Abstract
Biodiesel production was examined with Scenedesmus obliquus in a recirculatory aquaculture system with fish pond discharge and poultry litter to couple with waste treatment. Lipid productivity of 14,400 liter ha−1 year−1 was projected with 11 cultivation cycles per year. The fuel properties of the biodiesel produced adhered to Indian and international standards.
TEXT
Biodiesel has emerged as the most suitable alternative to petroleum diesel fuel owing to its ecofriendly characteristics and renewability (14). It burns in conventional diesel engines with or without any modification or can be used as a blend with petrodiesel, exhibiting lower exhaust emissions than conventional diesel fuel (19). The third-generation biodiesel, i.e., biodiesel from microalgae, is emerging as highly promising, with a projected yield of 58,700 to 136,900 liter ha−1 year−1 (7). However, microalga cultivation is expensive, as it involves huge consumption of water resources in addition to the inorganic nutrients (16). In our previous report (18), we examined simultaneous biodiesel production and waste recycling by the green microalga Scenedesmus obliquus (Trup.) Kütz (SAG 276-3a; SAG Culture Collection, Gottingen, Germany) with three types of wastes, viz. poultry litter (PL), fish pond discharge (FPD), and municipal secondary settling tank discharge (MSSTD) under laboratory batch culture conditions. In this report, we have extended our experiments to outdoor conditions by assessing biodiesel production with the same microalga in a recirculatory aquaculture system (RAS) using FPD and PL.
Nutrient removal and lipid accumulation potential of S. obliquus in RAS.
The RAS was developed at the Agricultural and Food Engineering Department, Indian Institute of Technology Kharagpur, Kharagpur, West Bengal, India, with fiber-reinforced plastic (FRP) tanks (length, 125 cm; breadth, 60 cm; depth, 45 cm), as detailed in Samantaray et al. (22). The effects of various physical parameters, such as sedimentation, mixing, culture depth, seasonal variation, and artificial light, on the bioremediation and lipid accumulation potential of S. obliquus were examined. S. obliquus was maintained in the laboratory in N 11 medium (24) at 25 ± 2°C (mean ± standard deviation) and with cycles of 14 h of light (75 μmol photons m−2 s−1 photosynthetic active radiation) and 10 h of dark.
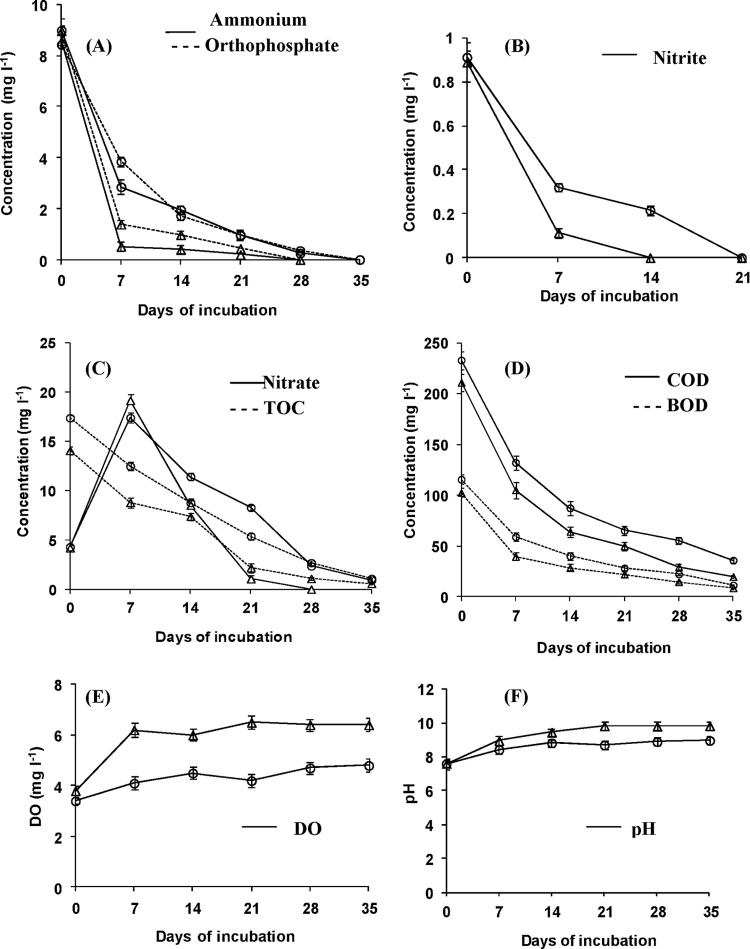
