Abstract
Objective
As human blastocyst-derived extravillous trophoblasts (EVTs) invade the early decidua, they are positioned to interact with immune cells and resident decidual cells, and remodel spiral arteries into high capacity vessels that increase blood flow to the developing fetal-placental unit. Shallow EVT invasion elicits incomplete vascular transformation and reduces uteroplacental blood flow that presages adverse pregnancy outcomes. Excess macrophages in the decidua induce EVT apoptosis via tumor necrosis factor-alpha (TNF-α) secretion. Our previous observation that pro-inflammatory cytokines enhance neutrophil and macrophage activator granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in first trimester decidual cells is now extended to include: 1) the specific macrophage activator M-CSF; 2) macrophage activation and subsequent enhancement of EVT apoptosis by both GM-CSF and M-CSF.
Study design
Quantitative reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay assessed M-CSF expression in first trimester decidual cells incubated with interleukin-1 beta (IL-1β) or TNF-α. Peripheral monocyte-derived macrophages pre-incubated with conditioned media from decidual cell cultures were co-cultured with a first trimester EVT cell line, HTR-8/SVneo cells. Macrophage activation was examined and EVT apoptosis evaluated by DNA fragmentation, caspase activation and cell membrane asymmetry.
Results
IL-1β or TNF-α significantly enhanced M-CSF expression in first trimester decidual cells. The conditioned media from these cultures activates macrophages, which promote caspase 3/7-dependent EVT apoptosis with antibodies against GM-CSF or M-CSF blocking this effect.
Conclusions
Pro-inflammatory cytokines increases synthesis of M-CSF in first trimester decidual cells. Both GM-CSF and M-CSF activate macrophages, which initiate caspase-dependent EVT apoptosis.
Keywords: First trimester decidual cells, Macrophage, Extravillous trophoblast, Apoptosis, GM-CSF, M-CSF
1. Introduction
Blastocyst-derived extravillous trophoblasts (EVTs) invade an underlying decidua comprised primarily of resident decidual cells (50%) and immune cells (40%) [1]. Invasion of the decidua and inner third of the myometrium is accompanied by conversion of uterine spiral arteries into high capacity vessels that increase blood flow to the developing fetal-placental unit [2]. By contrast, shallow decidual invasion is associated with incomplete vascular remodeling. The resulting impaired uteroplacental blood flow is inadequate to support the developing fetal-placental unit [2] and is associated with such disorders of pregnancy as preeclampsia, intrauterine growth restriction (IUGR), miscarriage, preterm birth and placental abruption [3].
Shallow EVT invasion of the decidua reflects decreased EVT invasiveness and/or exaggerated apoptosis that produces lower numbers of invading EVTs [4,5]. Aberrant augmentation of EVT apoptosis has been reported in pregnancies complicated by gestational trophoblast disease [6], preeclampsia [7], IUGR [8] and preterm birth [9]. The consequent increased shedding of EVT debris into the maternal circulation is associated with excess inflammation [10] that may lead to rejection of fetus. Conversely, over invasion of the decidua with insufficient EVT apoptosis is associated with blastocyst implantation on impaired decidua and frequently leads to placenta creta, which carries life threatening risks [11].
At the maternal interface of first trimester human decidua, invading EVTs interact with resident decidual cells and such immune cells as natural killer cells, dendritic cells and Mϕs [12]. Comprising 20–25% of first trimester human decidual leukocytes, Mϕs are major antigen-presenting cells that confer immune tolerance of the semi-allogeneic fetal transplant [13]. They act as major scavengers of apoptotic cells thereby reducing local inflammation [14]. Close apposition of significant number of Mϕs to invading EVTs positions decidual Mϕs to mediate normal EVT invasion by remodeling the decidua secondary to phagocytosis of apoptotic cells. Conversely, an excess of Mϕs in the preeclamptic decidua reported by our [15] and other laboratories has been shown to induce excess apoptosis in invading EVTs via the synthesis and release of TNF-α [16], thereby contributing to shallow preeclampsia-related EVT invasion.
CSFs are myeloid growth factors secreted by various cell types and exhibit pleiotropic functions. Specifically, GM-CSF is a potent differentiation-inducer and activator of Mϕs [17]. Previously, our laboratory found that pro-inflammatory cytokines associated with preeclampsia, miscarriage and preterm birth [18-20], IL-1β and TNF-α, markedly up-regulated GM-CSF production in cultured first trimester decidual cells. Complementing these in vitro observations, immunostaining revealed aberrantly high GM-CSF levels in preeclamptic versus gestational-age matched decidual cells [23]. In view of the established link between Mϕ-induced apoptosis of EVTs in preeclampsia [16] taken together with several reports indicating that macrophage-CSF (M-CSF) is a highly specific and potent inducer of differentiation and activation of Mϕs [21] and mediates Mϕ infiltration in the normal decidua [22], the current study: 1) evaluated the effects of IL-1β and TNF-α on M-CSF expression in first trimester decidual cells; 2) determined whether Mϕs can be activated by excess GM-CSF and M-CSF secreted by first trimester decidual cells; 3) assessed whether CSF mediated the enhancement of Mϕ-induced EVT apoptosis.
