Abstract
Load bearing porous biodegradable scaffolds are required to engineer functional tissues such as bone. Mechanical improvements to porogen leached scaffolds prepared from silk proteins were systematically studied through the addition of silk particles in combination with silk solution concentration, exploiting interfacial compatibility between the two components. Solvent solutions of silk up to 32 w/v% were successfully prepared in hexafluoroisopropanaol (HFIP) for the study. The mechanical properties of the reinforced silk scaffolds correlated to the material density and matched by a power law relationship, independent of the ratio of silk particles to matrix. These results were similar to the relationships previously shown for cancellous bone. The mechanism behind the increased mechanical properties was a densification effect, and not the effect of including stiffer silk particles into the softer silk continuous matrix. A continuous interface between the silk matrix and the silk particles, as well as homogeneous distribution of the silk particles within the matrix were observed. Furthermore, we note that the roughness of the pore walls was controllable by varying the ratio of particles matrix, providing a route to control topography. The rate of proteolytic hydrolysis of the scaffolds decreased with increase in mass of silk used in the matrix and with increasing silk particle content.
Keywords: scaffolds, silk, particles, reinforcement, tissue engineering, composite
INTRODUCTION
Around 1.3 million people suffer accidental or diseased skeletal defects requiring bone graft surgery each year in the United States.1 Currently, bone tissue engineering using biomaterials has seen increasing interest as a potential alternative for current therapies, such as autologous bone grafts.2 One aim of tissue engineering is to use biomaterials as functional load-bearing templates during native tissue regrowth, with the mechanical role of slowly degrading biomaterials supplanted by tissue ingrowth with time. This strategy, if successful, could replace prosthetic implants or temporary stabilization therapies based previously on nondegradable materials such as metals or ceramics, by eliminating the need for second surgeries or eventual implant failure since full native tissue regeneration would be the goal. Despite this goal, many challenges remain to satisfy most clinical needs related to new options for bone grafts.3 A major one, for example, is the compressive properties of the implanted bone tissue. This is a crucial prerequisite for functional bone tissue engineering, because the engineered bone substitute is often exposed to high levels of mechanical stress in vivo in load bearing treatments, therefore, compressive strength and stiffness of the biomaterials used in such systems are critical and commonly employed design metrics.4–6
Although many natural7–10 and synthetic11–14 biodegradable polymers have been investigated for bone engineering scaffolds, most polymers are limited by the lack of a combination of mechanical properties, degradability, controllable porosity, structural stability for extended time during tissue replacement, biocompatibility, or processability into porous matrixes. For example, porous collagen-based scaffolds lack of mechanical stability (e.g. ca. 0.034 MPa of compressive modulus15) despite of a variety of osteogenic studies 7,16,17, while cancellous bone ranges from 10 to 50 MPa18–20. Another degradable synthetic polymer that is hydrophobic and resorbable, polycaprolactone (PCL), has been studied in tissue regeneration due to its biocompatibility and slow degradation rate.21,22 However, PCL scaffolds does not promote an osteogenic response without preminerialzation,23 and the very slow rate of degradation in vivo can be problematic. Silk is a potential candidate biomaterial scaffolding for bone regeneration due to biocompatibility, degradability and excellent mechanical properties.24,25 Three-dimensional porous silk-based sponges, have been prepared by aqueous26,27 or organic solvent-based (hexafluoroisopropanol; HFIP) processes,28 and have shown the ability to support bone tissue regeneration.25,27,29–34 However, silk scaffolds used in these studies still fall short of initial mechanically features required to meet clinical needs related to bone support during initial stages of implantation and remodeling.
Silks are the strongest natural fibrous materials found in nature, however, the challenge to match bone properties may require control of assembly and processing during porous scaffold formation, unlike ligament research where the native properties can be directly exploited.35 One approach toward mechanically augmenting porous silk scaffolds was pursued by mineralization of silk sponges suing an alternate soaking process with CaCl2 and Na2HPO4 solutions.32 However, the mineralization deposits did not increase the mechanical strength of the sponges, although the presence of minerals had a positive impact on osteogenic outcomes. Preminerization was not sufficient to develop mechanically-robust pore architectures necessary to enhance mechanical properties. Recently, silk/calcium phosphate macroporous scaffolds exhibited compressive properties comparable to cancellous bone.36 However, their architecture, pore size, and density were not controllable and pore size distribution was in a range of 50~100 microns, generally too small for bone reconstruction. We recently demonstrated the ability to reinforce porous silk scaffolds through the addition of silk particles.37 The particles enhanced the compressive modulus and the yield strength of HFIP-based scaffolds 100-fold, 40-fold when these matrices were normalized by the scaffold bulk density. However, the mechanisms underlying these mechanical improvements and further elucidation of the range of mechanical properties achievable required further study, as reported here.
In the present study, porous silk sponges were generated with controllable architecture but significantly improved mechanical properties, as a major step toward silk-based biomaterials with suitable features for directly use in load bearing orthopedic repairs. Increasing silk concentration and the addition of silk fibroin particles were used to achieve these goals. Increased compressive properties of the silk porous scaffolds by density increase and interfacial compatibility between the silk matrix and silk particles were the major mechanisms underlying the vastly improved mechanical properties found.