The effect of sedimentation was studied in two sets, each set with 3 FRP tanks. One set was filled with FPD to a depth of about 15 cm directly from the fish culture pond, whereas another set was filled after settling for 24 h in a 1,000-liter settling tank followed by passage through the inclined plate settler (23). The water quality parameters of the nonsedimented FPD were as follows: orthophosphate, 8.96 ± 0.19 mg liter−1; ammonium, 8.99 ± 0.47 mg liter−1; nitrate, 4.31 ± 0.26 mg liter−1; nitrite, 0.91 ± 0.03 mg liter−1; total organic carbon (TOC), 17.4 ± 0.27 mg liter−1; biological oxygen demand (BOD), 115.7 ± 3.19 mg liter−1; chemical oxygen demand (COD), 233.4 ± 8.91 mg liter−1; dissolved oxygen (DO), 3.4 ± 0.13 mg liter−1; and pH, 7.6 ± 0.3. The nutrient removal efficiency of S. obliquus was studied, following the standard protocols of the American Public Health Association (8), and found to be higher in the sedimented FPD than in the nonsedimented FPD (Fig. 1), which coincided with the greater biomass (0.32 g liter−1) and lipid yield (60.9 mg liter−1) in the sedimented tanks (data not shown).
Fig 1.
Removal of nutrients and changes in DO and pH by S. obliquus from sedimented (△) and nonsedimented (○) FPD. l, liter.
Mixing was provided by stirrers with pitched blades (model no. RQ-24A, rotating intermittently at a speed of about 20 × g; Remi Instruments Ltd., Vasai, India) either in combination with aerators sparging compressed air (12 liter min−1) through polyvinyl chloride (PVC) porous tubing spread on the bottom of the tanks or without aeration. On day 21, under conditions of mixing, the levels of PO43−, NH4+, and NO2− dropped below the detectable limits, corresponding to 100% biofiltration. Falls in TOC, BOD, and COD values and increases in DO content and pH were clearly evident (data not shown). The bioremediation efficiency of S. obliquus was maximized under stirring conditions, followed by stirring with aeration and aeration alone. In general, significant increases in biomass and lipid yield were evident under mixing conditions, reaching up to 0.53 g liter−1 and 112.6 mg liter−1, respectively, with stirring alone (Fig. 2).
Fig 2.
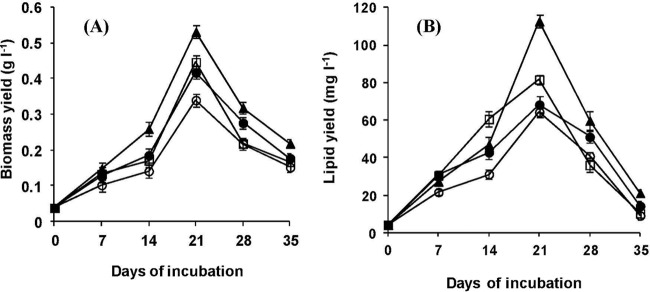
Effect of mixing on biomass (A) and lipid yield (B) of S. obliquus grown in sedimented FPD. ○, control; ▲, stirring; ●, aeration; □, stirring plus aeration; l, liter.