2. Materials and methods
2.1. Cell culture
2.1.1. First trimester decidual cell isolation
First trimester decidual cells were isolated as previously described [15]. Briefly, decidual specimens from elective terminations between 6 and 12 weeks of gestation were obtained under Yale University Human Investigation Committee approval. After digestion with 0.1% collagenase type IV and 0.01% DNase in Ham’s F-10, the digestate was purified on 60/50/40% Percoll gradient. Cells were then cultured in basal medium, a phenol red-free 1:1 v/v mixture of DMEM and Ham’s F-12 (Sigma–Aldrich, St. Louis, MO) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 ng/ml fungizone (Invitrogen, Carlsbad, CA) and 10% charcoal-stripped calf serum (Sigma–Aldrich). Cell purity was determined by flow cytometric analysis of anti-CD14 and anti-CD45 (BD Pharmingen, San Diego, CA) to monitor the presence of leukocytes. Cultured decidual cells were vimentin-positive and cytokeratin 7-negative and displayed decidualization-related morphological and biochemical changes during incubation with progestin, including enhanced prolactin and plasminogen activator inhibitor-1 and inhibited interstitial collagenase and stromelysin-1 expression (results not shown). After 6 passages, confluent leukocyte-free decidual cells were primed with estradiol (10−8 M) + medroxyprogesterone acetate (10−7 M) for 7d, then stimulated in serum-free fresh medium ± 10 ng/ml IL-1β or TNF-α (R&D Systems, Minneapolis, MN) for 24 h. Conditioned media (CM) were stored at −80 °C.
2.1.2. Isolation of peripheral blood monocytes and development of macrophages
Peripheral blood mononuclear cells were isolated from healthy reproductive age female donors by gradient Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) centrifugation. The monocytes (MOs) were purified with anti-CD14 paramagnetic beads from Miltenyi Biotec (Auburn, CA) according to the manufacturer’s instructions. Mϕs were developed from MOs as previously described [22]. Briefly, MOs were cultured in AIM V serum-free medium (Invitrogen) for 5d. The purity of MOs and attached Mϕs was determined by flow cytometric analysis of CD14 and CD11b expression, respectively.
2.1.3. Co-culture
HTR-8 cells, a generous gift from Dr. Charles Graham [23], were labeled with PKH67 (green fluorescence) or PKH26 (red fluorescence) according to the manufacturer’s instructions (Sigma–Aldrich). MO-derived Mϕs were pre-incubated in CM from first trimester decidual cells ± IL-1β or TNF-α ± anti-GM-CSF or anti-M-CSF neutralizing antibody for 48h. After pre-incubation, Mϕs were harvested and co-cultured with HTR-8 cells (HTR-8: Mϕ = 2:1) for 6, 12 or 24 h. After pre-incubation with CM, Mϕs were stained with anti-human CD16-PerCP-Cy5.5, CD18-APC, CD86-FITC and HLA-DR-PE antibodies to examine the expression of activation markers [24-26] on an LSRII flow cytometer using FACSDiva (BD Biosciences, San Jose, CA) and FlowJo software (FreeStar, Ashland, OR).
2.2. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Reverse transcription was carried out using Omniscript kit (Qiagen, Valencia, CA). Each RT reaction contained 2 μg of total RNA, 2 μl 1× buffer RT, 0.5 mM dNTPs, 1 μM T7-(dT)24 oligo-primer and 4 units of Omniscript reverse transcriptase. Specific primer pairs (Table 1) were designed and synthesized at the Yale DNA Synthesis Laboratory (Critical Technologies) for qPCR. A standard curve was created utilizing various concentrations of RT products by monitoring the increasing fluorescence of PCR products during amplification. Quantification of unknowns was determined and adjusted to the quantitative expression of β-actin from each unknown. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation.
Table 1.
Sequences of primer sets for M-CSF.
| Gene | Forward primers | Reverse primers |
|---|---|---|
| β-actin | 5′-GCTTCTTTGCAGCTCCTTCGTT-3′ | 5′-GTTGTCGACGACCAGCGC-3′ |
| M-CSF | 5′ -GTAGCCACATGATTGG-3′ | 5′-GTTATCTCTGAAGCGC-3′ |
2.3. Enzyme-linked immunosorbent assay (ELISA)
Total cell protein levels of whole decidual cell lysates were measured by the Bio-Rad Assay (Bio-Rad, Hercules, CA). A commercial ELISA kit was used to measure the levels of M-CSF in CM according to manufacturer’s instructions (R&D Systems). The ELISA has a sensitivity of <11.5 pg/ml. The intra-assay and inter-assay coefficients of variation vary from 2.1 to 7.8 and from 5.4 to 10.7, respectively.