MATERIALS AND METHODS
Silk solution preparation
Silk solution was prepared from Bombyx mori silkworm cocoons according to the procedures described in our previous studies.26,38 Cocoons of B. mori silkworm silk were supplied by Tajima Shoji Co (Yokohama, Japan). Briefly, the cocoons were degummed in a boiled 0.02-M Na2CO3 solution for 30 min, dissolved in a 9.3-M LiBr solution yielding a 20% (w/v) solution, and subsequently dialyzed against distilled water for 2 days to obtain silk fibroin aqueous solution (ca. 8 wt %). The silk solution obtained from the process was freeze-dried. To obtain 16 or 32 wt % silk solution in Hexafluoroisopropanol (HFIP), the freeze-dried silk was mixed with the desired amount of HFIP, and subsequently the container was tightly covered and heated overnight at 55°C.
Preparation of milled silk powder
B. mori (1 kg) were degummed in a laboratory dyeing machine (Thies Corp., Rock Hill, SC) using 0.02 M sodium carbonate and 0.6 g/L sodium dodecyl sulphate (Sigma-Aldrich, St Louis, MO) at 100°C for 20 minutes with a fiber (g) to liquid (L) ratio of 1:25. Degummed fibers were milled as previously described.39 Briefly, fibers were chopped into snippets using a cutter mill (Pulverisette 19, Fritsch, Idar-Oberstein, Germany). A stirred ball mill (1S Attritor, Union Process Inc., Akron, OH) was used for wet grinding of the chopped snippets using 20 kg yttrium treated zirconium oxide grinding media (5 mm) in a 9.5 liter tank. The silk fibers were stirred at 280 rpm for 10 hours. Distilled water was used in the wet grinding operation. Dry powders from the wet milled slurry were recovered using a laboratory spray dryer (B-290, Buchi Labortechnik AG, Postfach, Switzerland).
Preparation of scaffolds
HFIP-derived silk scaffolds with silk particles were fabricated by modifying our previously reported method.28 HFIP-derived silk particle-reinforced silk scaffolds were prepared by adding 1 ml of 16 or 32 w/v % silk fibroin in HFIP into the glass container (12 mm inner diameter) containing the granular NaCl (Sigma-Aldrich, St Louis, MO) of 3.4 g (particle size; 210–300 or 500–600 μm) premixed with silk particles of 80, 160, or 320 mg. The weights of the mixed silk particles are equivalent to 8 w/v %, 16 w/v %, or 32 w/v % of silk in the loaded 1 ml of silk solution. For convenient denomination of the mechanically reinforced silk scaffolds, 1) the non-reinforced (no silk particles) scaffolds from 16 w/v% and 32 w/v% silk/HFIP solution are denoted as 1:0 and 2:0, respectively, 2) the reinforced scaffolds from 16 w/v% silk/HFIP solution (1 ml) with silk particles of 80, 160, and 320 mg are denoted as 1:0.5, 1:1, and 1:2, respectively, 3) the reinforced scaffolds from 32 w/v% silk/HFIP solution (1 ml) with silk particles of 320 mg are denoted as 2:1.
After loading silk solution on the NaCl/silk particle mixture, the containers were tightly covered, heated at 55°C for 10 min, and centrifuged at 3,000 rpm for 10 min to place viscous silk solution into the void volume among the NaCl porogen. This process was repeated until the silk solution was fully embedded into the void volume. Subsequently, the containers were opened for 3 days to evaporate the HFIP from the silk/porogen matrix and subsequently 1 ml of 80% aqueous MeOH was added in the containers to induce β-sheet structure in the silk matrix. After MeOH treatment for 2 days, the matrices then immersed in deionized water with stirring for 3 or 4 days to remove residual NaCl (Figure 1).
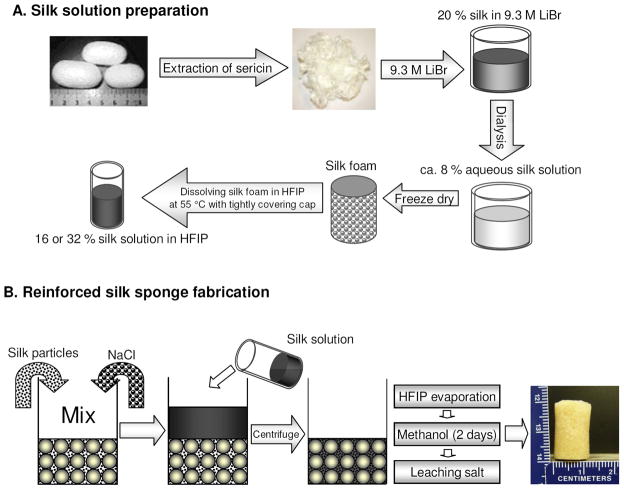
FIGURE 1.
Silk processing to obtain 16 or 32 % silk solution in HFIP (A), and preparation of silk particle-reinforced silk scaffolds (B).