The nutrient removal efficiency of S. obliquus was also studied in sedimented FPD at different culture depths (10, 15, and 20 cm) in FRP tanks with stirring. An inverse relationship was observed between culture depth and nutrient removal (data not shown). Although reducing the culture depth from 15 to 10 cm increased the biomass yield per litter of medium marginally (from 0.51 to 0.59 g liter−1), the overall productivity in terms of total yield or areal density was significantly higher at the 15-cm depth (Table 1). Maximum biomass productivity recorded at an areal density of 76.5 g m−2 (total yield, 57.4 g) in FRP tanks of the 15-cm depth. The maximum lipid yield of 11.4 g was also observed at the 15-cm culture depth.
Table 1.
Biomass and lipid yields of S. obliquus grown at various culture depthsa
| Culture depth (cm) | Biomass |
Lipid |
||||
|---|---|---|---|---|---|---|
| Volumetric yield (g liter−1) | Areal density (g m−2) | Total yield (g) | Volumetric (mg liter−1) | Areal density (g m−2) | Total yield (g) | |
| 10 | 0.59 ± 0.04 b | 59.1 ± 0.41 a | 44.3 ± 0.51 a | 115.6 ± 0.9 b | 11.6 ± 0.08 b | 8.7 ± 0.09 a |
| 15 | 0.51 ± 0.02 b | 76.5 ± 0.23 b | 57.4 ± 0.28 b | 101.5 ± 1.1 b | 15.2 ± 0.12 c | 11.4 ± 0.13 b |
| 20 | 0.28 ± 0.01 a | 56.0 ± 0.19 a | 42.0 ± 0.23 a | 47.9 ± 0.6 a | 9.6 ± 0.07 a | 7.2 ± 0.07 a |
Values are means ± standard errors (n = 3). Values within a column followed by different letters are significantly different from each other (P < 0.05, Duncan's new multiple range test). A separate analysis was done for each column.
The effects of seasonal variation on biomass and lipid production were studied in RAS with sedimented FPD at the 15-cm culture depth with stirring. The average temperatures were 37.8, 31.2, and 19.5°C and the hours of sunshine were 13.3, 12.5, and 10.5 h in summer, the rainy season, and winter, respectively (meteorological data are from the Physics and Meteorology Department, Indian Institute of Technology Kharagpur, Kharagpur, India). A maximum biomass yield of 0.61 g liter−1 with lipid content of 138.5 mg liter−1 was obtained during summer. The lipid yields for the rainy season and winter were 112.7 and 98.2 mg liter−1, respectively (data not shown).
As the hours of sunshine were found to be relatively short during winter, the tanks were illuminated with artificial lights for 2 h (4.30 pm to 6.30 pm). The lighting system was comprised of various numbers of fluorescent lamps mounted about 12 cm above the tanks. The tanks were illuminated with 1 to 4 fluorescent lamps and received light intensities of ∼20, 30, 50, and 70 μE m−2 s−1, respectively. One set of tanks was not illuminated with fluorescent lamps and was kept as a control. On day 21, some of the water quality parameters, such as the concentrations of PO43−, NH4+, and NO2−, dropped below the detection levels in all sets of tanks. The water quality parameters of all of these treated tanks were compared with the prescribed aquaculture limits (Table 2); the values were found to be within the permissible limits for fish culture ponds (11). An increase in lipid yield up to 129.8 mg liter−1 was recorded in the FRP tanks illuminated with a light intensity of 70 μE m−2 s−1 for 2 h, which could be comparable with the yield during the summer season (Table 2). During the rainy season, the lipid yield also increased up to 141.3 mg liter−1 in FRP tanks illuminated with a light intensity of 30 μE m−2 s−1 for 1 h (data not shown).
Table 2.
