Abstract
Investigations were carried out on the manner by which pulsed–high intensity focused ultrasound (HIFU) enhances the effectiveness of tissue plasminogen activator (tPA) in whole blood clots, in vitro. Scanning electronic microscope (SEM) of the surface of the clots showed that the exposures increased exposed fibrin, as well as the number of openings to more interior regions. These findings were supported by fluorescent antibody labeling of tPA in frozen sections of clots treated post-HIFU. Here, improved accumulation at the surface and penetration of the tPA into the clots were observed in those treated with HIFU. Fluorescence recovery after photobleaching was also performed, indicating that the diffusion coefficient increased 6.3-fold for fluorescently labeled dextrans, comparable in size to tPA, in the HIFU-treated clots. Improved understanding of the manner by which pulsed–HIFU exposures can improve the effectiveness of thrombolytics will help optimize the exposures for this application and potentially facilitate translation to the clinic.
Index Terms: Acoustic radiation forces, fluorescence recovery after photobleaching (FRAP), fluorescent antibody staining, pulsed–high intensity focused ultrasound (HIFU), radio-frequency (RF), SEM, tissue plasminogen activator (tPA), whole blood clots
I. Introduction
A variety of procedures has been evaluated for improving thrombolysis using ultrasound, both with and without thrombolytic agents [1]. We recently evaluated the use of pulsed-high intensity focused ultrasound (HIFU) exposures with tissue plasminogen activator (tPA) both in vitro and in vivo in a novel clot model. tPA combined with pulsed–HIFU significantly enhanced thrombolyis in vitro compared to tPA alone. Levels of D-dimers were also found to be significantly higher when HIFU was combined with tPA, compared to tPA alone, being proportional to the degree of thrombolysis for each treatment [3]. Whole blood clots were formed in the marginal ear vein of rabbits, where combining pulsed–HIFU with tPA significantly enhanced the rate of thrombolyis with tPA. Pulsed–HIFU alone was not found to have any effects on the clot, nor did it create any damaging effects in the vein [2]. In both studies, the pulsed–HIFU exposures alone did not have a significant effect detected.
The results of the studies described earlier indicate that pulsed–HIFU exposures improved the effectiveness of the tPA, and pulsed–HIFU alone did not substantially degrade the clots. Furthermore, these data suggest that pulsed–HIFU may have improved the bioavailability of tPA in clots, perhaps by increasing the transport of tPA. In the present study our goal was to use a variety of in vitro techniques to characterize the effects of pulsed–HIFU exposures on the transport of tPA in whole blood clots. Some preliminary investigations into the mechanisms for these effects were also carried out.
II. Materials and Methods
A. Clot Formation
In vitro, whole blood clots were produced, as previously described [3]. In short, 1 ml of fresh venous blood from healthy volunteers was inserted into sections of latex penrose tubing (5/16 in by 12 in, Kendall Healthcare Products, Ontario, CA) and sealed at each end with dialysis tubing closures separated by 3 cm (in order to be compatible with the HIFU system). These constructs were incubated at 37 °C in a water bath for 1.5 h to form a clot, whose typical dimensions were 60 × 40 × 2 mm. The clots were treated with pulsed–HIFU as will be described.
B. HIFU Exposures
HIFU exposures were carried out as previously described [3]. The device was custom modified from an image guided commercial system (Sonoblate 500, Focus Surgery, Indianapolis, IN). The dual probe was comprised of a spherical, concave 1 MHz therapeutic transducer (focal length: 4 cm; diameter: 5 cm) and a coaxial 10 MHz imaging transducer (aperture 8 mm). The focal zone of the therapeutic transducer had the shape of an elongated ellipsoid, with a radial diameter (−3 dB) of 1.38 mm and axial length (−3 dB) of 7.2 mm.
Exposures were carried out in a bath of degassed water (for coupling) kept at 37 °C. Clots were adhered horizontally, two at a time, to a specially made holder attached to a 3-D stage. This setup was then placed over the tank so that the clots were inserted into the water directly opposite the transducer. Using the imaging transducer, the clots were positioned within the focal zone of the therapeutic transducer, as indicated on the graphic user interface. Once in position, the treatment points were designated over the ultrasound image in a grid pattern of 12 points (2 × 6), with a spacing of 2 mm between the points. At each point, exposures consisted of 60 pulses at a pulse repetition frequency of 1 Hz, 10% duty cycle (100 ms ON; 900 ms OFF), and total acoustic power of 60 W (spatial averaged intensity = 4022 W/cm2). Complete treatment of an individual clot lasted 12 min. For sham treatments, clots were similarly inserted into the tank but not given exposures.
