Abstract
In this study with the model organism Agrobacterium tumefaciens, we used a combination of lacZ gene fusions, reverse transcriptase PCR (RT-PCR), and deletion and insertional inactivation mutations to show unambiguously that the alternative sigma factor RpoN participates in the regulation of AsIII oxidation. A deletion mutation that removed the RpoN binding site from the aioBA promoter and an aacC3 (gentamicin resistance) cassette insertional inactivation of the rpoN coding region eliminated aioBA expression and AsIII oxidation, although rpoN expression was not related to cell exposure to AsIII. Putative RpoN binding sites were identified throughout the genome and, as examples, included promoters for aioB, phoB1, pstS1, dctA, glnA, glnB, and flgB that were examined by using qualitative RT-PCR and lacZ reporter fusions to assess the relative contribution of RpoN to their transcription. The expressions of aioB and dctA in the wild-type strain were considerably enhanced in cells exposed to AsIII, and both genes were silent in the rpoN::aacC3 mutant regardless of AsIII. The expression level of glnA was not influenced by AsIII but was reduced (but not silent) in the rpoN::aacC3 mutant and further reduced in the mutant under N starvation conditions. The rpoN::aacC3 mutation had no obvious effect on the expression of glnB, pstS1, phoB1, or flgB. These experiments provide definitive evidence to document the requirement of RpoN for AsIII oxidation but also illustrate that the presence of a consensus RpoN binding site does not necessarily link the associated gene with regulation by AsIII or by this sigma factor.
INTRODUCTION
Evidence of microbial arsenite (AsIII) oxidation was first reported nearly a century ago (18). Subsequent progress has been sporadic, with work that identified some organisms capable of AsIII oxidation (46, 48, 60) and then a study of a Pseudomonas arsenitoxidans strain reported to grow chemolithoautotrophically with AsIII as a sole electron donor (23). Subsequent follow-up characterizations of this organism and this process failed to materialize; however, approximately 2 decades later, Santini et al. (52) described the isolation and initial characterization of a Rhizobium-like bacterium (strain NT-26) that could grow chemolithoautotrophically with AsIII as a sole electron donor for energy generation and with CO2 as a sole carbon source. Soon thereafter, and in part stimulated by the massive arsenic poisoning disaster in Bangladesh (2), a series of studies initiated the characterization of microbial AsIII oxidation in natural environments, including geothermal springs (9, 11, 12, 17, 19, 24, 25, 35, 51) and soils (41); in mining-contaminated environments (6, 13, 40); and, most recently, in anoxic photosynthesis (21, 33). Likewise, progress has been made in the understanding of the biochemistry of the AsIII oxidase enzyme (1, 14, 37).
Studies examining the genetics and regulatory control of AsIII oxidation have advanced, particularly within the past decade. Muller et al. (43) cloned and described the genes from a betaproteobacterium (subsequently identified as Herminiimonas arsenicoxydans [44]) that encode the small (aoxA [now aioB]) and large (aoxB [now aioA]) subunits of the AsIII oxidase enzyme. The AsIII oxidase structural genes were later cloned from the above-mentioned Rhizobium NT-26 organism (53). The symbols for genes coding for functions associated with AsIII oxidation have recently been changed from aox, aro, or aso to aio (36), and we will use this new aio gene nomenclature throughout this report and in the future.
Kashyap et al. (28) used random transposon mutagenesis to identify and characterize a two-component signal transduction pair, aioS and aioR, that is immediately adjacent to the aioBA genes in an Agrobacterium tumefaciens soil isolate, defining what was assessed at the time to be the aio operon. Parallel studies by Kashyap et al. (29) also identified a molybdate transporter and an Na+/H+ antiporter that were also found to be essential for AsIII oxidase oxidation. Later, and using a similar transposon mutagenesis approach, Koechler et al. (31) also identified the aioSR two-component pair and molybdate transporter as being essential for AsIII oxidation. While transposon mutation-based experiments (28, 31) indicated the role and importance of the sensor kinase AioS and its putative regulatory partner AioR (a bacterial enhancer binding protein), direct proof of these two proteins working together as part of a putative AsIII signal perception and transduction cascade was just recently provided by Sardiwal et al. (54), who demonstrated the autophosphorylation of an AioS component and the AioS-specific phosphorylation of AioR. Recently, our work has expanded this regulatory model to now include a third component, AioX, which is a periplasmic AsIII binding protein that is also essential for aioBA expression (39).
Koechler et al. (31) also isolated rpoN::Tn5::lacZ2 and dnaJ::Tn5::lacZ2 mutants that were defective in AsIII oxidation, although those observations were not accompanied by complementation experiments to demonstrate that the loss of function with these mutants was due to the interrupted genes as opposed to polar effects of the transposon on the transcription of adjacent downstream genes. However, ancillary data presented by Koechler et al. (31) provided additional indirect evidence of the rpoN gene product (also referred to as σ54, σN, and NtrA) being important for aioBA expression. In the current study, with A. tumefaciens strain 5A, we focused on the importance of RpoN, by introducing specific and precise mutations that removed the putative RpoN binding site immediately upstream of the aioBA genes as well as insertionally inactivating rpoN. In addition, we examined the genome for additional putative RpoN-dependent promoters to investigate the role and importance of this sigma factor in AsIII-dependent and AsIII-independent expression. We provide unambiguous evidence that RpoN is required for AsIII oxidation but apparently not for the expressions of other genes associated with a consensus RpoN promoter.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used for this study are listed in Table 1. The A. tumefaciens strains were cultured at 30°C in a defined minimal mannitol medium (MMN) (58) containing mannitol as a carbon and energy source and 50 μM phosphate, with aeration by shaking at 200 rpm. For some experiments, log-phase MMN-grown cells were washed via centrifugation and resuspended in MMN that lacked ammonium to simulate N starvation. Escherichia coli strains Top10 and S17-1 were grown at 37°C in Luria-Bertani (LB) medium. Bacterial growth was monitored via measurements of the culture optical density at 595 nm (OD595) by use of a SpectraMax (Molecular Devices, CA) microtiter plate reader. Where indicated, A. tumefaciens growth media were amended with 80 μg ml−1 gentamicin (Gen) and/or 15% sucrose for the selection of double recombinants by using levansucrase selection (see below). Where required for mutant construction, E. coli cells were grown with 20 μg ml−1 Gen. The genome of A. tumefaciens 5A has been sequenced and deposited in the NCBI database (20).