Effect of artificial light on bioremediation efficiency, biomass, and lipid yield of S. obliquus on day 21 of incubation during wintera
| Parameter | Initial concn | Control (without artificial light) | Light intensity (μE m−2 s−1)b |
Aquaculture limitc | |||
|---|---|---|---|---|---|---|---|
| 20 | 30 | 50 | 70 | ||||
| Orthophosphate (mg liter−1) | 8.3 ± 0.18 | ND | ND | ND | ND | ND | <0.2 |
| Ammonium (mg liter−1) | 8.6 ± 0.26 | ND | ND | ND | ND | ND | <1.0 |
| Nitrate (mg liter−1) | 4.2 ± 0.33 | 0.62 ± 0.05 | 0.58 ± 0.04 | 0.55 ± 0.04 | 0.46 ± 0.07 | 0.41 ± 0.04 | <150 |
| Nitrite (mg liter−1) | 0.89 ± 0.09 | ND | ND | ND | ND | ND | <2.0 |
| TOC (mg liter−1) | 14.2 ± 0.51 | 1.5 ± 0.04 | 1.3 ± 0.03 | 0.84 ± 0.04 | 1.2 ± 0.03 | 1.2 ± 0.03 | <30 |
| COD (mg liter−1) | 211.3 ± 8.2 | 47.2 ± 0.52 | 53.1 ± 0.82 | 41.3 ± 0.55 | 48.1 ± 0.35 | 51.7 ± 0.32 | <200 |
| BOD (O2 mg liter−1) | 102.5 ± 4.8 | 24.7 ± 0.68 | 24.3 ± 0.50 | 18.7 ± 0.58 | 20.4 ± 0.24 | 21.3 ± 0.18 | |
| DO (mg liter−1) | 3.8 ± 0.22 | 6.7 ± 0.04 | 6.6 ± 0.04 | 6.3 ± 0.07 | 6.8 ± 0.06 | 6.7 ± 0.05 | >5 |
| pH | 7.6 ± 0.06 | 8.8 ± 0.04 | 8.9 ± 0.05 | 8.7 ± 0.11 | 8.8 ± 0.12 | 8.7 ± 0.09 | >6.5 |
| Biomass yield (g liter−1) | 0.04 ± 0.01 | 0.48 ± 0.04 | 0.54 ± 0.09 | 0.57 ± 0.06 | 0.60 ± 0.04 | 0.62 ± 0.06 | |
| Lipid yield (mg liter−1) | 5.2 ± 0.04 | 98.2 ± 1.2 | 100.9 ± 3.7 | 113.2 ± 2.9 | 116.5 ± 3.3 | 129.8 ± 3.1 | |
All values are means ± standard errors (n = 3). ND, not detected.
Artificial light was provided for 2 h.
From reference 11.
Experiments with poultry litter supplementation.
Experiments conducted outdoors in RAS with PL-supplemented (5 g liter−1) FPD demonstrated a significant increase in the biomass yield (∼3-fold rise) (Table 3). Similarly, the lipid yield was increased up to 269.7 mg liter−1 (∼2.5-fold rise). In our earlier report (17), when S. obliquus cultures pregrown in N 11 medium supplemented with 15 g of glucose per liter under laboratory batch culture conditions were subjected to the optimized conditions, i.e., transfer of the cultures to medium containing reduced concentrations of nitrate and phosphate (0.04 g nitrate liter−1 and 0.03 g phosphate liter−1) compared to the concentrations in N 11 medium and with the presence of 1.0 g thiosulphate liter−1 for a culture period of 8 days, lipid accumulation of 2,160 mg liter−1 was obtained, which was ∼18-fold higher than the yield obtained for the N 11-grown control. Experiments with three types of wastes (PL, FPD, and MSSTD) under laboratory batch culture study conditions resulted in lipid yields ranging between 947 and 1,049 mg lipid liter−1 under the above-described optimized conditions, which was ∼50% of the yield recorded under the previously described conditions (18). In RAS, when S. obliquus cultures pregrown in FPD supplemented with 5 g PL liter−1 were transferred to the same volume of medium under the optimized conditions at the second stage, an increase in the lipid pool of up to 780.8 mg liter−1 was observed during the summer season (Table 3). During the rainy and winter seasons, lipid yields ranging from 767.3 to 792.9 mg liter−1 were recorded by providing artificial light for 1 h (30 μE m−2 s−1) in the rainy and 2 h (70 μE m−2 s−1) in the winter season.
Table 3.