Scanning electron microscopy (SEM)
Fractured sections of the silk scaffolds were obtained in liquid nitrogen using a razor blade. The fracture surfaces were sputter coated with Pt/Pd. The morphology was examined with a Field Emission Scanning Electron Microscope (FESEM) Zeiss Ultra55 or Supra55VP (Carl Zeiss AG, Germany). Pore size and wall thickness of silk scaffolds were analyzed with ImagePRO Plus 6.0 (Media Cybernetics, Inc., MD)
Confocal Laser Scanning Microscopy (CLSM)
The three dimensional porous sponges in Phosphate Buffered Saline (PBS) were visualized by a Leica (Wetzlar, Germany) DMIRE2 Confocal Laser Scanning Microscopy (CLSM) with a TCS SP2 scanner equipped with 488 nm argon and 543 nm He/Ne lasers. The scaffolds were excited at 488 nm and visualized using a 500–700 nm emission bandpass, in order to capture the intrinsic fluorescence of silk. Analysis was performed with the Leica Confocal Software (Wetzlar, Germany) and ImagePRO Plus 6.0. To examine the distribution of silk particles in the composite scaffolds, silk particles were labeled with a fluorescent dye. For fluorescent labeling, the silk particles, 300 mg, were suspended in 5 ml of PBS for 30 min to hydrate the particles. The carboxylic groups on the silk particle surfaces were activated by adding 5 ml of PBS containing 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC, 5 mg, Pierce, Rockford, IL) and N-hydroxysuccinimide (NHS, 7 mg, Pierce, Rockford, IL) to the suspended silk particle solution. The reaction was carried out for 15 min at room temperature. After the activation of silk particles to have amine-reactive NHS-esters, the particles were washed three times by repeated centrifugation and resuspension in 10 ml of fresh PBS. After the reaction, 10 ml PBS containing fluoresceinyl glycine amide (5 mg) (Invitrogen, Inc., Grand Island, NY) was added to the centrifuged silk particles. The coupling reaction was carried out for 2 h under slow stirring at room temperature. Finally, the silk particles were thoroughly washed three times in PBS and then three times in deionized water by repeated centrifugation and resuspension, and dried at room temperature. A 160 mg amount of the labeled silk particles (e.g. 16 w/v % in 1 ml HFIP) was premixed with 340 mg of NaCl (500–600 μm in size) and 1 ml of silk solution (16 w/v %) in HFIP was loaded. The porous sponge fabrication process were carried out as described above. The images of FITC-silk particles embedded in scaffolds were captured with 488 nm excitation and 510–530 nm emission and gain and offset values adjusted to eliminate background silk autofluorescence.
FTIR analysis
FTIR spectra were obtained using a JASCO FT/IR-6200 (Easton, MD) spectrophotometer. An Attenuated Total Reflectance (ATR) accessory was used for the and all analyses were performed with an average of 32 repeated scans and 4 cm−1 scan resolution. To identify the secondary structures we performed Fourier Transform Self-Deconvolution of the spectra in the amide I region (1590 ~1710 cm-1), using the Opus 5.0 software, as we have described previously.40
Mechanical Evaluations
Mechanical characterization of the compressive properties of the dried or hydrated porous scaffolds were tested on an Instron (Norwood, MA) 3366 testing frame equipped with a 0.1 kN load cell. The tests for hydrated scaffolds were carried out in 0.1 M PBS at 37°C. The scaffolds had been hydrated at least for 1 day before tests. In this test, cylinder-shaped samples were prepared 12 mm in diameter and 4 mm in height. The tests for dried scaffolds were performed in ambient conditions (50% RH and 22°C). Scaffolds for dry testing were 4 mm diameter and 3 mm in height and dried at 65°C overnight in vacuum before testing. All tests were accessed with a conventional open-sided (non-confined) configuration. All tests were performed using a displacement control mode at a rate of 5 mm/min, and all other conditions followed ASTM standard D1621-04a (Standard Test Method for Compressive Properties of Rigid Cellular Plastics) with the following alterations. After the tests, the compressive stress and strain were graphed based on the measured cross-sectional area (diameter of 12 mm for wet, 4 mm for dry) and sample height (nominal ~4–5 mm, measured automatically @ 0.02N tare load), respectively. The yield strength as well as the compressive modulus and standard deviation were determined after testing was complete, based on previously reported methods. Briefly, the elastic modulus was calculated based on a linear regression fitting of a small strain section that precedes an identifiable plateau region. The compressive yield strength was determined using an offset-yield approach. A line was drawn parallel to the modulus line, but offset by 0.5% of the initial sample gauge length. The corresponding stress value at which the offset line crossed the stress-strain curve was defined as the compressive yield strength of the scaffold, and is an estimate of the linear elastic and collapse plateau transition point.
Liquid displacement porosity measurements
The porosity of the scaffolds was determined with hexane by a modified liquid displacement method.41,42 The scaffolds were dried at 65°C overnight in vacuum, weighed (W1), and then immersed in hexane. A series of quick evacuation–repressurization cycles were performed to force the liquid into the pores of the dry scaffolds. All scaffolds sank in the liquid. Next, the scaffolds were kept in hexane for 5 min. The hexane impregnated scaffolds were moved to a pre-weighed bottle containing hexane and the weight difference (W2) was measured. Thus, the volume of hexane impregnated in scaffold was (W2 − W1)/ρh, where ρh is the hexane density. The volume of silk scaffolds was calculated using the known density (ρs) of silk 43. Thus, the total volume of scaffold and impregnated hexane was [(W2 − W1)/ρh + W1/ρs]. The density (d) of the scaffolds was obtained by:
and the porosity (ε)was calculated by:
In vitro enzymatic degradation
The scaffolds (4 mm diameter and 3 mm height) were incubated in protease XIV solution (5 U/ml in PBS, pH 7.4, Sigma-Aldrich) for desired time periods at 37°C. Enzyme solutions were replaced every two days to maintain enzyme activity. After the specific time, samples were washed with PBS and deionized water. Subsequently, the samples were dried in air for 24 h and further dried in vacuum for 24 h before measuring weight.