C. Scanning Electron Microscope
Clots were prepared for SEM and imaged using previously described techniques [4]. In short, clots were removed from the penrose tubing immediately after sham and pulsed exposures. They were fixed, dehydrated, dried, and sputter coated with gold-palladium. Representative images were captured of sham and pulsed–HIFU-treated clots at 100× and 700× magnification (n = 3) using a JSM-6510LV SEM (Jeol, Tokyo, Japan).
D. Fluorescent Antibody Labeling
Clots receiving either sham or HIFU exposures were immediately treated with tPA, as previously described [3]. Antibody (Ab) labeling of the tPA was performed using standard techniques [5]. After incubations in tPA, clots (sham and HIFU exposed) were frozen and sections were prepared. The tPA in the sections was labeled with a primary monoclonal antibody specific to human tPA that was conjugated with the fluorophore fluorescein isothiocayanate (FITC) (Abcam, Cambridge, MA). Sections were viewed with a fluorescent microscope (Leica, Wetzlar, Germany) at 100× magnification (excitation: 488 nm; emission: 530 nm) and representative images were captured and saved in Tagged Image File Format (TIFF) (n = 5).
E. Fluorescence Recovery After Photobleaching
For these experiments, clots were formed using 900 μL of fresh venous blood mixed with 100 μL of dextran (1 mg/mL in phosphate buffered saline (PBS), fluorescein labeled, Invitrogen, CA), using the same procedure described earlier. Each clot contained 3, 10, 40, 70, 500, or 2000 kDa dextran to investigate diffusion as a function of molecular size. Following HIFU and sham exposures, clots were removed from the penrose tubing and placed in saline filled imaging chambers (CoverWell & Secure-Seal, Electron Microscopy Sciences, Hatfield, PA). Fluorescence recovery after photobleaching (FRAP) [6] was immediately performed on samples, at randomly chosen locations, with a Zeiss 710 laser scanning confocal microscope equipped with Zen 2008 software (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using an excitation of 488 nm and emission bandpass filter of 492–625 nm. In addition, an identical FRAP procedure was performed on the same range of dextran molecular weights but in water, 1%, and 2% agarose. Diffusion coefficients were obtained by analyzing the spatial evolution of fluorescent intensity. A Gaussian diffusion model [7] was fit to a smoothed background subtracted time series. The fitting process computed parameters including spread (ω(t)), amplitude (A(t)), and center (χc) of a Gaussian model. The diffusion coefficient was obtained by fitting a line through ω2 (t) versus time using a robust random sample consensus (RANSAC) [8] algorithm. An image series was excluded from the presented data if it produced less than 30 data points (i.e., valid ω2 (t) and t pairs) after Gaussian fitting stage or the image series had less than 50% inliers (n = 7–11).
F. Displacement Imaging
Displacements induced by the pulsed–HIFU exposures were visualized using a technique previously described [9]. Briefly, individual pulses were given and radio-frequency (RF) data were collected using the 10 MHz collinear imaging transducer, at a pulse repetition frequency of 2.54 kHz and with a sampling rate of 50 MHz. Data collection commenced 1 ms prior to the cessation of the pulse and continued 122 ms after that. Data were processed offline using a custom MATLAB (MathWorks, Natick, MA) script.
III. Results
A. SEM
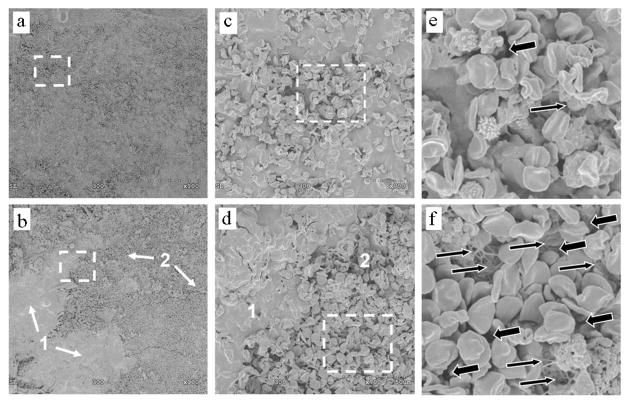
Lower magnification (100×) SEM images of the surface of sham-treated clots appeared visibly different than HIFU-treated ones, where two distinct morphologies were observed. At the higher magnification of 700×, sham controls appeared to be a uniform composite of cellular and acellular components. In contrast, HIFU-treated clots appeared to have two distinct regions with a relatively greater and lesser cellular fraction. Enlarging these distinct regions with higher magnification images revealed that compared to sham controls, the HIFU-treated clots had more exposed fibrin, as well as more openings at their surface. Representative images appear in Fig. 1.