Table 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer pair | Relevant marker(s) and/or characteristic(s) or sequence | Reference, source, or purpose |
|---|---|---|
| Bacterial strains | ||
| Agrobacterium tumefaciens | ||
| 5A | Wild type, soil isolate, AsIII oxidizing | 41 |
| 5A(ΔPaioB) | aioB promoter region deletion mutant | This study |
| 5A(rpoN::aacC3) | Genr; rpoN mutant by gentamicin cassette interruption | This study |
| 5A(rpoN::aacC3)(pCPP30-rpoN) | Genr Tetr; rpoN::aacC3 complemented strain | This study |
| 5A(PrpoN) | Kmr; 5A with pLSP-PrpoN | This study |
| 5A(PaioB) | Kmr; 5A with pLSP-PaioB | Laboratory stock |
| 5A(PphoB1) | Kmr; 5A with pLSP-PphoB1 | Laboratory stock |
| 5A(PpstS1) | Kmr; 5A with pLSP-PpstS1 | Laboratory stock |
| Escherichia coli | ||
| S17-1 | Pro− Mob+; conjugation donor | 57 |
| Top10 | High-competency cloning host | Invitrogen |
| Plasmids | ||
| pCR2.1-TOPO | PCR TA cloning vector | Invitrogen |
| pCPP30 | Broad host range; tetA | 22 |
| pJQ200sk | Genr traJ oriT sacB; suicide vector | 49 |
| pLSP-kt2lacZ | Kmr oriV; lacZ fusion vector used for lacZ fusion constructs | L. Pierson, Texas A&M University |
| pCR2.1-PaioB | pCR2.1-TOPO with PaioB region | Laboratory stock |
| pCR2.1-rpoN | pCR2.1-TOPO with rpoN region | This study |
| pJQ200sk-PaioB | pJQ200sk with deleted PaioB region | This study |
| pJQ200sk-rpoN::aacC3 | pJQ200sk with rpoN gene interrupted with gentamicin cassette | This study |
| pCPP30::rpoN | pCPP30 containing the complete rpoN gene | This study |
| pLSP-PrpoN | pLSP-kt2lacZ containing rpoN promoter region | This study |
| pLSP-PaioB | pLSP-kt2lacZ containing aioB promoter region | Laboratory stock |
| pLSP-PphoB1 | pLSP-kt2lacZ containing phoB1 promoter region | Laboratory stock |
| pLSP-PpstS1 | pLSP-kt2lacZ containing pstS1 promoter region | Laboratory stock |
| Primer pairs | ||
| rpoN-f/rpoN-r (2,124 bp) | 5′-GGCGTTCTCATCACCGACCAC-3′/5′-TTTACCAGATACACGCACACTCAT-3′ | For construction of rpoN::aacC3 |
| PaioBMu-1f/PaioBMu-1r (540 bp) | 5′-GCAGCGACGCCAGTTCCTT-3′/5′-CCCATCCACTAAACTTAAACAGTGTGGGGGTTCGGTTTTC-3′ | For deletion of aioB promoter |
| PaioBMu-2f/PaioBMu-2r (547 bp) | 5′-TGTTTAAGTTTAGTGGATGGGCTTCGACATTCAGTGGAGGAG-3′/5′-CGTTGGACAGGCGGCCGTAGATGA-3′ | For deletion of aioB promoter |
| PrpoN-f/PrpoN-r (454 bp) | 5′-GGCGTTCTCATCACCGACCAC-3′/5′-GATTGCGCTCCACCTCCTG-3′ | For construction of PrpoN::lacZ |
| B22Gm-f/B22Gm-r (471 bp) | 5′-ACAAAGTTAGGTGGCTCAA-3′/5′-TGGGTCGATATCAAAGTGC-3′ | Gentamicin cassette-specific primer |
| P4/P5 | 5′-GACGTTGCCTATCCCGATGAAGAT-3′/5′-GTTTGTTGATTGGCCAGGTGTAGG-3′ | For RT-PCR of aioBa |
| phoB1-2f/phoB1-2r (361 bp) | 5′-TGTTTAAGTTTAGTGGATGGGAGGGGCGCCGGCTATTCAA-3′/5′-CGCTCTAGAGACACCGACGACCTCCCTCAG-3′ | For RT-PCR of phoB1 |
| flgBRT-f/flgBRT-r (313 bp) | 5′-CAACATCGCCAACGCCAACACA-3′/5′-CCTCTTTACCGTCATCAGCATCAT-3′ | For RT-PCR of flgB |
| nrtART-f/nrtART-r (400 bp) | 5′-CCGGCAAGGTGACGCAGAA-3′/5′-TCCCCGGTGGTACAGGCAGTGAA-3′ | For RT-PCR of nrtA |
| glnART-f/glnART-r (356 bp) | 5′-AGGCCACTCTTCGGATTG-3′/5′-CATTGATGCCTTCGTGGTTGAT-3′ | For RT-PCR of glnA |
| glnBRT-f/glnBRT-r (227 bp) | 5′-CAAGCTCGATGAAGTGAAGG-3′/5′-CGATACGTCCGGTCTGTGC-3′ | For RT-PCR of glnB |
| dctART-f/dctART-r (359 bp) | 5′-CGACAAGGCCCATGAGCAGACCA-3′/5′-CAGCGCCGAGGACCACGAACAC-3′ | For RT-PCR of dctA |
See reference 28.
Construction of A. tumefaciens 5A mutant strains.
