from the Llobregat River.
Aerobic bacteria were evaluated in TSA at 37°C for 18 h. E. coli was determined with the membrane filtration method (3). Somatic coliphages, proposed as suitable indicators of viral fecal pollution, were enumerated by using the ISO method (4). Cdt phages were isolated from 100 ml of the sample and evaluated using real-time qPCR.
Data analysis and statistics.
For each experiment, a linear regression of the values was calculated between the intervals containing measurable data (above the limit of detection), and the regression equation was generated using Excel software (Microsoft Excel 2010). The results present the average of the three regression-line slopes and their corresponding standard deviations for the data obtained in the three independent replicas of each experiment.
RESULTS
Induction of Cdt-V phages.
The two Cdt-V phages used in the present study were obtained from E. coli wild-type strains and from S. sonnei lysogens S(Φ62) and S(Φ125) (2). We confirmed that these phages were spontaneously induced from their respective lysogens, since no significant (P > 0.05) differences were observed in phage determinations (infectivity assays or qPCR) when using mitomycin C (0.5, 2, and 5 μg/ml), EDTA, or UV induction compared to the noninduced cultures. Since Cdt phages are known to carry only one cdt copy (5), the cdt gene copy (GC) values can be extrapolated to give the number of Cdt phages in each sample.
Stability of Cdt phages.
Cdt phages, obtained from lysogens without an inducing agent, were evaluated for infectivity (plaque assay and hybridization) and using real-time qPCR. The presence of cdt-V in two to five plaques positive by hybridization was further confirmed by conventional PCR and sequencing.
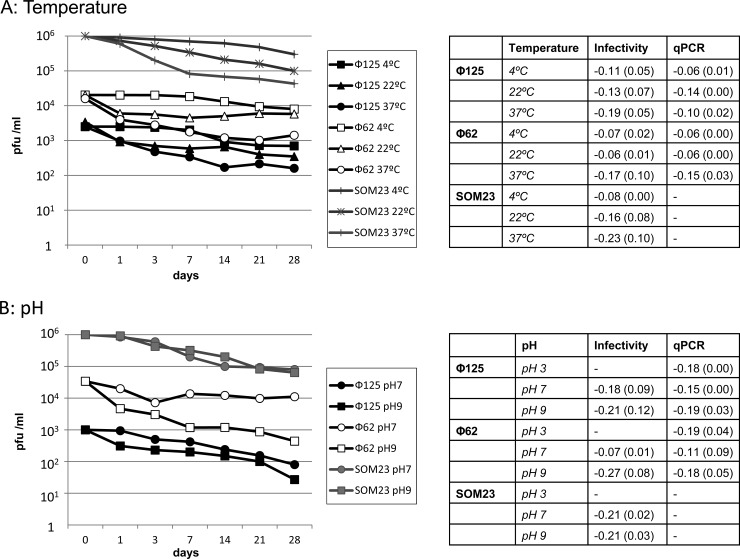
Similar reductions were observed over time for both Cdt phages and SOM23 (Fig. 1A). Storage at 4 and 22°C showed inactivation of <1 log10 units after 1 month of storage, and there were no significant differences between the temperatures (Student t test, P > 0.05). Phages showed less stability at 37°C and decreased more than 1 log10 unit after 1 month. qPCR showed a <1-log10 reduction in GC at the three temperatures after 1 month.
Fig 1.
Stability of Cdt phages and SOM23 at various temperatures (4, 22, and 37°C) (A) and different pHs (3, 7, and 9 after 1 month of storage at 4°C) (B). The results of infectivity presented (PFU/ml) for Φ62 and Φ125 correspond to plaque blot hybridization results. Infectivity of phage SOM23 was evaluated using plaque assays, which was included as a control. The charts show the results of one representative experiment. The tables present the averages (with the standard deviations [SD] indicated in parentheses) of the slopes of the logarithmic regression lines obtained in three replicas of each experiment for infectious phages and qPCR.