Fig. 1.
SEMs of (upper) sham-treated clots and (lower) HIFU-treated clots. (a) and (b) Taken at 100× magnification; (c) and (d) taken at 700× magnification (dotted boxes in (a) and (b), respectively); (e) and (f) enlarged 3.5-fold from (c) and (d) (dotted boxes). In (b) and (d), 1 and 2 indicate morphologically distinct regions. Large arrows: openings at the clot surface; small arrows: exposed fibrin.
B. Fluorescent Antibody Labeling
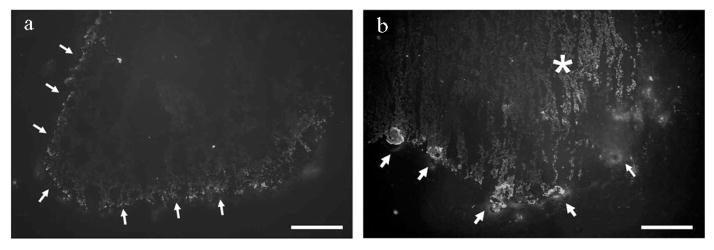
A moderate level of fluorescence was observed at the surface of the sham-treated clots. In comparison, greater focal fluorescent intensity was found on the surface of the clots treated with HIFU. In addition, the HIFU-treated clots demonstrated the presence of tPA in the core of the clot, which was not observed in those that were sham treated. Representative images appear in Fig. 2.
Fig. 2.
Fluorescent antibody labeling of tPA in sections of (a) sham-treated clots and (b) HIFU-treated clots. Arrows indicate tPA accumulation at the clot surface. The asterisk indicates tPA in the core of the clots, which was observed only in those treated with HIFU. Scale bar = 200 μm.
C. Fluorescence Recovery After Photobleaching
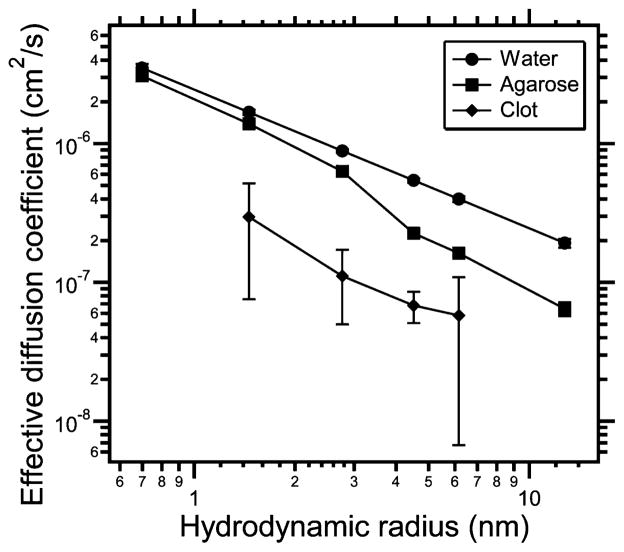
Over the range of dextran sizes that were evaluated, diffusion coefficients in water were highest, followed by 1% agarose, 2% agarose, and finally, the untreated blood clots. These results are summarized in Fig. 3. Over the same range, the increase in diffusion coefficient for the HIFU-treated clots compared to sham controls was increased 1.2- to 6.3-fold. For the 10, 40, and 70 kDa dextrans, these differences were significant (P -value < 0.05, t-test). These experiments were repeated three separate times with similar results in each case. The results appear in Table I.
Fig. 3.
Effective diffusion coefficients as a function of hydrodynamic radius (according to the Stokes–Einstein equation) assuming 25 °C. Diffusion coefficients were measured in water (●), 1% agarose (■), 2% agarose (▲), and blood clots not treated with HIFU (◆). 3 and 2000 kDa for the clot did not satisfy the inclusion criteria. Data are mean± standard deviation (SD) (n = 7–11).
TABLE I.
Diffusion Coefficients for Sham and HIFU-Treated Clots Over Range of Dextrans Evaluated
| DEFF([cm2 s−1] × 10−8)
| |||
|---|---|---|---|
| MW (kDa) | Sham | HIFU | Fold Increase |
| 10 | 29.5 ± 22.0 | 35.8 ± 34.5 | 1.21* |
| 40 | 11.1 ± 6.1 | 20.6 ± 13.7 | 1.86* |
| 70 | 6.8 ± 1.73 | 43.0 ± 10.3 | 6.32* |
| 500 | 5.76 ± 5.09 | 8.07 ± 4.98 | 1.40 |
Significant difference (P-value < 0.05, Mest).