Statistical Methods
Results were statistically analyzed using one-way analysis of variance (ANOVA). A statistically significant difference was reported if p ≤ 0.05. Data are reported as the mean ± standard deviation (SD) from at least three separate experiments.
RESULTS AND DISCUSSION
Porous silk scaffolds fabrication
Porous silk scaffolds have been studied for potential reconstruction of bone in our previous in vitro and in vivo studies because of the intrinsically high mechanical robustness of silk biomaterials as well as the other benefits of this material described earlier 30–32. However, mechanical-improvements of these porous silk sponges would provide options for direct support of load bearing tissue, thus improving potential outcomes in vivo. To progress towards this goal, in the present study, reinforced silk scaffolds were investigated by controlling both silk solution concentration and silk particle loading. By using the same silk material for both phases (solution and powder) and HFIP-based processing, the mechanical properties were significantly improved due to interfacial compatibility.
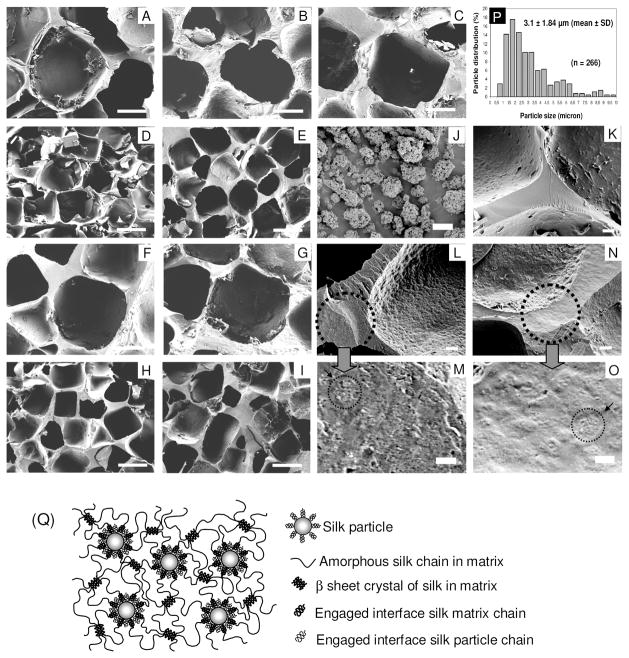
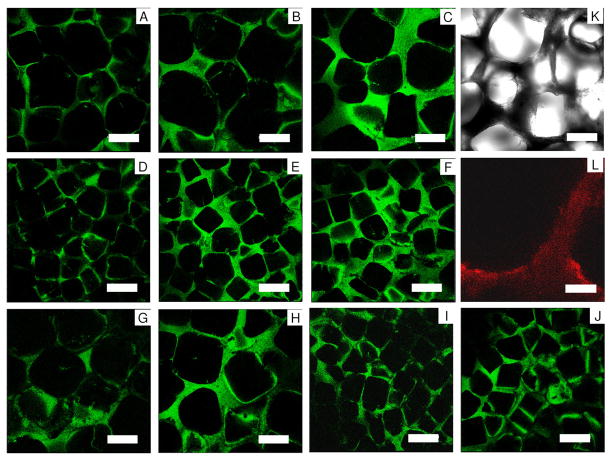
HFIP-derived scaffolds with high silk concentration and silk particle loading were prepared by modifying our conventional NaCl porogen leaching method28 (Figure 1). In order to prepare silk solution up to 32 w/v % the HFIP mixture had to be kept at 55°C overnight just below the boiling point of HFIP to increase silk solubility. The pore size of the scaffolds was regulated by salt porogen leaching using granular NaCl, with particle sizes of diameter 210~300 or 500~600 μm utilized. Silk particles were premixed with the NaCl particles before loading the silk solution. The silk particle size was sufficiently small (3.1±1.84 μm, Figure 2-J,P) compared to the large pores (>200 μm) or pore walls (>20 μm in thickness) so the particles could fill the gaps between the NaCl particles during scaffold processing. This was important for maximizing particular loading without sacrificing interconnectivity of neighboring pores in the final fabricated scaffolds. The bottles containing the scaffold samples were centrifuged immediately after the they were removed from a heating oven at 55°C to help the viscous silk solution permeate into the NaCl particle bed and the silk particle/NaCl particle mixture. This process was repeated until the silk solution was totally integrated into the mixture. The combination of 2:2 (1 ml of 32 w/v% silk/HFIP solution + 320 mg silk particles) failed to generate viable scaffolds because the silk solution could not be adequately loaded into the highly dense silk particle/NaCl particle mixture. An image of a scaffold (the 1:2 scaffolds) is shown in Figure 1. The yellow color from the silk particles indicates that the silk particles were well distributed in the scaffold walls. The fluorescence-dye conjugated silk particles were also used to address the distribution of the silk particles in the silk matrix by CLSM (Fig. 3-L). The fluorescent signal was observed throughout the cross-section of the scaffolds, again demonstrating that the particles were well distributed in the matrix.
FIGURE 2.