Stability at different pH values was evaluated at 4°C, since that temperature showed the lowest reduction in the number of infectious Cdt phages. When exposed to low pH (pH 3), all three phages lost their infectivity after only 1 day, and no plaques were observed; hence, pH 3 is not presented in the chart (Fig. 1B). Cdt phages presented remarkable stability at pH 7 and 9. At pH 7, the phages maintained their infectivity throughout the month, with inactivation at day 28 of only 1.1 (Φ125) and 0.5 (Φ62) log10 PFU/ml. Stability was also observed at pH 9, with reductions of only 1.6 and 1.7 log10 PFU/ml after 1 month (Fig. 1B). The qPCR reflected the stability at different pH with reductions of <1 log10 GC/ml after 1 month.
Inactivation treatments.
The resistance of the two Cdt phages and SOM23 to high temperatures (60 and 70°C) showed similar trends. When treated at 60°C, inactivation of infectious particles was of 1.3 log10 units at 60 min, whereas treatment at 70°C showed inactivation greater than 2.4 log10 units at 30 min and greater than 4.5 log10 units at 60 min. Cdt phage particles were still detectable by qPCR (Fig. 2A), and there was no remarkable reduction at any time interval or temperature, which confirms that the phage DNA remained intact. In contrast, a drastic reduction of 5 log10 units of CFU of E. coli strain 125 (Cdt-STEC) was observed after treatment at 60°C for 30 min.
Fig 2.
Persistence of Cdt phages Φ62 and Φ125, SOM23, and Cdt-STEC under thermal treatment at 60 and 70°C (A) to UV treatment (B) to 10 ppm of chlorine (C) through time. The charts on the left show logarithmic values of PFU, CFU or GC/ml. The tables on the right present the averages (SD) of the slopes of the logarithmic regression lines obtained in three replicas of each experiment. Dotted lines indicate values below the limit of detection.
Although the UV inactivation approach used does not allow calculation of the UV dose applied, it allows comparison between microorganisms. Cdt phages were very sensitive to the UV treatment used and showed reductions of infectious phages of 3.5 (Φ125), 3.1 (Φ62), and 4.0 (SOM23) log10 units after 30 min, but they were still more resistant than the Cdt-STEC strain (Fig. 2B), which showed a reduction of 5 log10 units after only 5 min. However, qPCR showed Cdt phage particles were not affected by the UV treatment, and GC did not decrease significantly (Student t test, P > 0.05).
Chlorination completely inactivated all parameters after 30 min of contact (Fig. 2C). However, Cdt phages took more than 20 min to decrease by 5 log10 units compared to 1 min for Cdt-STEC. Nevertheless, the strongest inactivation of phages was observed after 1 min of contact. qPCR values of GC/ml showed inactivation below 1 log10 unit after 30 min.
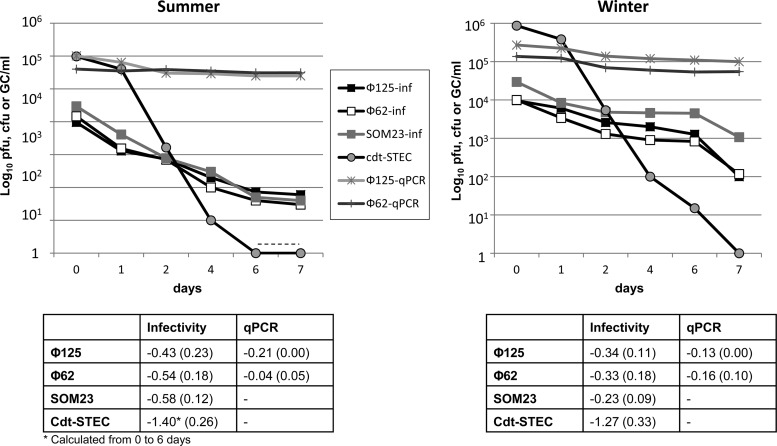
The inactivation of Cdt phages, SOM23, and Cdt-STEC in a mesocosm varied with season (Fig. 3). All of the microorganisms inactivated significantly faster (Student t test, P < 0.05) in summer than in winter. Inactivation of Cdt-STEC was faster than that of Cdt phages, whose inactivation was 2.7 and 2.2 log10 units for Φ125 and Φ62, respectively, in summer and 1.9 log10 units for both in winter. These inactivation values were similar to those of the control phage SOM23. qPCR values for both Cdt phages showed a very small reduction (Fig. 3) below 0.6 log10 units throughout the experiment in summer and below 0.4 log10 units in winter.