S. obliquus biomass and lipid yields under laboratory and outdoor RAS conditionsa
| Experiment | Culture conditions (reference) | Biomass yield (g liter−1) | Lipid yield |
|
|---|---|---|---|---|
| Volumetric yield (mg liter−1) | % Dry cell weight | |||
| Laboratory batch culture | Controld | 1.0 ± 0.14 c | 117.8 ± 1.8 a | 11.7 ± 0.81 a |
| Optimized conditionsc (17) | 0.38 ± 0.04 a | 221.5 ± 3.2 b | 58.3 ± 1.01 d | |
| 15 g liter−1 glucose (17) | 5.1 ± 0.17 g | 585.9 ± 9.2 c | 10.5 ± 0.87 a | |
| 15 g liter−1 glucose under optimized conditionsc (17) | 4.4 ± 0.09 f | 2,160.1 ± 19.5 g | 48.6 ± 0.58 c | |
| 20 g liter−1 PL under optimized conditionsc (18) | 1.9 ± 0.22 e | 957.4 ± 2.1 e | 51.2 ± 1.19 c | |
| MSSTD + 15 g liter−1 PL under optimized conditionsc (18) | 2.0 ± 0.21 e | 1048.9 ± 7.7 f | 52.7 ± 1.31 c | |
| FPD + 15 g liter−1 PL under optimized conditionsc (18) | 1.8 ± 0.23 de | 947.4 ± 5.8 e | 53.8 ± 0.92 cd | |
| Outdoor RAS | FPD | 0.53 ± 0.12 b | 103.9 ± 1.4 a | 19.6 ± 0.21 b |
| FPD + 5 g liter−1 PL | 1.5 ± 0.33 d | 269.7 ± 2.5 b | 18.0 ± 1.34 b | |
| FPD + 5 g liter−1 PL,c summer | 1.4 ± 0.29 d | 780.8 ± 3.8 d | 55.8 ± 2.04 d | |
| FPD + 5 g liter−1 PL,c rainy seasone | 1.4 ± 0.22 d | 767.3 ± 4.3 d | 54.8 ± 1.4 cd | |
| FPD + 5 g liter−1 PL,c wintere | 1.4 ± 0.31 d | 792.9 ± 2.5 d | 56.6 ± 1.14 d | |
Values are means ± standard errors (n = 3).
Values within a column followed by different letters are significantly different from each other (P < 0.05, Duncan's new multiple range test). A separate analysis was done for each column.
Optimized conditions: the cultures were grown in medium containing 0.04 g liter−1 nitrate, 0.03 g liter−1 phosphate, and 1.0 g liter−1 thiosulphate at the second stage for 8 days (17).
Control: grown in N 11 medium for 21 days (24).
Artificial light was provided for 1 h (30 μE m−2 s−1) in the rainy season and 2 h (70 μE m−2 s−1) in winter.
Fuel properties of S. obliquus biodiesel.
Lipids were transesterified according to our previously described method (17), and the biodiesel yield was 69% of the crude lipids. The fatty acid methyl ester composition of the biodiesel was determined by gas chromatography-mass spectrometry (GC-MS) (Autosystem XL, PerkinElmer, Shelton, CT) with methylpentadecanoate as the internal standard. Interestingly, biodiesel produced from S. obliquus oil cultivated in FPD and PL, alone or in combination, showed an increased palmitic acid content compared to that of the N 11-grown control. Conversely, the linolenic and linoleic acid contents were decreased in the waste-grown cultures (data not shown). These altered patterns would contribute significantly to a decrease in the total unsaturated lipid level of the biodiesel. The waste-grown S. obliquus achieved maximum reductions in polyunsaturated lipid levels of up to 9.9%, whereas the palmitate plus oleate levels reached up to 91.1%, which is desired for a good quality biodiesel. Interestingly, in outdoor cultivation, the linolenic acid content dropped to <12% in all types of samples, which was within the specified limit of biodiesel standards (10).