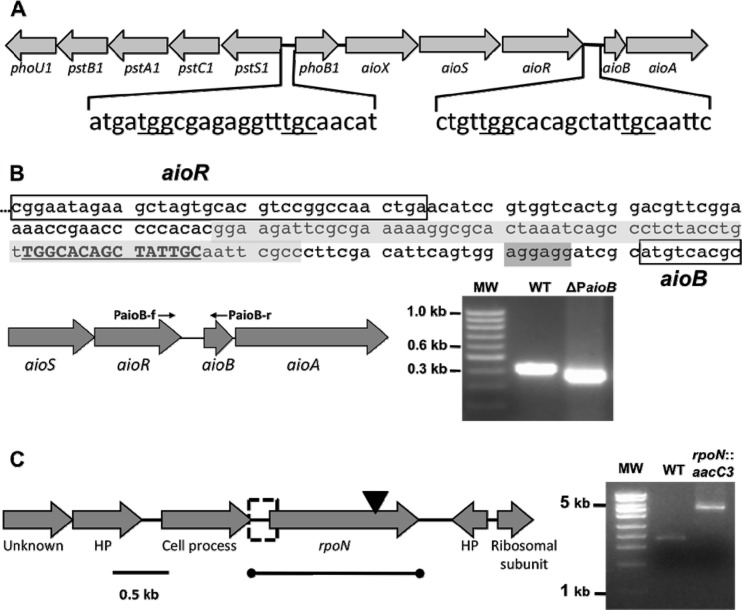
Primers used for the construction of A. tumefaciens deletion mutations, the insertional inactivation mutation, as well as the lacZ reporter fusions are provided in Table 1. The deletion of the RpoN binding site was constructed by crossover PCR as described previously (38) and by use of a levansucrase resistance strategy that we described previously (59). Briefly, the 497-bp C-terminal region of aioR was PCR amplified by using primers PaioBMu-1F and PaioBMu-1R (540-bp amplicon that includes 43 bp of the intergenic region). A second PCR amplified the region from 27 bp upstream of the aioB translational start site using primers PaioBMu-2F and PaioBMu-2R. A mixture of these two amplicons was used as the template for a third PCR that included primers PaioBMu-1F and PaioBMu-2R, yielding a product that contained a 67-bp deletion that removed the RpoN binding region of the aioB promoter (Fig. 1B). This product was ligated into pCR2.1-TOPO, yielding plasmid pCR2.1-PaioB. The 1,087-bp fragment was then subcloned into a BamHI- and XbaI-digested pJQ200sk vector, giving pJQ200sk-PaioB. This plasmid was transformed into E. coli S17-1 cells and then mobilized to A. tumefaciens 5A by conjugation. Merodiploids were selected on MMN agar plus Gen (MMNGen agar), with double crossovers then being selected on MMN agar containing 15% sucrose, which selects for the loss of the plasmid-borne sacB gene. Gentamicin-sensitive, sucrose-resistant colonies were screened and confirmed by PCR and sequencing, yielding the in-frame ΔPaioB deletion mutant.
Fig 1.
Physical arrangement and locations of genes involved in this study, and PCR-based evidence of mutation constructs. (A) Relative positions of the putative RpoN binding sites associated with the aioB gene and located between the phoB1 and pstS1 coding regions. (B) Sequence of the aioB promoter region that was deleted. The 3′ end of aioR and the 5′ end of aioB are boxed, and the ribosome binding site for aioB is highlighted in dark gray. The consensus RpoN binding site is highlighted with boldface uppercase type, and the region deleted (ΔPaioB mutant) is highlighted in light gray. In addition, PCR evidence to document the 67-bp deletion created in the aioB promoter region is provided. (C) Position of rpoN in strain 5A relative to the flanking genes, the approximate insertion point of the aacC3 cassette (2.5 kb) (inverted black arrowhead) in the rpoN coding region (1.48 kb), the region of DNA that was PCR cloned and used for complementation of the rpoN::aacC3 mutant, and the promoter region of rpoN used for the rpoN::lacZ reporter construct (dashed box). Also, PCR evidence documents the insertion of aacC3 in the rpoN gene (4.6-kb construct). HP, hypothetical protein; WT, wild type; MW, molecular weight marker.
Primer pair rpoN-f/rpoN-r was used to construct an insertion mutation in the rpoN coding region. The 2.1-kb amplified fragment was ligated and cloned into pCR2.1-TOPO, creating pCR2.1-rpoN. The rpoN gene harbored by pCR2.1-rpoN was then inactivated by the insertion of a gentamicin cassette (aacC3) at a ClaI site at bp 1378. The gentamicin cassette was prepared by the digestion of Tn5-B22 with BamHI and HindIII, polished with Klenow fragment (Promega) to create blunt ends, and ligated into the Klenow fragment-treated ClaI site. The rpoN::aacC3 construct (4.6 kb) was then cloned into the BamHI and XbaI cloning sites of pJQ200sk, creating pJQ200sk-rpoN::aacC3, which was then transformed into S17-1 cells, followed by conjugation into strain 5A. Mutants wherein the wild-type allele was replaced by this construct were generated by using the above-described levansucrase selection strategy. For the complementation of the rpoN::aacC3 insertion mutant, the same rpoN reading frame and upstream promoter region were PCR cloned by using primer pair rpoN-f/rpoN-r. The rpoN fragment from pCR2.1-rpoN was subcloned into the EcoRI cloning site of the pCPP30 vector, creating pCPP30-rpoN, which was transformed into S17-1 cells and then introduced into the rpoN::aacC3 mutant by conjugation.
Arsenic chemical species analysis.
AsIII oxidation was assayed in the wild type and the rpoN::aacC3 mutant by using HPLC-ICP-MS (high-performance liquid chromatography–inductively coupled plasma mass spectrometry) (Agilent 7500) to separate (ammonium carbonate mobile phase at a flow rate of 1 ml min−1) and quantify AsIII and AsV in culture supernatants. For calibration, standard solutions of 25, 50, 75, and 100 μM combined AsIII and AsV were employed. Cell-free culture fluids (0.22-μm filtration of centrifugation supernatants; Millipore) from cells grown in low-phosphate MMN containing 100 μM AsIII were harvested at time zero and at the log and stationary phases.
Promoter activity analysis.
The aioB, rpoN, phoB1, and pstS1 promoter regions of the A. tumefaciens 5A genome were amplified by PCR using primers (Table 1) that incorporated EcoRI and BamHI restriction sites that allowed for directional cloning into lacZ reporter plasmid pLSP-kt2lacZ (Table 1). The resulting constructs were designated pLSP-PaioB, pLSP-PrpoN, pLSP-PphoB1, and pLSP-PpstS1, respectively, and were used for the analysis of promoter activity after the introduction of each construct into A. tumefaciens wild-type strain 5A via conjugation using E. coli strain S17-1. The lacZ reporter assays used cells grown in 50 ml of MMN with kanamycin under various conditions (with or without AsIII) and incubated at 30°C on a rotary shaker at 200 rpm. After the various incubation times, 1-ml aliquots from each flask were collected and used for assays of β-galactosidase activity according to a method described previously by Miller (42).
Reverse transcriptase PCR analysis of gene expression.
Cells were incubated in MMN with or without 100 μM AsIII for 4 h. The total RNA of the cells (OD595 of ∼0.2 to 0.3) was extracted and purified with an RNeasy kit (Qiagen) in accordance with the manufacturer's instructions. DNA contamination was removed by DNase I treatment and cleanup with a Turbo DNA-free kit (Ambion). The mRNA was then reverse transcribed into cDNA by using avian myeloblastosis virus (AMV) reverse transcriptase (RT) (Promega) with gene-specific primers (Table 1), according to the manufacturer's instructions, and with 500 ng RNA as the template in each RT reaction. PCR programs were standard, with primer-specific annealing temperatures, but all programs included final extension steps performed at 68°C for 5 min.
RESULTS
RpoN is required for AsIII oxidation.