2.4. DNA content analysis
After co-culture, both adherent and floating cells were harvested by 0.5%Tripsin/EDTA and fixed in 70% ice-cold ethanol for >1 h at −20 °C. Permeablized cells were washed and resuspended in 1× PBS and incubated with propidium iodide (PI)/RNase staining buffer (BD Pharmingen) at room temperature for 30min prior to flow cytometric analysis using CellQuest Pro (BD Biosciences) and FlowJo software. An individual population of cells was selected by gating on an area versus width dot plot to exclude cell debris and for doublet discrimination. Difference in DNA degradation was examined by gating on subdiploid peaks of PKH67-labeled HTR-8 cells then analyzing the mean value obtained from 4 experiments.
2.5. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
DeadEnd™ Fluorometric TUNEL System from Promega (Madison, MI) was used to examine DNA fragmentation. After co-culture of PKH26 (red)-labeled HTR-8 cells with Mϕs in CM for 24 h, cells were fixed in 4% formaldehyde for 25 min at 4 °C. After permeablization in 0.2% Triton X-100, addition of equilibration buffer was followed by incubation with the TdT reaction mix for 1 h at 37 °C. Finally, the cover slips were mounted with VECTASHIELD® Mounting Medium containing 4,6-diamidino-2-phenylindole (Vector Lab, Burlingame, CA). The numbers of TUNEL-positive and -negative nuclei were counted in 3 randomly selected microscopic fields at 200 × magnification. The DNA fragmentation index was calculated as the number of cells with green fluorescence divided by the number of total red PKH26-stained cells.
2.6. Western blotting analysis
After co-culture with Mϕs for 24 h, HTR-8 cells were harvested in whole cell lysis buffer containing Complete Lysis-M (Roche, Indianapolis, IN). Protein concentration was determined by DC Protein Assay II (Bio-Rad). Fifty microgram of each sample were electrophoresed on 4–20% Mini-Protean TGX Ready Gels (Bio-Rad) and transferred to a nitrocellulose membrane. Membranes were then blocked in Odyssey blocker (LI-COR Biosciences, Lincoln, NE) and incubated with rabbit anti-human caspase 3 or anti-human caspase 7 (Cell Signaling, Beverly, MA) and mouse anti-human hsp90 overnight at 4 °C. After 1 h incubation in secondary IRDye 800-conjugated donkey anti-rabbit or anti-mouse (1:8000) antibody (Rockland, Gilbertsville, PA) at room temperature, membranes were washed in PBS + 0.1% Tween-20 × 3. Signals were detected by an Odyssey Infrared Imaging System (LI-COR Biosciences).
2.7. Caspase Glo-3/7 analysis
After co-culture with Mϕs pre-incubated with CM from first trimester decidual cells for 24 h, HTR-8 cells were incubated with 100 μl caspase 3/7 reagent (Promega, Madison, WI) at room temperature for 2h. Luminescence was measured at 450 nm. Activity of caspase 3/7 was expressed as arbitrary luminescence units.
2.8. Annexin V-APC/7-AAD staining
After co-culturing PKH67-labeled HTR-8 cells with Mϕs in CM in the presence or absence of 40 μM pan-caspase inhibitor, z-VAD (R & D Systems, Minneapolis, MN), cells were resuspended in 100 μl of 1 × annexin V binding buffer (BD Pharmingen) with annexin V-allophycocyanin (APC) (5 μl/106 cells) and 7-amino-actinomysin D (7-AAD) (5 μl/106 cells) (BD Biosciences) and incubated for 15 min at room temperature in the dark. The cells were analyzed on a FACSCalibur using CellQuest Pro and FlowJo software. The percentage of annexin V positive and annexin V/7-AAD double positive cells was calculated by gating on green PKH67-labeled cells.
2.9. Statistics
Normally and non-normally distributed data were distinguished by the Kolmogorov–Smirnov one-sample test before applied the appropriate parametric or non-parametric test. Results are expressed as mean ± SEM. Differences were evaluated by Wilcoxon Signed Ranks test or the Student’s t-test, when appropriate. A p < 0.05 was considered significant.
3. Results
3.1. M-CSF expression is up-regulated by IL-1β and TNF-α in first trimester decidual cells
Fig. 1A indicates that incubation of first trimester decidual cells with either IL-1β or TNF-α significantly enhanced M-CSF mRNA levels by 10-fold (n = 5, p < 0.01) and 9-fold (n = 5, p < 0.005), respectively. Secreted levels of M-CSF in parallel incubations are significantly up-regulated by IL-1β or TNF-α by 14-fold (n = 6, p < 0.005) and 13-fold (n = 6, p < 0.05), respectively (Fig. 1B).
Fig. 1.