SEM images of the silk particle-reinforced silk scaffolds. The 1:0 scaffolds-500~600 μm pore size (A), 1:1 scaffolds-SP-500~600 μm pore size (B), 1:2 scaffolds-500~600 μm pore size (C), 1:0 scaffolds--210~300 μm pore size (D), 1:2 scaffolds-210~300 μm pore size (E), 2:0 scaffolds-500~600 μm pore size (F), 2:1 scaffolds--500~600 μm pore size (G), 2:0 scaffolds-210~300 μm pore size (H), 2:1 scaffolds-210~300 μm pore size (I), B. mori silk fibroin particles (J), wall fracture image of the 2:0 scaffolds-500~600 μm pore size (K), wall fracture image of the 1:2 scaffolds-500~600 μm pore size (L and M), wall fracture image of the 2:1 scaffolds-210~300 μm pore size (N and O). Histogram of silk particle size distribution (P), Schematic model showing a good miscibility between silk matrix and silk particles (Q): Random coiled silk chains on the interface of silk matrix and silk particles are wetted together because of good miscibility. Scale bars 200 μm in (A–I), 20 μm in (K, L, N), and 5 μm in (J, M, O).
FIGURE 3.
CLSM images of the silk particle-reinforced silk sponges in wet state. The 1:0 scaffolds-500~600 μm pore size (A), 1:1 scaffolds-500~600 μm pore size (B), 1:2 scaffolds-500~600 μm pore size (C), 1:0 scaffolds-210~300 μm pore size (D), 1:1 scaffolds-210~300 μm pore size (E), 1:2 scaffolds-210~300 μm pore size (F), 2:0 scaffolds-500~600 μm pore size (G), 2:1 scaffolds-500~600 μm pore size (H), 2:0 scaffolds-210~300 μm pore size (I), 2:1 scaffolds-210~300 μm pore size (J), Transmittance Image of the 1:2 scaffolds-500~600 μm pore size from CLSM (note. same section as image (C)) (K). The 1:1 scaffolds-500~600 μm pore size with fluorescence-dye conjugated silk particles (L); Image is in false color. Red indicates fluorescence-dye conjugated silk particles in the silk matrix, while silk matrix alone without fluorescence-dye conjugated silk particles is shown in dark (data not shown). Scale bars 300 μm in (A–K) and 50 μm in (L).
Scaffold architecture – pore size, porosity, and surface feature
Figures 2 and 3 show SEM and CLSM section morphologies, respectively, of the scaffolds. The pore sizes and wall thicknesses of the silk scaffolds from SEM and CLSM images are listed in Tables 1 and 2. Scaffolds should be designed to have interconnected pores where new bone tissue regeneration can be integrated with adequate neovascularization and nutrient/metabolic waste diffusion.3,27,30 Therefore, high porosity with interconnectivity is required for positive bone regeneration. Controlled pore sizes in scaffolds to mimic bone features, where different architectural features (pore geometry and size) related to distinct anatomical bone sites are also desirable.18,44,45 The pore sizes of silk scaffolds in the study could be controlled by using different NaCl sizes as reported in our previous studies, regardless of different silk solution concentration and silk particles utilized in the study. The pore sizes of all scaffolds matched those of the NaCl porogen size used (210 to 300 μm, or 500 to 600 μm). Feasible pore sizes for new bone tissue growth by tissue engineering is generally considered to be 100–900 μm;46–48 outside of this range, pore sizes are too small for adequate transport over time, and inhibits cell migration and neovascularization, while larger pores reduce surface to volume ratios resulting in slow neotissue formation.
Table 1.
Pore size (mean ± SD, N=15) and wall thickness (mean±SD, N=15) of mechanically reinforced silk sponges (Pore size: 210 – 300 μm).
| Samples (Pore size: 210 – 300 μm) | Pore size (μm) | Wall thickness (μm) | ||
|---|---|---|---|---|
|
| ||||
| Dry (by SEM) | Hydrated (by CLSM) | Dry (by SEM) | Hydrated (by CLSM) | |
| 1:0 | 266.4 ± 26.5 | 248.8 ± 22.1 | 6.6 ± 3.3 | 8.1 ±1.9 |
| 1:1 | 265.9 ± 27.0 | 265.4 ± 20.6 | 15.8 ± 4.3 | 15.2 ± 3.6 |
| 1:2 | 262.6 ± 26.5 | 288.2 ± 25.4 | 22.2 ± 11.4 | 24.7 ± 5.2 |
| 2:0 | 258.8 ± 37.2 | 266.6 ± 21.9 | 16.1 ± 7.8 | 17.0 ± 5.8 |
| 2:1 | 270.1 ± 22.8 | 267.4 ± 17.2 | 24.9 ± 12.8 | 23.4 ± 9.5 |
Table 2.
Pore size (mean ± SD, N=15) and wall thickness (mean±SD, N=15) of mechanically reinforced silk sponges (Pore size: 500 – 600 μm).
| Samples (Pore size: 500 – 600 μm) | Pore size (μm) | Wall thickness (μm) | ||
|---|---|---|---|---|
|
| ||||
| Dry (by SEM) | Hydrated (by CLSM) | Dry (by SEM) | Hydrated (by CLSM) | |
| 1:0 | 588.3 ± 38.4 | 518.8 ± 78.3 | 17.0±7.7 | 16.4 ± 3.4 |
| 1:1 | 540.7 ± 38.5 | 525.4 ± 41.4 | 28.0±12.5 | 29.9 ± 9.8 |
| 1:2 | 549.8 ± 50.0 | 528.0 ± 48.1 | 42.3±23.7 | 42.9 ± 10.7 |
| 2:0 | 515.4 ± 26.5 | 529.3 ± 38.0 | 31.3±16.9 | 32.8 ± 10.9 |
| 2:1 | 532.5 ± 45.0 | 524.4 ± 47.4 | 39.9±20.6 | 41.1 ± 10.3 |
We note that the wall thickness, density, and porosity were also tunable with varying silk concentration and silk particle content. The wall thickness of the scaffolds increased with higher silk concentration and mixing in more silk particles, demonstrating that wall thickness was related to the total silk mass either from the silk solution or particles. The dimensions and shapes of the porous features of all scaffolds in PBS were nearly identical to those shown by SEM in the pre-hydrated state.