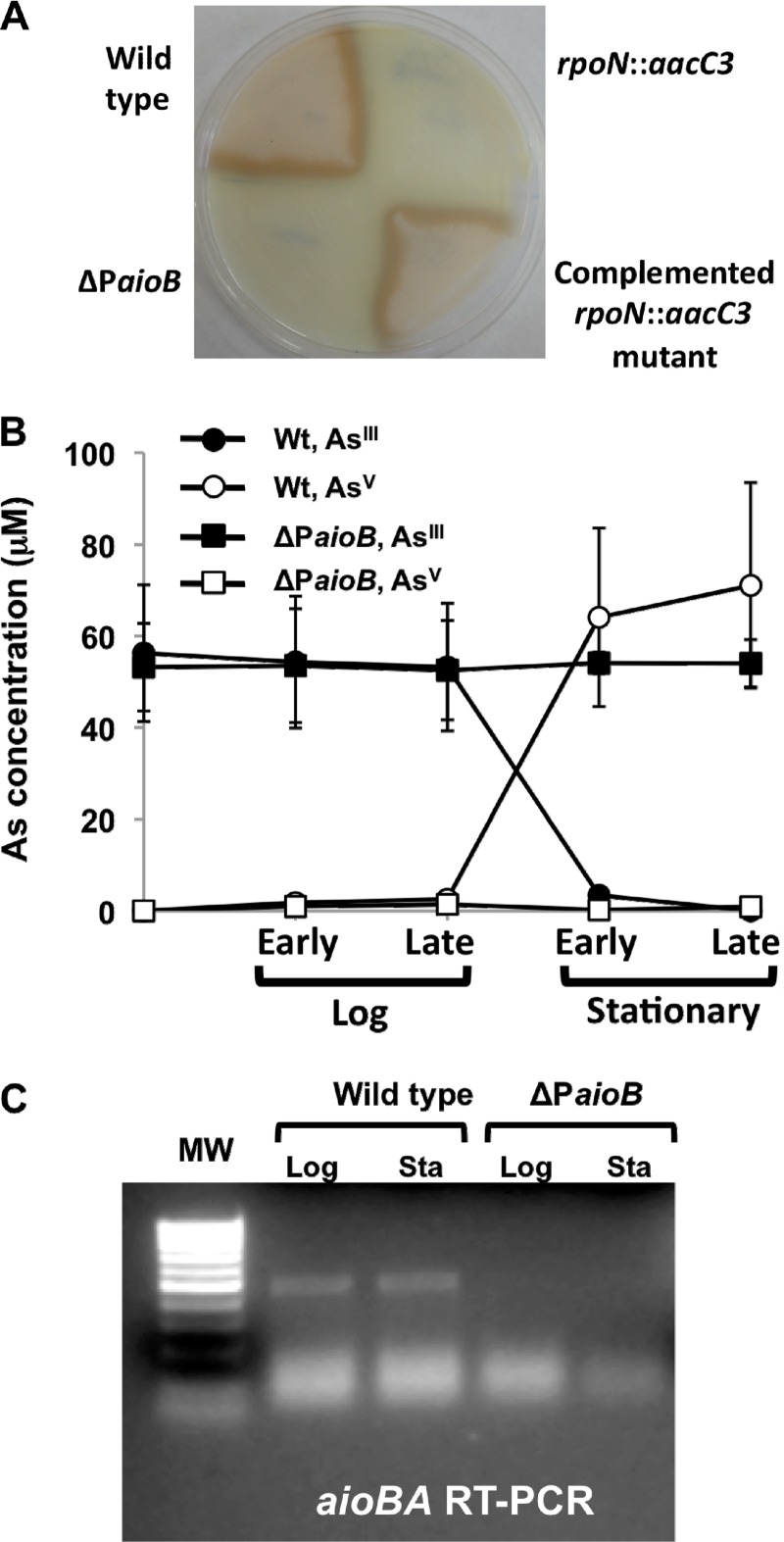
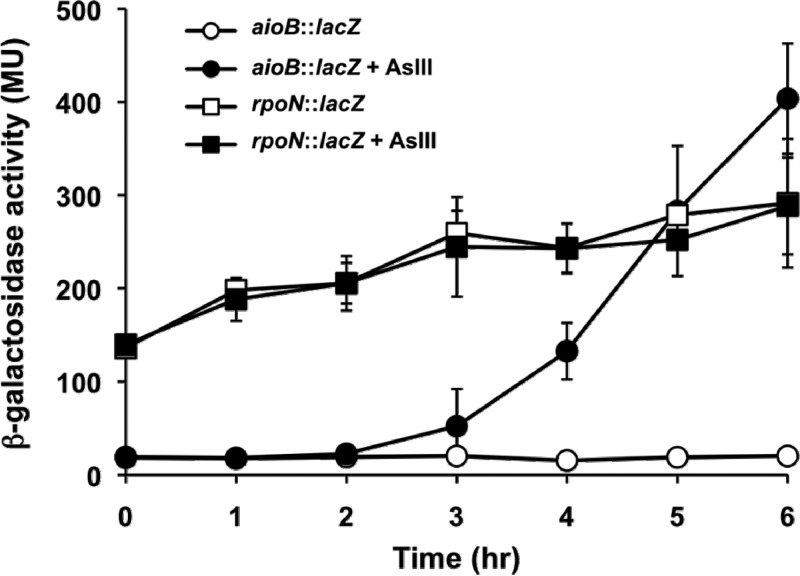
Kashyap et al. (28) previously described an aio gene cluster comprised of aioS-aioR-aioB-aioA-cyt-moeA and provided RT-PCR evidence for these genes being expressed in a monocistronic fashion. Those authors also described the isolation of an aioR mutant having a Tn5-B22 transposon inserted into the 3′ region of the coding frame and which was interpreted as having polar effects on the transcription of the AsIII oxidase-encoding genes aioBA. However, the intergenic region separating aioR and aioB (Fig. 1) was noted to be significant, perhaps accommodating another independently regulated promoter. This was consistent with a previous suggestion by Sardiwal et al. (54) and an earlier personal communication with J. M. Santini that a σ54-dependent promoter was directly upstream of the Rhizobium NT-26 aioBA genes. An analysis of this region in A. tumefaciens strain 5A yielded a similar conclusion (Fig. 1A), and thus, experiments were designed to assess this possibility. Specifically, a deletion mutation was precisely constructed in the region directly upstream of aioB which included the entire predicted RpoN binding site but which excluded any of the flanking coding frames for aioR or aioB and likewise excluded the putative ribosome binding site for aioB (Fig. 1B); this mutant is referred to as the ΔPaioB mutant. In parallel, the wild-type rpoN allele in strain 5A was replaced with an insertionally inactivated rpoN allele (rpoN::aacC3), creating an RpoN− mutant (Fig. 1C). Both mutations yielded organisms incapable of oxidizing AsIII (Fig. 2A and B), but the complementation of the rpoN::aacC3 mutant with a defined region of DNA that included only the rpoN reading frame and the upstream promoter region (Fig. 1C) reversed the defect back to wild-type AsIII oxidation (Fig. 2A). Further analyses of the ΔPaioB deletion mutant illustrated that the expression of aioBA was silenced (Fig. 2C). An analysis of aioB::lacZ and rpoN::lacZ reporters illustrated the expected AsIII-dependent expression of aioBA, whereas rpoN expression appeared to be constitutive and not influenced by AsIII (Fig. 3).