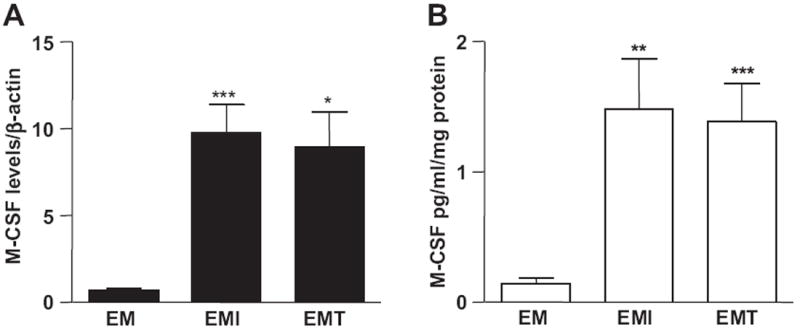
IL-1β and TNF-α increase M-CSF mRNA and protein expression in first trimester decidual cells. Leukocyte-free first trimester decidual cells were primed with estradiol (E) + medroxyprogesterone acetate (M), then incubated with steroids alone (EM) or with steroids plus IL-1β (EMI) or TNF-α (EMT) for 6 h (for qRT-PCR) or 24 h (for ELISA). The results are normalized to β-actin and total cell protein for (A) qRT-PCR and (B) ELISA for M-CSF mRNA and protein, respectively. The results are reported as the mean ± SEM. (n = 3–6; *P < 0.05, **P < 0.01, ***P < 0.005).
3.2. First trimester decidual cell-derived GM-CSF and M-CSF mediate the activation of macrophages
The expression of HLA-DR (Fig. 2A), CD16 (Fig. 2B), CD18 (Fig. 2C) and CD86 (Fig. 2D) in peripheral MO-derived Mϕs was induced by CM from IL-1β- or TNF-α-treated first trimester decidual cells. The treatment of anti-GM-CSF antibody effectively blocked such induction (Fig. 2A, C, D) except CD16 expression induced by IL-1β- or TNF-α-treated first trimester decidual cells (Fig. 2B), while anti-M-CSF antibody inhibited the up-regulation of HLA-DR (Fig. 2A), CD16 (Fig. 2B), CD18 (Fig. 2C) and CD86 (Fig. 2D).
Fig. 2.
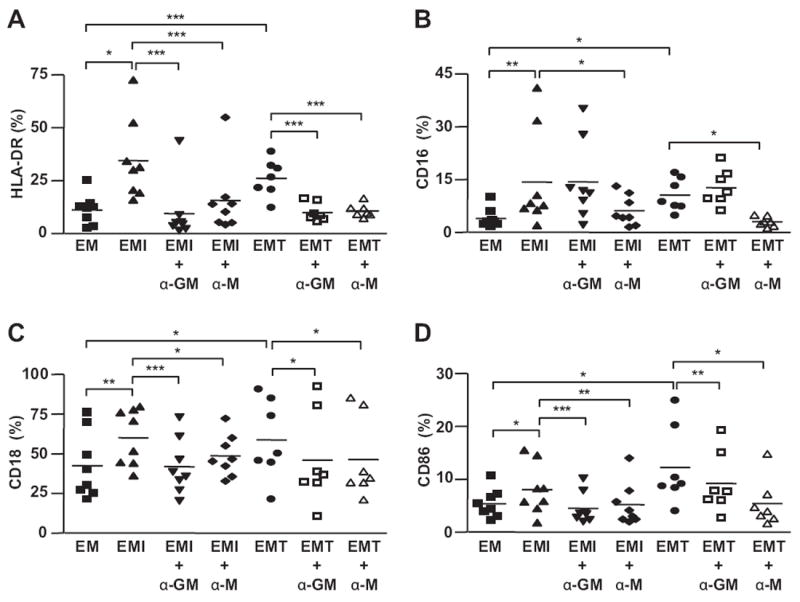
First trimester decidual cell-derived GM-CSF and M-CSF mediate the activation of macrophages. Flow cytometric analysis of the expression of activation markers, (A) HLA-DR, (B) CD16, (C) CD18, (D) CD86, on Mϕs cultured with CM from 1st trimester decidual cells treated with or without IL-1β (EMI) or TNF-α (EMT) in the presence or absence of anti-human GM-CSF (α-GM) or anti-human M-CSF (α-M) neutralizing antibody for 48 h. The results are reported as the mean ± SEM. (n = 7–8; *P < 0.05, **P < 0.01, ***P < 0.005).
3.3. DNA fragmentation in HTR-8 cells was induced by Mϕs and enhanced by IL-1β- or TNF-α-stimulated first trimester decidual cells
The subdiploid peak in a DNA histogram reflects DNA fragmentation, a hallmark of cell death. After co-culture with Mϕs, detachment of HTR-8 cells with membrane blebbing was observed in the HTR-8 cells (Supplementary Fig. 1E and G). Compared to the controls, Mϕ-induced HTR-8 DNA fragmentation was enhanced by pre-incubation of Mϕs with CM from IL-1β- or TNF-α-treated first trimester decidual cells from 20% to 29% or 31%, respectively (Fig. 3A and B). The localization of DNA fragmentation in HTR-8 cells was also observed by TUNEL assay. As indicated by Fig. 3C, numbers of TUNEL-positive (turquoise) PKH26-labeled HTR-8 cells (red) with apoptotic body formation were increased by co-culturing with Mϕs pre-incubated with CM from first trimester decidual cells treated by IL-1β (Supplementary Fig. 1E and F) or TNF-α (Supplementary Fig. 1G and H).
Fig. 3.