The porosity and density of sponges are important features to evaluate relative to biomaterial properties for tissue engineering and were investigated using a hexane displacement method in the present work (Table 3). The porosities were linearly related to total silk % (% of silk solution + % of silk particle, R2= 93.8 %), suggesting that the distributed pores were interconnected. The porosity of the scaffolds varied from 92.7 ± 0.9 % to 74.6 ± 0.9 % (74.8 ± 1.5 %) and the density of the scaffolds ranged from 101.7 ± 12.5 to 356.3 ± 12.3 (353.3 ± 21.1) mg/ml, for the 1:0 scaffolds to the 1:2 (2:1) scaffolds, respectively, which is governed by mixing both types of silk matrix and silk particles. The porosities were comparable to those of HFIP-derived silk scaffolds prepared by salt leaching or gas forming (84–98%).28 However, the pore size of the scaffolds did not significantly affect porosity and density.
Table 3.
Porosity (mean ± SD, N=3), and density (mean ± SD, N=3) of mechanically reinforced silk sponges.
| Samples (210 – 300 μm) | Porosity (%) | Density (mg/ml) | Samples (500 – 600 μm) | Porosity (%) | Density (mg/ml) |
|---|---|---|---|---|---|
| 1:0 | 92.7 ± 0.9 | 101.7 ± 12.5 | 1:0 | 90.1 ± 0.5 | 138.0 ± 7.4 |
| 1:1 | 81.0 ± 1.3 | 266.7 ± 17.9 | 1:1 | 84.7 ± 0.7 | 213.7 ± 9.9 |
| 1:2 | 77.2 ± 1.3 | 319.2 ± 17.7 | 1:2 | 74.6 ± 0.9 | 356.3 ± 12.3 |
| 2:0 | 83.7 ± 1.0 | 227.9 ± 14.6 | 2:0 | 86.1 ± 1.5 | 194.0 ± 21.4 |
| 2:1 | 76.7 ± 0.3 | 325.6 ± 4.6 | 2:1 | 74.8 ± 1.5 | 353.3 ± 21.1 |
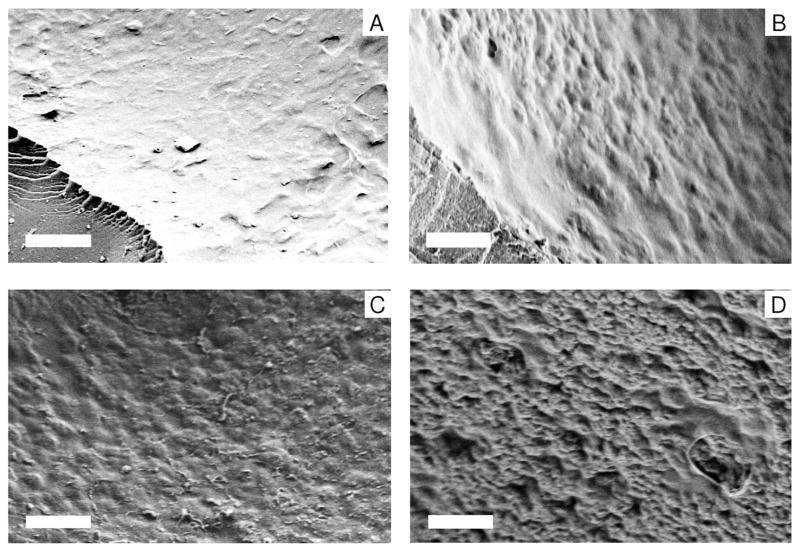
Figure 4 exhibits the surface topographical changes with different ratios of silk particles and silk matrix. The silk scaffolds without silk particles (the 2:0 scaffold) (Figure 4A) showed relatively smooth surface features, while surface roughness was induced with the inclusion of the silk particles. Surface roughness was more pronounced with a greater ratio of silk particles to silk matrix (Figure 4B–D). The greater surface roughness may have biological implications. For instance, it has been reported that a rough surface will arrest fibrin clot formation, a factor necessary to regulate osteroconduction.49–51 Consequently, rough surfaces may promote migration of osteogenic cells over smooth surfaces. Therefore, these changes in pore surface roughnesses could effect osteoconduction and new bone tissue in-growth in a positive way pending outcomes from future studies in vivo.
FIGURE 4.
Surface features of the silk particle-reinforced silk scaffolds by SEM. The 2:0 scaffolds-500~600 μm pore size (A), 2:1 scaffolds-500~600 μm pore size (B), 1:1 scaffolds-500~600 μm pore size (C), 1:2 scaffolds-500~600 μm pore size (D). Scale bar 20 μm.