Fig 2.
AsIII oxidation phenotype of A. tumefaciens wild-type strain 5A and mutants described in this study. (A) Qualitative assessment of AsIII oxidation using the AgNO3 staining method for wild-type strain 5A, the ΔPaioB mutant, the rpoN::aacC3 mutant, and the rescued rpoN::aacC3 mutant carrying pCPP30::rpoN. The brown precipitate indicates the presence of AsV. (B) HPLC-ICP-MS-based measurement of aqueous AsIII and AsV in AsIII oxidation assays with wild-type strain 5A and the ΔPaioB mutant. Error bars represent the standard deviations of data from two replicate cultures. (C) RT-PCR monitoring of aioBA expression in the wild type and the ΔPaioB mutant in the log and stationary phases of cells grown in the presence of 100 μM AsIII.
Fig 3.
Reporter gene assays to track the expressions of aioB and rpoN as a function of time after the addition (or not) of 100 μM AsIII. β-Galactosidase activity is presented as Miller units (MU), and error bars represent the standard deviations of data from two replicate cultures.
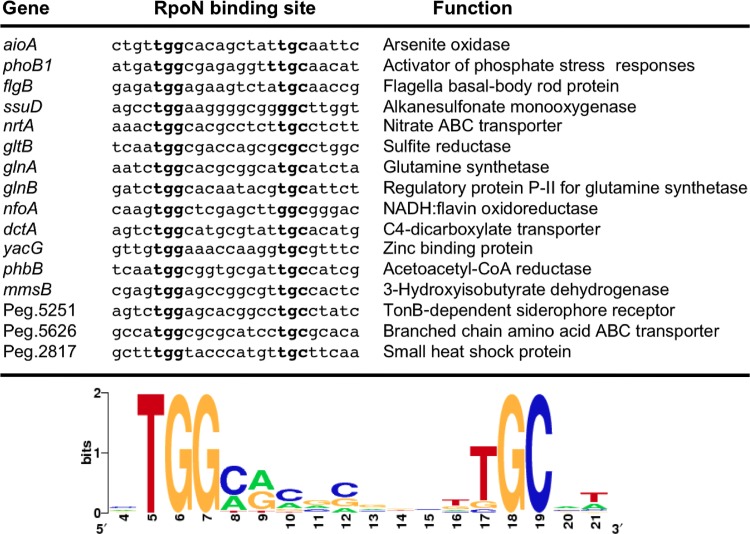
An examination of the strain 5A genome for genes/operons known in other bacteria to be controlled by RpoN found putative RpoN binding sites associated with 16 genes coding for a variety of functions and which displayed the well-defined GG-(N10)-GC consensus RpoN binding site (Fig. 4). For all of these genes, the putative RpoN binding sites contained a perfectly conserved thymine at the 5′ end and a near-perfect consensus addition of a thymine at the 3′ end (Fig. 4). These genes code for functions associated with the phosphate stress response (phoB1 and pstS1), flagellum construction (flgB), nitrogen metabolism (nrtA, glnA, and glnB), poly-β-hydroxybutyrate synthesis (phbB), and others (Fig. 4).
Fig 4.
Alignment of the RpoN binding sites in the promoter regions from a sample of genes of the genome of strain 5A. Nucleotides highly conserved are highlighted in boldface type, and the consensus sequence depicting the relative conservation of each predicted position is shown at the bottom. CoA, coenzyme A.
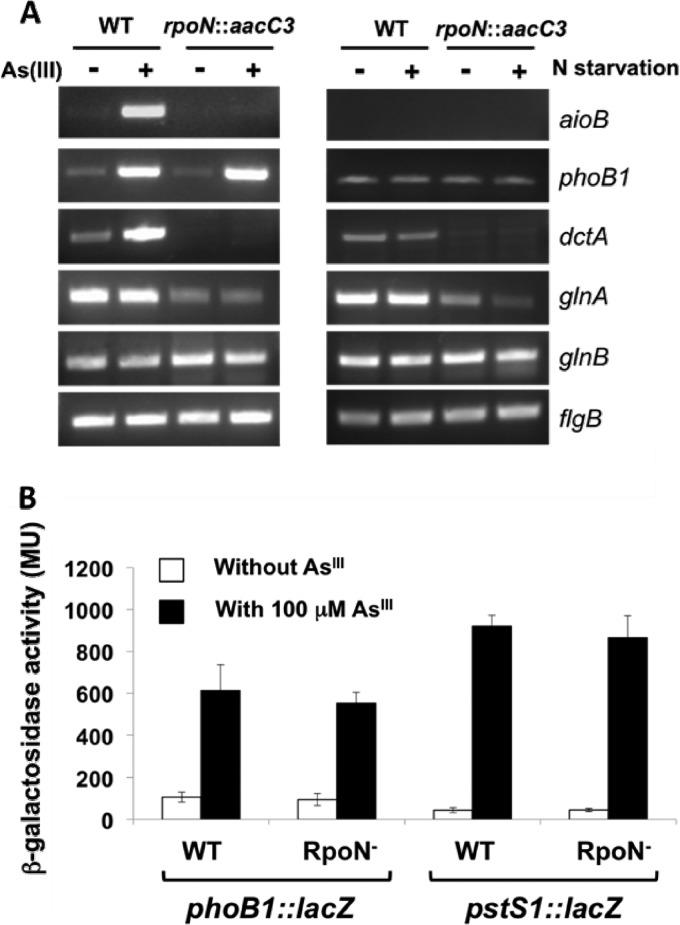
The importance of RpoN for the expression of a random sample of these genes was examined by qualitative RT-PCRs using RNA extracted from cells that were exposed to AsIII or that were starved for nitrogen; the latter analysis was included because of the known association of RpoN with the regulation of N metabolism (e.g., see reference 16). Consistent with the experiments summarized in Fig. 2C, aioB expression was significantly upregulated in AsIII-exposed wild-type cells but was not detectable in the rpoN::aacC3 mutant regardless of the incubation conditions (Fig. 5A). Surprisingly, dctA was also upregulated by AsIII and, like aioB, was silent in the rpoN::aacC3 mutant. The glnA gene required RpoN for full expression, but it was found to be expressed under all incubation conditions and in both strains although at reduced levels in the rpoN::aacC3 mutant and at further reduced levels in the rpoN::aacC3 mutant incubated under N starvation conditions (Fig. 5A). Examples of genes where transcription appeared to be unaffected by the loss of functional RpoN included phoB1, glnB, and flgB (Fig. 5A). RT-PCR analysis showed that phoB1 is upregulated by AsIII (Fig. 5B). A quantitative lacZ reporter gene analysis of phoB1 and the adjacent (but divergently transcribed) pstS1 gene showed that both genes were dramatically induced by AsIII; however, neither gene was affected by the rpoN::aacC3 mutation (Fig. 5B). A significantly more expansive characterization of the AsIII and phosphate coregulation of the aio, pst, and pho genes will be presented elsewhere (Y. S. Kang, B. Bothner, C. Rensing, and T. R. McDermott, submitted for publication).