Various fuel properties of S. obliquus biodiesel were analyzed according to the standard protocols, and the data are compiled in Table 4. The density of the algal biodiesel was determined in accordance with ASTM D4052-96 (3) using relative density bottles with a 10-ml capacity (Borosil). The viscosity was determined with the help of a Cannon Fensky viscometer tube in a kinetic viscometer bath (Maharana Instruments Mfg. Co., Ajmer, India) according to IS 1448 (11). For each measurement, 5 ml of biodiesel was required. The viscosity of the biodiesel was calculated from the measured flow time and the calibration constant of the viscometer and is expressed as centistokes (cSt) mm2 s−1.
Table 4.
Comparison of S. obliquus biodiesel with petroleum diesel and various biodiesel standards
| Property | Biodiesel from S. obliquus grown as indicateda |
Petroleum diesel | Biodiesel standard(s) (reference[s]) |
||||
|---|---|---|---|---|---|---|---|
| Controlb | FPD | FPD + PL | ASTM (2, 3, 4) | EN 14214 (10) | IS 15607 (6) | ||
| Density at 15°C (kg m−3) | 878 | 886 | 883 | 850 | 860–900 | 870–900 | |
| Viscosity at 40°C (mm2 s−1) | 3.9 | 4.4 | 4.2 | 2.6 | 1.9–6.0 | 3.5–5.0 | 3.5–5.0 |
| Calorific value (MJ kg−1) | 37.1 | 38.3 | 37.6 | 42.2 | |||
| Iodine value (g I2/100 g) | 79.2 | 62.8 | 66.3 | <120 | ≤115 | ||
| Acid value (mg KOH g−1) | 0.5 | 0.7 | 0.7 | 0.4 | <0.8 | <0.5 | ≤0.8 |
| Saponification value (mg KOH g−1) | 239.4 | 244.8 | 241.5 | ||||
| Cetane index | 51.3 | 54.5 | 54.0 | 49–55 | ≥47 | ≥51 | ≥51 |
| Ash content (%) | 0.01 | 0.01 | 0.01 | 0.01 | <0.02 | <0.02 | <0.02 |
| Water content (%) | 0.02 | 0.01 | 0.02 | 0.02 | <0.03 | <0.05 | ≤0.05 |
Values are the means of three observations.
Control: grown in N 11 medium (24).
Calorific value expresses the amount of heat generated by complete combustion of a unit weight of fuel. It was determined by bomb calorimeter (Maharana Instruments Mfg. Co., Ajmer, India) according to ASTM D240-02 (4). For each test, 1 g of biodiesel was burnt in an atmosphere of compressed oxygen in a “calorimetric bomb” immersed in water. The temperature rise of the water was measured with an extreme accuracy. The calorific value of the test sample was calculated and is expressed as MJ kg−1. The iodine value was determined by a titrimetry method using 0.2 to 0.3 g biodiesel (1).
Acid and saponification values were determined by a titrimetry method following EN 14104 (9) and Vicente et al. (25), respectively, using 0.3 g of algal biodiesel for each analysis. The cetane index measures the readiness of the fuel to autoignite when injected into the engine. The cetane index of biodiesel was calculated from the saponification and iodine values following Krisnangkura (15). The ash content of the biodiesel was determined in accordance with the procedure given in ASTM D482-07 (2). Five grams of biodiesel kept in a silica crucible was ignited and allowed to burn until only ash and carbon remained. The carbonaceous residue was further reduced to ash by heating in a muffle furnace (Suan Scientific Instruments and Equipments, Kolkata, India) at 775°C for 10 min. Then, the sample was cooled to room temperature and weighed again. The ash content was determined with an electronic balance (CP225D; Sartorious, Goettingen, Germany). The water content in the biodiesel was measured with a Karl Fisher (KF) titrator (TKF-55; Toshniwal Instruments Pvt. Ltd., Ajmer, India). About 1 g of biodiesel was dissolved in dried methanol (15 ml) contained in a moisture-free vessel. The sample was continuously stirred by a magnetic stirrer. Titration was done with the KF solution contained in a burette which was driven by a microprocessor-controlled stepper motor. The water content of the sample was determined by measuring the KF solution required to reach the endpoint of titration (13).