Structural analysis
Secondary structure of the silk scaffolds and silk particles were determined by FTIR (Figure 5). FTIR spectra of the 1:0 scaffolds, the 1:2 scaffolds, and silk particles alone showed characteristic peaks of silk II (beta sheet) at 1698 and 1623 cm−1 (amide I).40 These results indicate that the silk scaffolds with or without silk particles from silk solution induced a conformational transition from random coil to β-sheet. The crystallinity of the 1:0 scaffolds, the 1:2 scaffolds, and silk particles were 40.0%, 37.1%, and 42.3%, respectively (Table 4).
FIGURE 5.
FTIR absorbance spectra in the amide I and II region for the reinforced silk scaffolds. The 1:0 scaffolds-500~600 μm pore size (A), 1:2 scaffolds-500~600 μm pore size (B), silk particles (C). The secondary structure of the samples were analyzed by Fourier transform self-deconvolution of the FTIR absorbance spectra in amide I region as listed in Table 4.
Table 4.
Secondary structure of silk scaffolds and silk particles, determined by Fourier transform self-deconvolution.
| Samples | β-sheets | Random coils and α-helixes | turns | Side chains |
|---|---|---|---|---|
| 1:0 (A) | 40.0 | 21.6 | 21.0 | 20.4 |
| 1:2 (B) | 37.1 | 20.1 | 21.8 | 18.0 |
| Silk particles (C) | 42.3 | 23.5 | 15.3 | 18.9 |
Mechanical properties
The unconfined compressive modulus and strength of the porous scaffolds in dry and wet states (37°C in PBS buffer pH 7.4) are listed in Tables 5 and 6. The mechanical properties of the porous scaffolds at physiological conditions provides insight into performance under physiological load-bearing functions of the biomaterials. Most reports with porous polymeric biomaterial sponges report mechanical properties in a dry state. Therefore, we also measured mechanical properties of these new porous silk composite scaffolds under dry conditions to provide a systematic comparison with previously reported data.
Table 5.
Compressive modulus and yield stress of silk particle-reinforced silk sponges. (N=4, mean±SD) (Pore size: 210 – 300 μm).
| Silk sponges (210 – 300 μm) | Compressive modulus (MPa) | Compressive strength (MPa) | ||
|---|---|---|---|---|
|
| ||||
| Dry | Hydrated | Dry | Hydrated | |
| 1:0 | 2.7 ± 1.9 | 0.22±0.07 | 0.47 ± 0.33 | 0.040±0.006 |
| 1:0.5 | - | 0.63±0.29 | - | 0.097±0.046 |
| 1:1 | 30.6 ± 4.9 | 1.27±0.30 | 3.8 ± 0.8 | 0.176±0.050 |
| 1:2 | 37.7 ± 18.7 | 2.22±0.49 | 3.7 ± 0.9 | 0.251±0.028 |
| 2:0 | 36.5 ± 3.6 | 1.19±0.30 | 3.7 ± 1.1 | 0.150±0.023 |
| 2:1 | 60.4 ± 6.0 | 2.22±0.41 | 5.1 ± 0.1 | 0.273±0.039 |
Table 6.
Compressive modulus and yield stress of silk particle-reinforced silk sponges. (N=4, mean±SD) (Pore size: 500 – 600 μm).
| Silk sponges (500 – 600 μm) | Compressive modulus (MPa) | Yield stress (MPa) | ||
|---|---|---|---|---|
|
| ||||
| Dry | Hydrated | Dry | Hydrated | |
| 1:0 | 3.9 ± 1.5 | 0.43± 0.20 | 0.47 ± 0.22 | 0.056±0.030 |
| 1:0.5 | - | 0.66±0.14 | - | 0.108±0.032 |
| 1:1 | 31.2 ± 9.8 | 1.33±0.24 | 2.5 ± 0.6 | 0.152±0.20 |
| 1:2 | 35.6 ± 6.7 | 2.62±0.48 | 2.8 ± 0.4 | 0.283±0.033 |
| 2:0 | 33.4 ± 11.0 | 1.57±0.19 | 3.8 ± 0.9 | 0.168±0.017 |
| 2:1 | 66.3 ± 17.1 | 2.82±0.40 | 4.1 ± 0.6 | 0.283±0.017 |
In physiological conditions, all the reinforced scaffolds exhibited sponge-like mechanical behavior typical of open-celled foams (Figure 6A,B). The strain-stress curves exhibited an elastic region at the initial stage of strain, followed by a plateau level of stress corresponding to pore wall buckling and collapse, followed by, a densification region where the stress increased sharply, likely due to platen-platen contact. We observed that the mechanical strength and modulus consistently increased with both increased silk concentration and silk particle content. Data on the compressive properties of the reinforced silk scaffolds showed a strong correlation between total concentration of silk fibroin in the scaffolds and compressive modulus (this will be addressed with scaffold density below, see Table 5 and 6). Average compressive modulus and strength were in a range between 0.22±0.07 and 2.82±0.40, and between 0.040±0.006 and 0.283 ± 0.017 MPa, respectively. The compressive modulus and strength of the silk scaffolds with 500 ~ 600 μm pore size were higher than those with 210 ~ 300 μm pore size; however, the results were not significantly different. The influence of porogen size on mechanical behavior has been described elsewhere.26,52–54
FIGURE 6.
Plots of compressive stress against compressive strain measured at physiological (wet) condition; (A) 210–300 μm pore size and (B) 500–600 μm pore size, and at dry (dry) condition; (C) 210–300 μm pore size and (D) 500–600 μm pore size. In all plots, (i): the 1:0 scaffolds, (ii) 1:1 scaffolds, (iii) 1:2 scaffolds, (iv) 2:0 scaffolds, (v) 2:1 scaffolds. The average compressive modulus and strength were obtained from 4 samples in each group and listed in Tables 5 and 6.