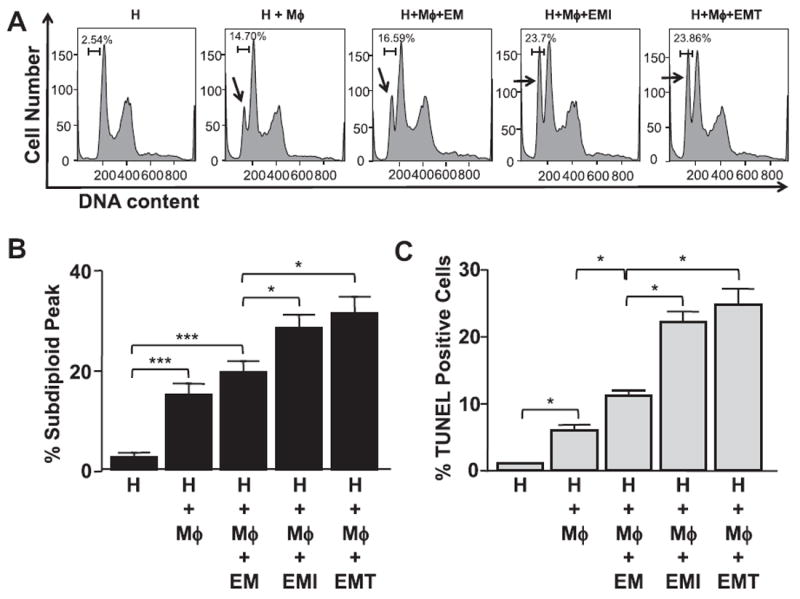
DNA fragmentation of HTR-8 cells. PI staining for DNA content in PKH26 (Red)-labeled HTR cells co-cultured with Mϕs in CM from 1st trimester decidual cells treated with or without IL-1β (EMI) or TNF-α (EMT) for 24 h. Arrows indicate the subdiploid peaks of DNA histogram. (A) Representative DNA histograms; (B) Percentage of cells in subdiploid peak; (C) TUNEL assay. The results are reported as the mean ± SEM. (n = 4; *P < 0.05, ***P < 0.005).
3.4. First trimester decidual cell-derived GM-CSF and M-CSF mediate caspase 3/7 activation in HTR-8 cells co-cultured with Mϕs
Apoptosis of HTR-8 cells was confirmed by the examination of caspase 3/7 activation. Activation of caspases reflects a reduction of pro-caspases accompanied by an increase of caspases. Compared to control, the expression of caspase 3/7 was induced in HTR-8 cells co-cultured with Mϕs following their incubation with CM from IL-1β- or TNF-α-treated first trimester decidual cells (Fig. 4A). Western blotting analysis demonstrated reduced expression of pro-caspase 3 and pro-caspase 7 and increased expression of caspase 3 (Fig. 4B) and caspase 7 (Fig. 4C) in HTR-8 cells after co-culture. The induction of caspase 3 and 7 was significantly inhibited by the treatment of anti-GM-CSF or -M-CSF neutralizing antibody (Fig. 4B–C). The enhancement of apoptosis by CM is blocked by a pan-caspase inhibitor (Supplementary Fig. 2).
Fig. 4.
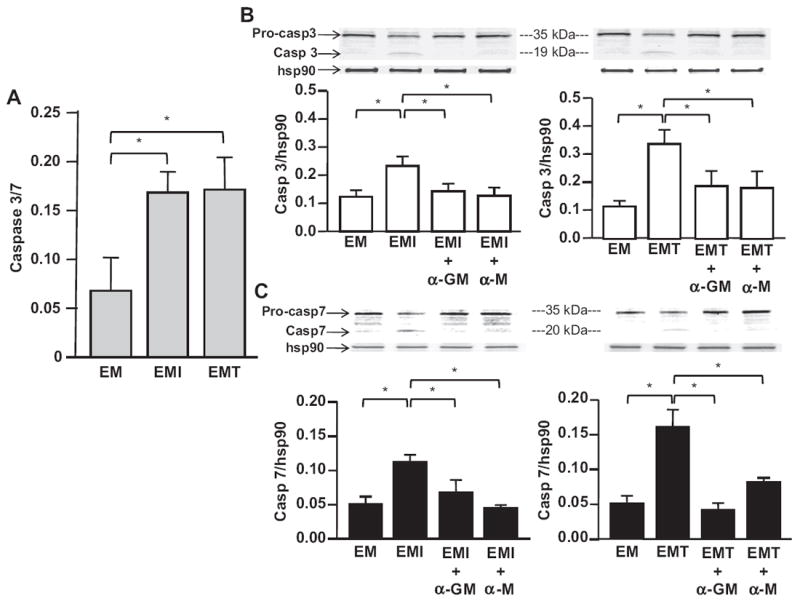
Caspase 3/7 expression in HTR-8 cells. (A) Quantitative caspase Glo-3/7 colorimetric assay; Western blotting analysis of (B) caspase 3 and (C) caspase 7 expression in HTR-8 cells (H) co-cultured with Mϕs in CM from 1st trimester decidual cells treated with or without IL-1β (EMI) or TNF-α (EMT) for 24 h. Caspase signaling intensity was normalized to the abundance of the housekeeping protein, hsp90, in Western blotting. The results are reported as mean ± SEM. (n = 3; *P < 0.05, **P < 0.01).