Fig 5.
Influence of the rpoN::aacC3 mutation on expressions of genes associated with an RpoN binding site. (A) Qualitative standardized RT-PCR expression analysis of genes (in the wild-type strain or the rpoN::aacC3 mutant) associated with an RpoN binding site. Cells were grown in the absence (−) or in the presence (+) of AsIII or under conditions with or without N. For each gene, RT-PCR amplicon volumes loaded into the gel were standardized. (B) β-Galactosidase activity (in Miller units) derived from the transcription of the phoB1::lacZ and pstS1::lacZ reporters in the wild type and the rpoN::aacC3 mutant and as a function of cells grown in the presence or absence of 100 μM AsIII.
DISCUSSION
The studies summarized in this report strengthen our understanding of transcriptional controls that regulate the expressions of key genes involved in AsIII oxidation. The alternative sigma factor RpoN is essential for aioBA expression and AsIII oxidation (Fig. 2), a conclusion supported by two independent lines of evidence. First, an insertional interruption of the rpoN coding region silenced aioBA expression and resulted in a null AsIII oxidation phenotype. However, this could be reversed to the wild-type status with a defined DNA fragment containing the rpoN coding region and minimal flanking DNA. Second, the introduction of a precise deletion in a sequence that exhibited the well-known GG-(N10)-GC signature RpoN binding site (4) directly upstream of the aioB gene (but leaving the aioB translational start site intact) also resulted in the silencing of aioBA expression and AsIII oxidation. A recent random transposon-based mutation study with the organism Herminiimonas arsenicoxydans identified an rpoN::Tn5 mutant as being defective in AsIII oxidation (31). However, the necessary complementation experiments were not performed to confirm that the phenotype was actually due to the interrupted rpoN gene as opposed to transposon polar effects on the expression of rpoX (an rpoS-like sigma factor [61]) and other genes immediately downstream of what Duquesne et al. (13) depicted previously as an H. arsenicoxydans operon that contains rpoN (31). The precisely targeted mutation and complementation experiments in the present study provide definitive evidence that the rpoN gene product is essential for aioBA expression and, hence, AsIII oxidation. This observation also allows a more definitive understanding of the AsIII oxidase-negative phenotype of the aioR::Tn5-B22 mutant described previously (28). Specifically, the Tn5-B22 insertion in the extreme 3′ region of aioR in the aioS-aioR-aioB-aioA operon likely disrupted the DNA binding component of the regulator AioR and is consistent with AioR participating as a bacterial enhancer binding protein in conjunction with RpoN to facilitate the induction of aioBA. Kashyap et al. (28) also reported evidence that aioSR are cotranscribed with aioBA in what appears to be an operon arrangement. In this specific context, it is not presently clear why the loss of the RpoN binding site would then interrupt aioBA expression in strain 5A.
The requirement for RpoN as an essential regulator of AsIII oxidation is not necessarily surprising given the wide variety of cellular functions that RpoN regulates (reviewed in references 34 and 56). Indeed, we found that the consensus GG-(N10)-GC RpoN binding-site signature associated with aioB was also found elsewhere in the genome (Fig. 4), being associated with a wide range of functions previously noted for other bacteria (34, 50, 56). Given such a range of functions, it is also not surprising that the expression of rpoN appears to be constitutive in A. tumefaciens strain 5A and is not influenced by a specific solute or ion such as AsIII (Fig. 3), which is in agreement with previous studies (7, 26).
What was surprising, however, was the observation of a C4-dicarboxylate transporter (dctA) (Fig. 4) upregulated by AsIII (Fig. 5A). It is not immediately apparent why, or how, such a transporter might be involved (or not) in AsIII oxidation or metabolism. Previously reported microarray studies also identified the AsIII induction of a C4-dicarboxylate transporter in H. arsenicoxydans (8). The Dct system in strain 5A is a secondary transporter operating without a periplasmic solute binding protein, whereas the AsIII-inducible dctPMQ dicarboxylate transporter in H. arsenicoxydans is annotated as a tripartite ATP-independent periplasmic (TRAP)-type transporter (reviewed in reference 45). While these two C4-dicarboxylate transporters are structurally different, their function is the same. Such shared regulatory and functional similarities would seem not to be a simple coincidence and suggest a potential involvement in some facet of arsenic metabolism. Rat and plant mitochondrial C4-dicarboxylate transporters have been shown to facilitate the exchange of malate and phosphate (15, 27), with arsenate robustly substituting for phosphate in the shuttle (15). In the context of a prokaryote cell, such a mechanism could result in AsV removal from the cell and is an issue that we are examining in more detail.
The other instance where RpoN appeared to be important involved glnA. Not surprisingly, AsIII had no influence on glnA expression in the wild-type or mutant strains. Also, glnA expression in the wild-type strain was observed under all conditions, although the level of expression varied depending on the incubation conditions. The glnA expression level was noticeably reduced in the rpoN::aacC3 mutant under N-replete conditions, similar to what was found previously for Xylella fastidiosa (10), and was even further reduced when the mutant was starved for N (Fig. 5A).