Fig 3.
Persistence of Cdt phages (qPCR and infectivity), SOM23 (plaque assay) and Cdt-STEC (colony counts) to inactivation processes in a mesocosm in summer and winter. Each chart shows the results of one representative experiment in each season. The table presents the averages (SD) of the slopes of the equation of the logarithmic regression lines obtained with three replicas of the experiment. The dotted line indicates values below the limit of detection.
Quantification of free Cdt phages in samples from the Llobregat River.
Eleven river-water samples were collected from the Llobregat River, a watercourse that receives mostly human fecal pollution. The samples showed fecal contaminants 4 log10 units below the levels present in wastewater samples from the same area (Table 1) (2). Cdt phages were detected by qPCR in 72.7% of river samples, which confirmed the circulation of Cdt phages in the environment.
Table 1.
Cdt phages sdetection in Llobregat River water
| Sample (n = 11) parameter | Bacterial indicators |
Bacteriophages |
||
|---|---|---|---|---|
| Aerobic bacteria (CFU/100 ml) | E. coli (CFU/100 ml) | Somatic coliphages (PFU/100 ml) | Cdt phages (GC/100 ml)a | |
| % Positive | 100 | 100 | 100 | 72.7 |
| No. | ||||
| Avg | 5.13 × 105 | 1.65 × 103 | 2.63 × 104 | 1.14 × 101 |
| Max | 6.32 × 105 | 5.21 × 103 | 5.51 × 104 | 1.9 × 101 |
| Min | 1.93 × 104 | 9.30 × 102 | 1.02 × 104 | 6.50 × 100 |
GC, gene copies.
DISCUSSION
The induction of Cdt phages in the present study does not seem to be stimulated by any inducing agent such as mitomycin C, UV light, or EDTA (16, 24, 28), in contrast to the behavior of the Cdt-I phage (5), which is induced by mitomycin C. Spontaneous induction of Cdt phages suggests that the diffusion of induced Cdt phages should be greater than for those phages that require specific conditions for their induction.
In previous work, Cdt phages were detected in wastewater samples (2), revealing the environmental presence of these phages released directly from the gut or induced from Cdt-positive bacteria. Here, Cdt phages were detected in 72.7% of river samples with lower levels of contamination than wastewater and primarily affected by human waste, indicating that the Cdt phages detected probably had an anthropogenic source. The potential capacity of Cdt phages to transduce cdt is a cause for some concern because of the potential conversion of nonlysogenic strains to Cdt-producing ones. Evaluating the persistence of Cdt phages in extraintestinal environments is necessary to provide insight into the spread and mobilization of cdt in the environment.
The persistence of Cdt phages was evaluated by qPCR, as well as by phage infectivity. The qPCR results do not necessarily correlate with infectivity of phages, which is essential for potential cdt transduction. Hybridization was also necessary for an accurate enumeration of Cdt-positive plaques, to discriminate them from other phages (for instance Stx phages) that may have been present in the phage lysates (2).
Cdt phages remain stable after 1 month of storage at various temperatures and pH values. Temperature determines the occurrence and viability of the phages in different environments. In terms of pH, some phages persist well in acidic environments (19), but this is not the case for Cdt phages, which lose their infectivity when stored for 1 day at pH 3. In contrast, Cdt phages retain their infectivity better at pH 7 and 9, which is in line with reports of λ phage (17).