3.5. Decidual cell-derived GM-CSF and M-CSF each mediates enhancement of Mϕ-induced HTR-8 apoptosis
Apoptosis is distinguished from necrosis by sequential phosphotidylserine (PS) externalization and DNA degradation in a kinetic study (Supplementary Fig. 3A and B). Apoptosis is characterized by early externalization of membrane-bound PS (annexin V staining) followed by DNA degradation (7-AAD staining). By contrast, PS/DNA degradation-double positive staining is evident by the absence of early PS externalization in necrosis. In the kinetic study carried out for Fig. 5, co-staining of annexin V and 7-AAD displays a shift from early annexin V-single positive staining to late annexin V/7-AAD double positive staining in apoptotic cell population confirming that HTR-8 cell death induced by Mϕs progressed from annexin V-single positive to annexin V/7-AAD double positive (Supplementary Fig. 3A and B). Fig. 5 demonstrates that HTR-8 apoptosis induced by Mϕs is enhanced following pre-incubation with CM from IL-1β- (Fig. 5A and B) or TNF-α-treated (Fig. 5A and C) first trimester decidual cells. As expected, pre-treatment with anti-human GM-CSF or M-CSF neutralizing antibody significantly inhibited such enhancement (Fig. 5A–C), suggesting that both GM-CSF and M-CSF play roles in promoting the apoptosis-inducing activity of Mϕs.
Fig. 5.
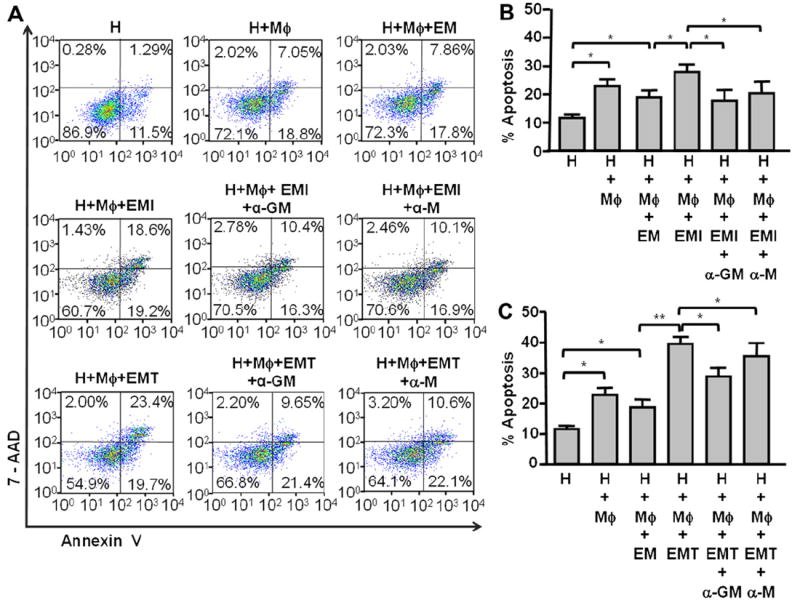
Apoptosis of HTR-8 cells. (A) Apoptosis in HTR-8 cells (H) co-cultured with Mϕs under the influence of CM from 1st trimester decidual cells treated with or without IL-1β (EMI) or TNF-α (EMT) in the presence or absence of anti-human GM-CSF (α-GM) or anti-human M-CSF (α-M) neutralizing antibody for 24 h was examined by 7-AAD/annexin V co-staining. (B) & (C) The total percentage of HTR-8 cells undergoing apoptosis was calculated as the sum of cells in early apoptosis with 7-AAD−/annexin V+ (right lower quadrant) and late apoptosis are 7-AAD+/annexin V+ (right upper quadrant). The results are reported as mean ± SEM. (n = 4; *P < 0.05, **P < 0.01).
4. Discussion
Successful pregnancy requires immunological coordination between a receptive endometrium and the implanting semi-allogeneic blastocyst. In the first trimester human decidua, 20–25% of the immune cells are Mϕs. Substantial numbers of these are closely associated with invasive EVTs throughout human pregnancy [27]. Mϕs are implicated in mediating both normal and abnormal placentation as well as in modulating the placental response to infection [28,29]. At the implantation site, recruited Mϕs accumulate adjacent to EVTs [27] where they phagocytose decidual apoptotic cells. The resulting remodeling of the decidua is proposed to promote EVT invasion [30]. However, the association of aberrant apoptosis of EVTs and excess Mϕs reflects an exaggerated maternal inflammatory response to the invading EVTs. The resulting impaired EVT invasion [16,31] is implicated in the pathogenesis of adverse pregnancy outcomes [32,33]. In addition, excess IL-1β and TNF-α production by Mϕs associated with infections [34,35] and polymorphisms [36] are accompanied by impaired EVT invasion that may in turn lead to adverse pregnancy outcomes. Although decidual cell is the predominant cell type encountered by recruited Mϕs and invading EVTs at the implantation site, direct evidence demonstrating interactions among these cells is lacking. Thus, the current study assessed interactions among EVTs, Mϕs and first trimester decidual cells with the goal of obtaining insight into how these interactions may go awry and contribute to the pathophysiology of adverse pregnancy outcomes associated with shallow EVT invasion, including miscarriage, IUGR, preterm birth and preeclampsia.