The density of the S. obliquus biodiesel ranged from 878 to 886 kg m−3, which was within the limits prescribed by the European (10) and Indian (6) standards (Table 4). The viscosity of the biodiesel was found to vary between 3.9 and 4.4 mm2 s−1, which was also within the range specified for biodiesel standards. The calorific value was within the range of 37.1 to 38.3 MJ kg−1, which is 8 to 12% lower than that of petroleum diesel but is comparable to those of Jatropha (37.2 MJ kg−1) and karanja (36.1 MJ kg−1) biodiesels (12, 20). The iodine value was within the limits specified in biodiesel standards, i.e., EN 14214 (<120 g I2/100 g) and IS 15607 (≤115 g I2/100 g). The saponification value ranged from 239.4 to 244.8 mg KOH g−1, while the cetane index was found to vary between 51.3 and 54.0. S. obliquus biodiesel was characterized as having a quantity of ash similar to that of petroleum diesel. The water content of S. obliquus biodiesel was found to vary between 0.01 and 0.02%, which also adhered to the international and Indian standards.
A major bottleneck relevant to the mass production of algae is the ability to maintain a monospecific culture of the laboratory-selected strain in outdoor conditions if it is not robust enough to withstand the field conditions. The selected microalgal strain must be productive in outdoor culture and flexible so as to adapt to the unavoidable changes in physicochemical parameters of an outdoor environment. In the RAS study, we found that a monospecific culture of Scenedesmus obliquus was maintained throughout the year with comparable levels of productivity. The ability to grow as a monoalgal culture in outdoor conditions demonstrates its potential as a model organism for mass cultivation.
The outdoor study in RAS demonstrated an average lipid yield of ∼780 mg liter−1 (Table 3). Thus, the lipid productivity achieved with the two-phase strategy (21-day culture period plus 8-day optimization period) is equivalent to ∼27 mg liter−1 day−1. This volumetric lipid productivity can be projected to an areal lipid productivity of 14,400 liters ha−1 year−1, assuming 11 cultivation cycles per year, leaving the rest of the period for cleaning and maintenance of the system. This projected value is close to the projection of Woertz et al. (26), where a consortium of native microalgae in dairy wastewater could produce 11,000 liters of lipid per hectare per year. Rodolfi et al. (21) also projected an annual lipid productivity of 20 tons per hectare with Nannochloropsis sp. F&M-M24.
Much higher lipid productivity (58,700 to 136,900 liter ha−1 year−1) has been envisioned by Chisti (7). The current productivity, however, could be enhanced further by increasing the culture depth in tanks with improved mechanization for proper mixing of large volumes of cultures or by reducing the growth period with well-designed photobioreactors, which in turn would result in an increase in the number of cultivation cycles and, thus, an increase in biomass vis-a-vis lipid yield. Such processes could become renewable and carbon neutral by combining them with CO2 sequestration from industrial emissions, such as flue gases, and with wastewaters as the nutrient supply.
ACKNOWLEDGMENTS
We are thankful to West Bengal Pollution Control Board, Kolkata, for GC-MS analysis.
Shovon Mandal thanks the Indian Institute of Technology Kharagpur, Kharagpur, India, for providing a fellowship to carry out the research work.