When assaying different inactivation conditions, there was a clear gradation in resistance, with bacteria being much more susceptible to inactivation than phages, as extensively reported for other phages (9, 12, 25, 26). Assuming that the natural host of Cdt-V phages is E. coli, their higher stability suggests that phages are the natural reservoir of cdt in the environment. Inactivation of Cdt phages showed similar trends to that of SOM23. This may be due to the fact that it is a tailored phage, which has been described as highly stable under different conditions (1, 11, 20). The results for Cdt phage inactivation were also in agreement with reports on other E. coli phages, either for thermal treatment (8, 20, 23), UV treatment (20, 31), or chlorination (10, 12, 26). Chlorinating water with 10 ppm of chlorine would provide water with no bacteria, which would be considered suitable for consumption according to current practices in many parts of the world. However, Cdt phages may still be detected in the chlorinated samples.
Inactivation of phages in a mesocosm provides the simultaneous analysis of different factors, except probably for the effect of grazing. However, protozoa grazing seems to be restricted to larger microorganisms than viruses, and the effect of grazing on phage numbers is not clear (14). It is difficult to determine which factor is the main cause of inactivation, although a detailed analysis may provide some insight. The pond did not experience pH variations during the experiment. In summer, all of the microorganisms were inactivated faster than in winter. The main differences in the physical factors between summer and winter were temperature and solar irradiation. The low inactivation of Cdt phages at similar temperatures, especially after 7 days, suggests that irradiation is the differential factor for the inactivation of Cdt phages in the mesocosm. Although our UV experiments cannot be compared to solar UV doses, UV light is a recognized inactivating factor (9, 11, 25).
Bacteriophages are an important source of new genetic variants. It is important to find out more about the induction and dissemination of extraintestinal phages in order to control the emergence of new pathogenic strains. The persistence shown by Cdt phages indicates that they spread in the environment and act as mobile vehicles of cdt, as observed previously for other bacteriophages harboring virulence genes, such as Shiga toxin phages (25).
ACKNOWLEDGMENTS
This study was supported by the Generalitat de Catalunya (2009SGR1043), the Spanish Ministry of Education and Science (AGL2009-07576), the Xarxa de Referència en Biotecnologia, and the bacteriophage network FAGOMA. A.A.-G. has an FI grant from the Generalitat de Catalunya (Spain).
Footnotes
Published ahead of print 8 June 2012
REFERENCES
- 1. Ackermann HW, Tremblay D, Moineau S. 2004. Long-term bacteriophage preservation. WFCC Newsl. 38:35–40 [Google Scholar]
- 2. Allué-Guardia A, GarcíA-Aljaro C, Muniesa M. 2011. Bacteriophage-encoding cytolethal distending toxin type V gene induced from nonclinical Escherichia coli isolates. Infect. Immun. 79:3262–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous 1998. Standard methods for the examination of water and wastewater, 20th ed American Public Health Association, American Works Association and Water Environmental Federation, Washington, DC [Google Scholar]
- 4. Anonymous 2000. ISO 10705-2: water quality: detection and enumeration of bacteriophages. 2. Enumeration of somatic coliphages. International Organisation for Standardisation, Geneva, Switzerland [Google Scholar]
- 5. Asakura M, et al. 2007. An inducible lambdoid prophage encoding cytolethal distending toxin (Cdt-I) and a type III effector protein in enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 104:14483–14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baggi F, Demarta A, Peduzzi R. 2001. Persistence of viral pathogens and bacteriophages during sewage treatment: lack of correlation with indicator bacteria. Res. Microbiol. 152:743–751 [DOI] [PubMed] [Google Scholar]
- 7. Boyd EF, Brüssow H. 2002. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 10:521–529 [DOI] [PubMed] [Google Scholar]
- 8. Cunault C, Pourcher AM, Burton CH. 2011. Using temperature and time criteria to control the effectiveness of continuous thermal sanitation of piggery effluent in terms of set microbial indicators. J. Appl. Microbiol. 111:1492–1504 [DOI] [PubMed] [Google Scholar]