In contrast to aioB and dctA, genes such as phoB1, pstS1, glnB, and flgB were examples where an association with a consensus RpoN binding site did not correlate with an RpoN requirement (Fig. 5), at least under the experimental conditions considered. Previous predictions suggested an RpoN involvement wherever the GG-(N10)-GC motif was present (30, 47), but others have documented observations similar to those reported here. For example, in Pseudomonas putida, the presence of the GG-(N10)-GC motif does not always imply RpoN-associated regulation (55), and the lack of functional RpoN in this organism was shown previously to be essentially irrelevant to bacterial environmental responses normally associated with RpoN-controlled regulator functions (5). Furthermore, there is evidence of RpoN potentially interacting with a repressor and, hence, participating in a negative regulatory sense (3, 32) or that the spacing between the GC doublet and the transcriptional start site might not always be optimal (55). These types of explanations likely apply to A. tumefaciens strain 5A as well.
ACKNOWLEDGMENTS
Support for this research was provided by U.S. National Science Foundation grant MCB-0817170 to T.R.M. and B.B. and by the Montana Agricultural Experiment Station (project 911310) to T.R.M.
Footnotes
Published ahead of print 1 June 2012
REFERENCES
- 1. Anderson CL, Williams J, Hille R. 1992. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J. Biol. Chem. 267:23674–23682 [PubMed] [Google Scholar]
- 2. Betts KS. 2001. Developing a good solution for arsenic. Environ. Sci. Technol. 35:414A–415A doi:10.1021/es0125117 [DOI] [PubMed] [Google Scholar]
- 3. Boucher JC, Schurr MJ, Deretic V. 2000. Dual regulation of mucoidy in Pseudomonas aeruginosa and sigma factor antagonism. Mol. Microbiol. 36:341–351 [DOI] [PubMed] [Google Scholar]
- 4. Cannon W, Claverie-Martin F, Austin S, Buck M. 1993. Core RNA polymerase assists binding of the transcription factor sigma 54 to promoter DNA. Mol. Microbiol. 8:287–298 [DOI] [PubMed] [Google Scholar]
- 5. Cases I, de Lorenzo V. 2001. The limits to genomic predictions: role of sigma(N) in environmental stress survival of Pseudomonas putida. FEMS Microbiol. Ecol. 35:217–221 [DOI] [PubMed] [Google Scholar]
- 6. Casiot C, et al. 2003. Bacterial immobilization and oxidation of arsenic in acid mine drainage (Carnoulès creek, France). Water Res. 37:2929–2936 [DOI] [PubMed] [Google Scholar]
- 7. Castano I, Bastarrachea F. 1984. glnF-lacZ fusions in Escherichia coli: studies on glnF expression and its chromosomal orientation. Mol. Gen. Genet. 195:228–233 [DOI] [PubMed] [Google Scholar]
- 8. Cleiss-Arnold J, et al. 2010. Temporal transcriptomic response during arsenic stress in Herminiimonas arsenicoxydans. BMC Genomics 11:709 doi:10.1186/1471-2164-11-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clingenpeel SR, D'Imperio S, Oduro H, Druschel GK, McDermott TR. 2009. Cloning and in situ expression studies of the Hydrogenobaculum arsenite oxidase genes. Appl. Environ. Microbiol. 75:3362–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Silva Neto JF, Koide T, Gomes SL, Marques MV. 2010. Global gene expression under nitrogen starvation in Xylella fastidiosa: contribution of the σ54 regulon. BMC Microbiol. 10:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Imperio S, Lehr CR, Breary M, McDermott TR. 2007. Autecology of an arsenite chemolithotroph: sulfide constraints on function and distribution in a geothermal spring. Appl. Environ. Microbiol. 73:7067–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donahoe-Christiansen J, Jackson CR, D'Imperio S, Inskeep WP, McDermott TR. 2004. Arsenite-oxidizing Hydrogenobaculum sp. isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl. Environ. Microbiol. 70:1865–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duquesne K, et al. 2008. Arsenite oxidation by a chemoautotrophic moderately acidophilic Thiomonas sp.: from the strain isolation to the gene study. Environ. Microbiol. 10:228–237 [DOI] [PubMed] [Google Scholar]
- 14. Ellis PJ, Conrads T, Hille R, Kuhn P. 2001. Crystal structure of the 100 kDa arsenite oxidase from Alcaligenes faecalis in two crystal forms at 1.64 Å and 2.03 Å. Structure 9:125–132 [DOI] [PubMed] [Google Scholar]
- 15. Furbank RT, Agostino A, Hatch MD. 1990. C4 acid decarboxylation and photosynthesis in bundle sheath ceils of NAD-malic enzyme-type C4 plants: mechanism and the role of malate and orthophosphate. Arch. Biochem. Biophys. 276:374–381 [DOI] [PubMed] [Google Scholar]
- 16. Garcia E, Bancroft S, Rhee SG, Kustu S. 1977. The product of a newly identified gene glnF, is required for synthesis of glutamine synthetase in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 74:1662–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gihring TM, Banfield JF. 2001. Arsenite oxidation and arsenate respiration by a new Thermus isolate. FEMS Microbiol. Lett. 204:335–340 [DOI] [PubMed] [Google Scholar]
- 18. Green HH. 1918. Isolation and description of a bacterium causing oxidation of arsenite to arsenate in cattle dripping baths. Rep. Dir. Vet. S. Afr. 6:593–599 [Google Scholar]
- 19. Hamamura N, et al. 2009. Linking microbial oxidation of arsenic with detection and phylogenetic analysis of arsenite oxidase genes in diverse geothermal environments. Environ. Microbiol. 11:421–431 [DOI] [PubMed] [Google Scholar]
- 20. Hao X, et al. 2012. Genome sequence of the arsenite-oxidizing strain Agrobacterium tumefaciens 5A. J. Bacteriol. 194:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoeft SE, Kulp TR, Han S, Lanoil B, Oremland RS. 2010. Coupled arsenotrophy in a hot spring photosynthetic biofilm at Mono Lake, California. Appl. Environ. Microbiol. 76:4633–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang HC, He SY, Bauer DW, Collmer A. 1992. The Pseudomonas syringae pv. syringae 61 hrpH product, an envelope protein required for elicitation of the hypersensitive response in plants. J. Bacteriol. 174:6878–6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilyaletdinov AN, Abdrashitova SA. 1981. Autotrophic oxidation of arsenic by a culture of Pseudomonas arsenitoxidans. Mikrobiologiia 50:197–204 (In Russian.) [PubMed] [Google Scholar]
- 24. Inskeep WP, et al. 2007. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ. Microbiol. 9:934–943 [DOI] [PubMed] [Google Scholar]
- 25. Jackson CR, Langner HW, Donahoe-Christiansen J, Inskeep WP, McDermott TR. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ. Microbiol. 3:532–542 [DOI] [PubMed] [Google Scholar]