D. Displacement Imaging
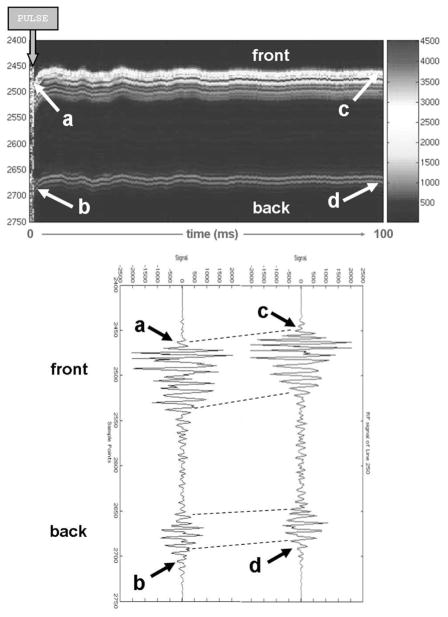
Displacements in the clots were found to be greatest immediately after the cessation of the pulse (243 ± 32 μm; n = 6), and gradually tapered off toward the end of the 100 ms data acquisition period, where displacement was typically less than 10% of the peak value. Displacements were also higher at the front end of the clots (i.e., closer to the transducer); at the back end of the clots, displacements were 176 ± 27 μm (n = 6). A representative time course in displacement in a clot for a single pulse is shown in Fig. 4.
Fig. 4.
Typical displacement profile for a single pulse given in a clot. (Upper image) Sequence of RF lines in the focal zone, indicating the displacement immediately after the pulse is given and the gradual relaxation over a period of 100 ms. (Lower image) Individual RF lines at the beginning (where the displacement is maximal) and end of this period. Relatively bright signals (upper image) and large amplitudes (lower image) correspond to the front and back of the tubing.
IV. Discussion
Pulsed–HIFU exposures used in this study were previously found to significantly enhance thrombolysis of whole blood clots when used in combination with the thrombolytic tPA, both in vitro [3] and in vivo [2]. The results of these studies indicated that HIFU alone did not produce substantial mechanical degradation of the clots, but did improve the effectiveness of tPA. The results of the present study provide more detailed evidence on how this might have occurred. The surface of the HIFU-treated clots observed by SEM showed subjectively that the exposures increased exposed fibrin, as well as the number of openings to more interior regions when compared to sham controls. The result of these openings is demonstrated by fluorescent labeling of tPA in the clot. Improved accumulation at the surface was observed in the HIFU-treated clots, compared to sham controls, as well as penetration into the core of the clots, which was not observed at all in the controls.
Whereas SEM and antibody labeling have previously been used in investigations in clot models [4], [5], to our knowledge, the use of FRAP has not. FRAP analysis has been applied to a range of human tissues to investigate macromolecular transport since its utility was first described as a means to determine 2-D lateral mobility rates [6]. Compared to a study carried out in human cartilage [10], a relatively high degree of heterogeneity was found for transport in the blood clots, both in sham-and HIFU-treated samples. The composition of clots is quite diverse consisting of cellular elements, including platelets, red, and white blood cells, as well as clotting factors. The exact density and orientation of these elements most likely influenced the diffusion coefficient and its high degree of heterogeneity. The more than sixfold increase in diffusion of the 70 kDa dextran through HIFU-treated clots, compared to sham controls, is a very promising result considering that tPA, a commonly used thombolytic, has a very similar molecular weight of 66 kDa. In fact, the rate-limiting step of therapeutic thrombolysis has been shown to be the transport of thombolytics in the clot [11]. This significant improvement with HIFU could represent a clinically relevant increase in transport.
The data presented here provide further detail to the previously reported studies, whose results suggest that pulsed–HIFU-mediated thrombolysis could be attributable to improved penetration and transport in the clots. It does not, however, contribute substantially to helping explain the underlying ultrasound mechanisms for producing these effects. To date, only a limited number of studies have investigated possible mechanisms for improving the effectiveness of thrombolytics using ultrasound. These studies have been restricted to lower intensity exposures using nonfocused ultrasound beams, where reported observations, such as fibrin desegregation [12] and improved penetration [13], were attributable to noninertial cavitation. Whereas a similar mechanism could also have been occurring in the present study, it does not appear likely for the more macroscopic effects observed, which were orders of magnitude greater in scale than the expected influence of local oscillating bubbles.