- 9. Davies-Colley RJ, Donnison AM, Speed DJ, Ross CM, Nagels JW. 1999. Inactivation of faecal indicator microorganisms in waste stabilization ponds: interactions of environmental factors with sunlight. Water Res. 33:1220–1230 [Google Scholar]
- 10. Dee SW, Fogelman JC. 1992. Rates of inactivation of waterborne coliphages by monochloramine. Appl. Environ. Microbiol. 58:3136–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durán AE, et al. 2002. Removal and inactivation of indicator bacteriophages in fresh waters. J. Appl. Microbiol. 92:338–347 [DOI] [PubMed] [Google Scholar]
- 12. Durán AE, et al. 2003. Usefulness of different groups of bacteriophages as model microorganisms for evaluating chlorination. J. Appl. Microbiol. 95:29–37 [DOI] [PubMed] [Google Scholar]
- 13. Ge Z, Schauer DB, Fox JG. 2008. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell Microbiol. 10:1599–1607 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez JM, Sherr EB, Sherr BF. 1990. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl. Environ. Microbiol. 56:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haghjoo E, Galan JE. 2004. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. U. S. A. 101:4614–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imamovic L, Muniesa M. 2012. Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PLoS One 7:e32393 doi:10.1371/journal.pone.0032393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jepson CD, March JB. 2004. Bacteriophage lambda is highly stable DNA vaccine delivery vehicle. Vaccine 22:3413–3419 [DOI] [PubMed] [Google Scholar]
- 18. Johnson WM, Lior H. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb. Pathog. 4:115–126 [DOI] [PubMed] [Google Scholar]
- 19. Jończyk E, Kłak M, Miêdzybrodzki R, Górski A. 2011. The influence of external factors on bacteriophages-review. Folia Microbiol. (Praha) 56:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee HS, Sobsey MD. 2011. Survival of prototype strains of somatic coliphage families in environmental waters and when exposed to UV low-pressure monochromatic radiation or heat. Water Res. 45:3723–3734 [DOI] [PubMed] [Google Scholar]
- 21. Martínez-Castillo A, et al. 2012. Type III effector genes and other virulence factors of Shiga toxin-encoding Escherichia coli isolated from wastewater. Environ. Microbiol. Rep. 4:147–155 [DOI] [PubMed] [Google Scholar]
- 22. Mayer MP, Bueno LC, Hansen EJ, DiRienzo JM. 1999. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect. Immun. 67:1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mocé-Llivina L, Muniesa M, Pimenta-Vale H, Lucena F, Jofre J. 2003. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol. 69:1452–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mühldorfer I, et al. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect. Immun. 64:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muniesa M, Lucena F, Jofre J. 1999. Comparative survival of free Shiga toxin 2-encoding phages and Escherichia coli strains outside the gut. Appl. Environ. Microbiol. 65:5615–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muniesa M, Lucena F, Jofre J. 1999. Study of the potential relationship between the morphology of infectious somatic coliphages and their persistence in the environment. J. Appl. Microbiol. 87:402–409 [DOI] [PubMed] [Google Scholar]
- 27. Muniesa M, Mocé-Llivina L, Katayama H, Jofre J. 2003. Bacterial host strains that support replication of somatic coliphages. Antonie Van Leeuwenhoek 83:305–315 [DOI] [PubMed] [Google Scholar]
- 28. Mustard JA, Little JW. 2000. Analysis of Escherichia coli RecA interactions with LexA, λ CI, and UmuD by site-directed mutagenesis of recA. J. Bacteriol. 182:1659–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okuda J, Kurazono H, Takeda Y. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167–172 [DOI] [PubMed] [Google Scholar]
- 30. Smith JL, Bayles DO. 2006. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 32:227–248 [DOI] [PubMed] [Google Scholar]
- 31. Tartera C, Bosch A, Jofre J. 1988. The inactivation of bacteriophages infecting Bacteroides fragilis by chlorine treatment and UV-irradiation. FEMS Microbiology Lett. 56:313–316 [Google Scholar]
- 32. Tóth I, et al. 2009. Cytolethal distending toxin type I and type IV genes are framed with lambdoid prophage genes in extraintestinal pathogenic Escherichia coli. Infect. Immun. 77:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Young VB, et al. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620–623 [DOI] [PubMed] [Google Scholar]