- 26. Jishage M, Iwata A, Ueda S, Ishihama A. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178:5447–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplan RS, Pedersen PL. 1985. Isolation and reconstitution of the n-butylmalonate-sensitive dicarboxylate transporter from rat liver mitochondria. J. Biol. Chem. 260:10293–10298 [PubMed] [Google Scholar]
- 28. Kashyap DR, Botero LM, Franck WL, Hassett DJ, McDermott TR. 2006. Complex regulation of arsenite oxidation in Agrobacterium tumefaciens. J. Bacteriol. 188:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kashyap DR, Botero LM, Lehr C, Hassett DJ, McDermott TR. 2006. A Na+:H+ antiporter and molybdate transporter are essential for arsenite oxidation in Agrobacterium tumefaciens. J. Bacteriol. 188:1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim Y, Watrud LS, Matin AC. 1995. A carbon starvation survival gene of Pseudomonas putida is regulated by σ54. J. Bacteriol. 177:1850–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koechler S, et al. 2010. Multiple controls affect arsenite oxidase gene expression in Herminiimonas arsenicoxydans. BMC Microbiol. 10:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohler T, Alvarez JF, Harayama S. 1994. Regulation of the rpoN, ORF102 and ORF154 genes in Pseudomonas putida. FEMS Microbiol. Lett. 115:177–184 [DOI] [PubMed] [Google Scholar]
- 33. Kulp TR, et al. 2008. Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science 321:967–970 [DOI] [PubMed] [Google Scholar]
- 34. Kustu S, Santero E, Keener J, Popham D, Weiss D. 1989. Expression of σ54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol. Rev. 53:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langner H, Jackson CR, McDermott TR, Inskeep WP. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302–3309 [DOI] [PubMed] [Google Scholar]
- 36. Lett MC, Muller D, Lièvremont D, Silver S, Santini J. 2012. Unified nomenclature for genes involved in prokaryotic aerobic arsenite oxidation. J. Bacteriol. 194:207–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lieutaud A, et al. 2010. Arsenite oxidase from Ralstonia sp. 22: characterization of the enzyme and its interaction with soluble cytochromes. J. Biol. Chem. 285:20433–20441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Link AJ, Phillips D, Church GM. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu G, et al. 19 December 2011. A periplasmic arsenite-binding protein involved in regulating arsenite oxidation. Environ. Microbiol. [Epub ahead of print.] doi:10.1111/j.1462-2920.2011.02672.x [DOI] [PubMed] [Google Scholar]
- 40. Macur RE, Wheeler JT, McDermott TR, Inskeep WP. 2001. Microbial populations associated with the reduction and enhanced mobilization of arsenic in mine tailings. Environ. Sci. Technol. 35:3676–3682 [DOI] [PubMed] [Google Scholar]
- 41. Macur RE, Jackson CR, McDermott TR, Inskeep WP. 2004. Bacterial populations associated with the oxidation and reduction of arsenic in an unsaturated soil. Environ. Sci. Technol. 38:104–111 [DOI] [PubMed] [Google Scholar]
- 42. Miller JH. 1972. Experiments in molecular genetics, p 431 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 43. Muller D, Lievremont D, Simeonova DD, Hubert JC, Lett MC. 2003. Arsenite oxidase aox genes from a metal-resistant β-proteobacterium. J. Bacteriol. 185:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller D, et al. 2006. Herminiimonas arsenicoxydans sp. nov., a metalloresistant bacterium. Int. J. Syst. Evol. Microbiol. 56:1765–1769 [DOI] [PubMed] [Google Scholar]
- 45. Mulligan C, Fischer M, Thomas GH. 2011. Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea. FEMS Microbiol. Rev. 35:68–86 [DOI] [PubMed] [Google Scholar]
- 46. Osborne FH, Ehrlich HL. 1976. Oxidation of arsenite by a soil isolate of Alcaligenes. J. Appl. Bacteriol. 41:295–305 [DOI] [PubMed] [Google Scholar]
- 47. Pandza S, et al. 2000. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol. Microbiol. 36:414–423 [DOI] [PubMed] [Google Scholar]
- 48. Philips SE, Taylor ML. 1976. Oxidation of arsenite to arsenate by Alcaligenes faecalis. Appl. Environ. Microbiol. 32:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quandt J, Hynes MF. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15–21 [DOI] [PubMed] [Google Scholar]
- 50. Reitzer L, Schneider BL. 2001. Metabolic context and possible physiological themes of σ54-dependent genes in Escherichia coli. Microbiol. Mol. Biol. Rev. 65:422–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salmassi TM, et al. 2002. Oxidation of arsenite by Agrobacterium albertinmagni, AOL15, sp. nov., isolated from Hot Creek, California. Geomicrobiol. J. 19:53–66 [Google Scholar]
- 52. Santini JE, Sly LI, Schnagl RD, Macy JM. 2000. A new chemolithotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santini JM, vanden Hoven RN. 2004. Molybdenum-containing arsenite oxidase of the chemolithoautotrophic arsenite oxidizer NT-26. J. Bacteriol. 186:1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sardiwal S, Santini JM, Osborne TH, Djordjevic S. 2010. Characterization of a two-component signal transduction system that controls arsenite oxidation in the chemolithoautotroph NT-26. FEMS Microbiol. Lett. 313:20–28 [DOI] [PubMed] [Google Scholar]
- 55. Savioz A, Zimmermann A, Haas D. 1993. Pseudomonas aeruginosa promoters which contain a conserved GG-N10-GC motif but appear to be RpoN-independent. Mol. Gen. Genet. 238:74–80 [DOI] [PubMed] [Google Scholar]
- 56. Shingler V. 2011. Signal sensory systems that impact σ54-dependent transcription. FEMS Microbiol. Rev. 35:425–440 [DOI] [PubMed] [Google Scholar]
- 57. Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 58. Somerville JE, Kahn ML. 1983. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J. Bacteriol. 156:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Summers ML, Elkins JG, Elliot BA, McDermott TR. 1998. Expression and regulation of phosphate stress inducible genes in Sinorhizobium meliloti. Mol. Plant Microbe Interact. 11:1094–1101 [DOI] [PubMed] [Google Scholar]
- 60. Turner AW. 1949. Bacterial oxidation of arsenite. Nature 164:76–77 [DOI] [PubMed] [Google Scholar]
- 61. Zhao JJ, Chen C, Zhang LP, Hu CQ. 2009. Cloning, identification, and characterization of the rpoS-like sigma factor rpoX from Vibrio alginolyticus. J. Biomed. Biotechnol. 2009:126986. [DOI] [PMC free article] [PubMed] [Google Scholar]