The redistribution of clot components into acellular and cellular rich regions was seen at the clot surface. The latter appeared to coincide with more exposed fibrin, as well as greater numbers of openings at the surface. This two-phased morphology may explain focal regions of enhanced accumulation of the tPA. A more likely mechanism for such effects was perhaps the repetitive displacements (60 per treatment point), being generated by acoustic radiation forces, which were on the order of hundreds of microns. The possibility that such robust displacements could have caused bulk movement of the clots’ acellular fraction—leading to improved bioavailability of the tPA—is plausible, especially since our previous in vitro study found a trend of increasing thrombolysis using tPA as we increased the power of our exposures and resulting radiation force [3]. Similar pulsed–HIFU exposures generating this type of local tissue movement were recently associated with increases in convective transport in skeletal muscle [9]. In this case, it was proposed that steep gradients in displacement occurring on the edge of the focal zone could generate localized shear forces for opening up cell-to-cell interfaces, and in this way improve transport. Whether the underlying mechanism is radiation forces or something else, or even a combination, will have to be determined in future studies. Ultimately, better understanding of how pulsed–HIFU can improve the effectiveness of thrombolytics will facilitate the optimization of these exposures as well as how and when to specifically take advantage of this tool for clinical applications.
It is important to point out the limitations of our study, perhaps most importantly, this clot model may not reproduce the biology of an organized, chronic in vivo clot, which has very different mechanical and physiological properties. Clinically, HIFU may be most relevant when applied to the chronic clot, which is most recalcitrant to conventional means of thrombolysis. The mechanisms studied could still have implications for clot lysis with HIFU of course.
Acknowledgments
The work of G. Jones and M. J. Stone was supported by the Howard Hughes Medical Institute. This work was supported in part by the intramural research program of the National Institutes of Health (NIH) Clinical Center.
The authors would like to than P. Merryman for her help with procuring blood for the clots, and Mr. K. Nagashima for his help with the SEM.
Contributor Information
Guy Jones, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA.
Finnie Hunter, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA.
Hilary A. Hancock, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA
Ankur Kapoor, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA.
Michael J. Stone, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA
Bradford J. Wood, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA
Jianwu Xie, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA.
Matthew R. Dreher, The Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA
Victor Frenkel, Email: vfrenkel@cc.nih.gov, The Molecular Imaging Laboratory, Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892 USA.
References
- 1.Pfaffenberger S, Devcic-Kuhar B, Kastl SP, Huber K, Maurer G, Wojta J, Gottsauner-Wolf M. Ultrasound thrombolysis. Thromb Haemost. 2005;94(1):26–36. doi: 10.1160/TH04-12-0818. [DOI] [PubMed] [Google Scholar]
- 2.Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res. 2007;121:193–202. doi: 10.1016/j.thromres.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenkel V, Oberoi J, Park M, Deng C, Stone MJ, Neeman Z, Wood BJ, Horne MK, III, Li KCP. Pulsed-high intensity focused ultrasound (HIFU) enhances thrombolysis in and in vitro model. Radiology. 2006;239(1):86–93. doi: 10.1148/radiol.2391042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gersh KC, Nagaswami C, Weisel JW, Lord ST. The presence of gamma’ chain impairs fibrin polymerization. Thromb Res. 2009;124:356–363. doi: 10.1016/j.thromres.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator–plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiffert S, Oppermann W. Systematic evaluation of FRAP experiments performed in a confocal laser scanning microscope. J Microsc. 2005;220:20–30. doi: 10.1111/j.1365-2818.2005.01512.x. [DOI] [PubMed] [Google Scholar]
- 8.Fischler MA, Bolles R. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Commun ACM. 1981;24:381–395. [Google Scholar]
- 9.Hancock HA, Smith LH, Cuesta J, Durrani AK, Angstadt M, Palmeri ML, Kimmel E, Frenkel V. Investigations into pulsed high-intensity focused ultrasound enhanced delivery: Preliminary evidence for a novel mechanism. Ultrasound Med Biol. 2009 Jul 16; doi: 10.1016/j.ultrasmedbio.2009.04.020. [Online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fetter NL, Leddy HA, Guilak F, Nunley JA. Composition and transport properties of human ankle and knee cartilage. J Orthop Res. 2006;24:211–219. doi: 10.1002/jor.20029. [DOI] [PubMed] [Google Scholar]
- 11.Blinc A, Francis CW. Transport processes in fibrinolysis and fibrinolytic therapy. Thromb Haemost. 1996;76:481–491. [PubMed] [Google Scholar]
- 12.Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78:1063–1068. [PubMed] [Google Scholar]
- 13.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]