Footnotes
Published ahead of print 1 June 2012
REFERENCES
- 1. American Oil Chemists' Society 1998. Official methods and recommended practices of the AOCS, 5th ed AOCS Press, Champaign, IL [Google Scholar]
- 2. ASTM International 2007. Standard test method for ash from petroleum products. ASTM D482-07. ASTM International, Villanova, PA: http://www.astm.org [Google Scholar]
- 3. ASTM International 2002. Standard test method for density and relative density of liquids by digital density meter. ASTM D4052-96. ASTM International, Villanova, PA: http://www.astm.org [Google Scholar]
- 4. ASTM International 2007. Standard test method for heat of combustion of liquid hydrocarbon fuels by bomb calorimeter. ASTM D240-02. ASTM International, Villanova, PA: http://www.astm.org [Google Scholar]
- 5. Bureau of Indian Standards 1976. Methods of test for petroleum and its product, part 25: determination of kinematic and dynamic viscosity. Indian Standard IS 1448. Bureau of Indian Standards, New Delhi, India: http://www.bis.org.in [Google Scholar]
- 6. Bureau of Indian Standards 2005. Biodiesel (B 100) blend stock for diesel fuel. Indian Standard IS 15607. Bureau of Indian Standards, New Delhi, India: http://www.bis.org.in [Google Scholar]
- 7. Chisti Y. 2007. Biodiesel from microalgae. Biotechnol. Adv. 25:294–306 [DOI] [PubMed] [Google Scholar]
- 8. Clesceri LS, Greenberg AE, Eaton AD. (ed). 1998. Standard methods for the examination of water and wastewater, 20th ed American Public Health Association, Washington, DC [Google Scholar]
- 9. European Committee for Standardization 2003. Fat and oil derivatives—fatty acid methyl esters (FAME)—determination of acid value. European Standard EN 14104:2003. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 10. European Committee for Standardization 2003. Automotive fuels—fatty acid methylesters (FAME) for diesel engines—requirements and test methods. European Standard EN 14214:2003. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 11. Hajek BF, Boyd CE. 1994. Rating soil and water information for aquaculture. Aquacult. Eng. 13:115–128 [Google Scholar]
- 12. Francis G, Becker K. 2002. Biodiesel from Jatropha plantations on degraded land, p 9 University of Hohenheim, Stuttgart, Germany: http://www.youmanitas.nl/pdf/Bio-diesel.pdf [Google Scholar]
- 13. International Organization for Standardization 2000. Petroleum products—determination of water—coulometric Karl Fischer titration method. International Standard ISO 12937:2000. International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 14. Krawczyk T. 1996. Biodiesel alternative fuel makes inroads but hurdles remain. Int. News Fats Oils Relat. Mater. 7:801–829 [Google Scholar]
- 15. Krisnangkura K. 1986. A simple method for estimation of cetane index of vegetable oil methyl esters. J. Am. Oil Chem. Soc. 63:552–553 [Google Scholar]
- 16. Li X, Hu H-Y, Yang J. 2010. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 27:59–63 [DOI] [PubMed] [Google Scholar]
- 17. Mandal S, Mallick N. 2009. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 84:281–291 [DOI] [PubMed] [Google Scholar]
- 18. Mandal S, Mallick N. 2011. Waste utilization and biodiesel production by the green microalga Scenedesmus obliquus. Appl. Environ. Microbiol. 77:374–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMillen S, Shaw P, Jolly N, Goulding B, Finkle V. 2005. Biodiesel: fuel for thought, fuel for Connecticut's future, p 56 Connecticut Center for Economic Analysis Report, University of Connecticut, Storrs, CT [Google Scholar]
- 20. Raheman H, Phadatare AG. 2004. Diesel engine emissions and performance from blends of karanja methyl ester and diesel. Biomass Bioenergy 27:393–397 [Google Scholar]
- 21. Rodolfi L, et al. 2009. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102:100–112 [DOI] [PubMed] [Google Scholar]
- 22. Samantaray S, Nayak JK, Mallick N. 2011. Wastewater utilization for poly-β-hydroxybutyrate production by the cyanobacterium Aulosira fertilissima in a recirculatory aquaculture system. Appl. Environ. Microbiol. 77:8735–8743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarkar S, Kamilya D, Mal BC. 2007. Effect of geometric and process variables on the performance of inclined plate settlers in treating aquacultural waste. Water Res. 41:993–1000 [DOI] [PubMed] [Google Scholar]
- 24. Soeder CJ, Bolze A. 1981. Sulphate deficiency stimulates the release of dissolved organic matter in synchronous culture of Scenedesmus obliquus. Plant Physiol. 52:233–238 [Google Scholar]
- 25. Vicente G, Martınez M, Aracil J. 2004. Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour. Technol. 92:297–305 [DOI] [PubMed] [Google Scholar]
- 26. Woertz I, Feffer A, Lundquist T, Nelson Y. 2009. Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J. Environ. Eng. 135:1115–1122 [Google Scholar]