Previously, our laboratory demonstrated that incubation of primary first trimester decidual cells with pro-inflammatory cytokines associated with adverse pregnancy outcomes enhances production of GM-CSF, which activated both neutrophils (granulocytes) and MOs/Mϕs, and increased the expression of several MO/Mϕ-recruiting chemokines [22,28]. Augmenting our observations on GM-CSF expression, the current study demonstrates that incubation of first trimester decidual cells with either IL-1β or TNF-α markedly enhances expression of more potent and more specific Mϕ activator, M-CSF. In the current study, Mϕ activation was reversed by incubation of CM from IL-1β- or TNF-α-treated first trimester decidual cells with neutralizing antibodies against either GM-CSF or M-CSF, suggesting that both CSFs play important roles in activating Mϕs. Among the activation markers examined in this study, CD16 was identified as an Fc receptor which binds to Fc portion of IgG antibodies and only expresses in a minor subset of macrophages. Consistent with a previous reported in neutrophils [37], the current study demonstrates that first trimester decidual cell-derived GM-CSF does not affect CD16 expression in macrophages. M-CSF has been demonstrated to induce the expression of CD16 in macrophages [38]. First trimester decidual cell-derived M-CSF is now revealed to induce CD16 expression in macrophages.
In view of the well-established role of Mϕs in initiating innate immune response against pathogens and inducing apoptosis in various cell types, the current observations suggest that excess activated Mϕs in decidua may also induce EVT apoptosis. Apoptosis is characterized by a sequential cell shrinkage, loss of cell membrane asymmetry, chromatin condensation, internucleosomal cleavage of chromatin, and apoptotic body formation [39]. In the current study, Mϕ-induced EVT cell death was assessed by assays that measure both early and late apoptotic events. The current study provides the first demonstration that first trimester decidual cells treated with pro-inflammatory stimuli promote Mϕ-induced EVT apoptosis. GM-CSF and M-CSF play crucial roles in this apoptosis induction in both early and late apoptotic events. Consequently, the balance between life and death of EVTs is shifted in favor of the latter. The resulting reduction in the numbers of viable EVTs leads to shallow EVT invasion and impaired vascular remodeling. In addition to Mϕs, NK cells are suggested to play a role in the pathogenesis of adverse pregnancy outcomes via their cytotoxicity as a result of immune maladaptation [40].
Observations presented in the current study were made with on primary first trimester decidual cells and Mϕs, from healthy donors to best mimic in vivo conditions and used a large number of donors to decrease the impact of individual variation among donors. A kinetic study of apoptosis at various time points using annexin V-APC and 7-AAD staining was used to track the apoptotic cells from annexin V and 7-AAD double negative (viable) to annexin V positive and 7-AAD negative (early apoptosis) and finally to annexin V and 7-AAD double positive (late apoptosis) (Supplementary Fig. 3A and B). Annexin V/7-AAD double positive cannot distinguish late apoptosis from necrosis. Annexin V positive/7-AAD negative cells will not be detected when the cells are undergoing necrotic cell death.
In summary, the current study reveals that stimulation of first trimester decidual cells by pro-inflammatory cytokines promotes Mϕ-induced EVT apoptosis through a caspase-dependent pathway and that decidual cell-derived GM-CSF and M-CSF mediate Mf activation that serves as a prelude to enhancing this apoptosis. These observations suggest that inflammation-mediated augmentation of decidual cell secreted GM-CSF and M-CSF plays a heretofore undisclosed role in activating Mϕs. Future studies will: a) dissect the mechanisms that regulate EVT apoptosis induced by macrophages activated during incubation with CM obtained from pro-inflammatory stimuli-treated first trimester decidual cells; b) determine whether the induction of EVT apoptosis is mediated by cellecell contact or paracrine effectors; c) evaluate the effects of GM-CSF and M-CSF on the development of adverse pregnancy outcomes studies using relevant in vivo mouse models. The results of such efforts are expected to provide insight into the patho-physiology of major complications of human pregnancy and will lead to the development of novel preventative and therapeutic strategies.
Supplementary Material
Acknowledgments
We are very grateful to the research nurses, Ms. Luisa Coraluzzi, Ms. Erin Kustan and Ms. Cheryl Danton, for their great efforts in the collection of clinical specimens. We also thank Ms. Lynn Buch-walder and Dr. Mizanur Rahman for their assistance in ELISA and cell culture, respectively.
Funding
This work is supported by National Institute of Child Health and Development, National Institutes of Health grant 5R01HD056123-03 (S. J. Huang), Albert Mckern Award (S. J. Huang) and March of Dime 21-FY05-1249 grants (C. J. Lockwood).
Footnotes
Appendix. Supplementary data
Supplementary data related to this article can be found online at doi:10.1016/j.placenta.2011.12.007.
Conflict of interest
None of the authors disclose any conflict of interest.
References
- 1.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63(1):1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 4.Whitley GS, Dash PR, Ayling LJ, Prefumo F, Thilaganathan B, Cartwright JE. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am J Pathol. 2007;170(6):1903–9. doi: 10.2353/ajpath.2007.070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26(7):877–97. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 6.Chiu PM, Ngan YS, Khoo US, Cheung AN. Apoptotic activity in gestational trophoblastic disease correlates with clinical outcome: assessment by the caspase-related M30 CytoDeath antibody. Histopathology. 2001;38(3):243–9. doi: 10.1046/j.1365-2559.2001.01065.x. [DOI] [PubMed] [Google Scholar]
- 7.Heazell AE, Buttle HR, Baker PN, Crocker IP. Altered expression of regulators of caspase activity within trophoblast of normal pregnancies and pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(10):1034–43. doi: 10.1177/1933719108322438. [DOI] [PubMed] [Google Scholar]
- 8.Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186(5):1056–61. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 9.Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34(12):1265–75. doi: 10.1016/j.humpath.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195(1):29–39. doi: 10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–45. doi: 10.1016/j.placenta.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Gorivodsky M, Torchinsky A, Shepshelovich J, Savion S, Fein A, Carp H, et al. Colony-stimulating factor-1 (CSF-1) expression in the uteroplacental unit of mice with spontaneous and induced pregnancy loss. Clin Exp Immunol. 1999;117(3):540–9. doi: 10.1046/j.1365-2249.1999.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17(3):209–18. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 14.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14(3):131–6. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 15.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214(3):328–36. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 16.Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81(8):1143–52. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- 17.Jokhi PP, King A, Loke YW. Production of granulocyte-macrophage colony-stimulating factor by human trophoblast cells and by decidual large granular lymphocytes. Hum Reprod. 1994;9(9):1660–9. doi: 10.1093/oxfordjournals.humrep.a138769. [DOI] [PubMed] [Google Scholar]
- 18.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. sSemin Immunopathol. 2007;29(2):151–62. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 19.Calleja-Agius J, Schembri-Wismayer P, Calleja N, Brincat M, Spiteri D. Obstetric outcome and cytokine levels in threatened miscarriage. Gynecol Endocrinol. 2011;27(2):121–7. doi: 10.3109/09513590.2010.487614. [DOI] [PubMed] [Google Scholar]
- 20.El-Shazly S, Makhseed M, Azizieh F, Raghupathy R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol. 2004;52(1):45–52. doi: 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 21.Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF-1–a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–9. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 22.Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, et al. The implication of aberrant GM-CSF expression in decidual cells in the pathogenesis of preeclampsia. Am J Pathol. 2010;177(5):2472–82. doi: 10.2353/ajpath.2010.091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 24.Andreesen R, Brugger W, Scheibenbogen C, Kreutz M, Leser HG, Rehm A, et al. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990;47(6):490–7. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 25.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 26.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156(1):191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 27.Bulmer JN, Johnson PM, Sasagawa M, Takeuchi S. Immunohistochemical studies of fetal trophoblast and maternal decidua in hydatidiform mole and choriocarcinoma. Placenta. 1988;9(2):183–200. doi: 10.1016/0143-4004(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 28.Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72(1–2):60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JS. Current topic: the role of macrophages in the uterine response to pregnancy. Placenta. 1990;11(6):467–75. doi: 10.1016/s0143-4004(05)80192-4. [DOI] [PubMed] [Google Scholar]
- 30.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174(5):1959–71. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155(1):293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 33.Bansal AS. Joining the immunological dots in recurrent miscarriage. Am J Reprod Immunol. 2010;64(5):307–15. doi: 10.1111/j.1600-0897.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 34.Rothermel CD, Schachter J, Lavrich P, Lipsitz EC, Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989;57(9):2705–11. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprecher E, Becker Y. Detection of IL-1 beta, TNF-αlpha, and IL-6 gene transcription by the polymerase chain reaction in keratinocytes, Langerhans cells and peritoneal exudate cells during infection with herpes simplex virus-1. Arch Virol. 1992;126(1–4):253–69. doi: 10.1007/BF01309699. [DOI] [PubMed] [Google Scholar]
- 36.Reid JG, Simpson NA, Walker RG, Economidou O, Shillito J, Gooi HC, et al. The carriage of pro-inflammatory cytokine gene polymorphisms in recurrent pregnancy loss. Am J Reprod Immunol. 2001;45(1):35–40. doi: 10.1111/j.8755-8920.2001.450106.x. [DOI] [PubMed] [Google Scholar]
- 37.Griffin JD, Spertini O, Ernst TJ, Belvin MP, Levine HB, Kanakura Y, et al. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990;145(2):576–84. [PubMed] [Google Scholar]
- 38.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, et al. M2 macrophages phagocytose rituximabopsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182(7):4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 39.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90(1):105–10. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