Mechanical stability of the implanted biomaterials should be considered for load-bearing bone tissue engineering applications. Although silk is a stiff and ductile polymer in natural fiber form, fabrication and processing ultimately determine scaffold mechanical behavior. In the current study, silk particles which are intrinsically similar to the bulk silk matrix, were used as a filler to enhance mechanical properties of the scaffolds. Composite fabrication processes with reinforcing fillers has been explored to enhance the mechanical strength of matrix materials.55–57 We have previously shown the benefit of increasing particle loading for mechanical benefits.37 However, from FTIR data (Figure 5, Table 4), the secondary structure of silk particles was similar to that of the silk matrix in the porous silk scaffolds. Furthermore, from comparison between the 1:1 scaffolds and the 2:0 scaffolds or between the 1:2 scaffolds and the 2:1 scaffolds (thus the same total silk concentration), the compressive modulus and strength were not significantly different. From these data we can conclude that the increased mechanical properties was due to a densification effect, and not due to the inclusion of stiffer silk particles into the softer silk matrix.
In dry conditions the samples reinforced with silk particles showed fluctuating saw-like stress-strain curves which presumably resulted from abrupt interfacial rupture between the silk matrix and the embedded silk particles with increasing strain. The scaffolds without silk particles displayed a smoother profile typical of continuous open-celled foams and pure silk scaffolds previously reported28 (Figure 6C,D). The fluctuation was more clearly observed in the scaffolds with higher silk particle ratios to silk matrix and in the scaffolds with 500~ 600 μm pore size, demonstrating their brittle nature. Nonetheless, the compressive modulus and strength of the reinforced silk scaffolds (~ 66.3 ± 17.1 MPa and ~ 5.1 ± 0.1 MPa, respectively) were significantly higher than those previously reported for HFIP-derived (17 wt% silk in HFIP) (~790±3 KPa and 250±4 KPa, respectively) and aqueous-derived (8–10 wt% silk in water) silk porous scaffolds. (3,330±500 KPa and 320±10 KPa, respectively).
We also note that the compressive modulus of the 1:0 scaffolds (the HFIP-derived 16% scaffolds) (2.7–3.9 MPa at dry state and 220–430 KPa at wet state) was higher than the of aqueous-derived silk scaffolds (8–10 wt% silk in water) (1.3–3.3 KPa)26 and previously reported HFIP-derived scaffolds (17 wt% of silk solution) (100–790 KPa in the dry state28 and ~20 KPa in the wet state37. This could be explained by the different MeOH treatment time used in the process. We treated samples with MeOH for 2 days instead of 30 min28 or 3 hours37 (e.g. treatment time in the previous work) in order to induce maximum β sheet content in the HFIP-derived scaffolds. This result agrees with the present FTIR data that the MeOH treatment for 2 days induced more silk II structure in the silk scaffolds, similar to the content found in native silk fibers, when compared to the FTIR results of the HFIP-derived scaffolds (17wt% silk in HFIP) with 30 min MeOH treatment in the previous study.28 Therefore, the results of the present study suggest a more beneficial MeOH treatment time to induce high β sheet content in the silk matrix to improve mechanical properties.
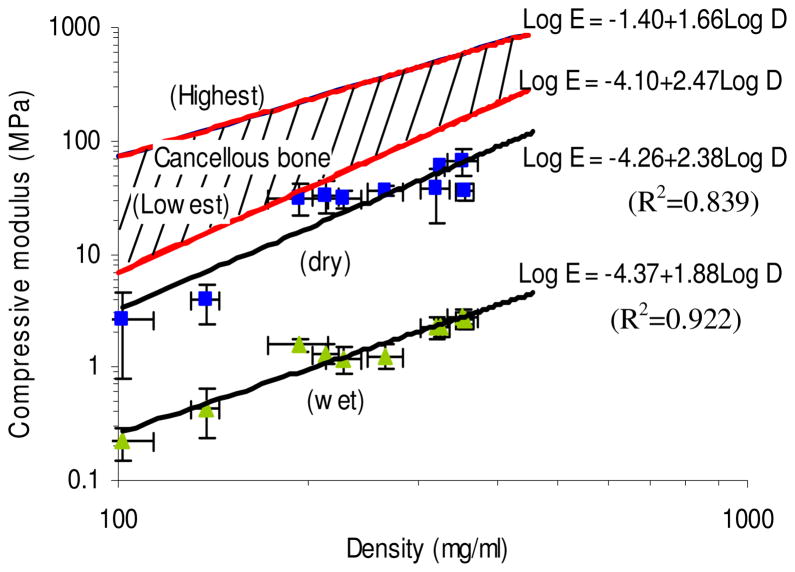
Based on the well-documented relationship between material density and compressive properties of cancellous bone, we sought to compare the density of the reinforced silk scaffolds controlled by silk solution concentration and silk particle content to their compressive properties (Figure 7). The compressive modulus of the scaffolds were strongly correlated to the density of the scaffolds following a power law equation, similar to cancellous bone 18. The density of porous silk scaffolds ranged from 101.7±12.5 to 325.6±4.6 mg/ml. The relationship between log(compressive modulus) and log(density) of the reinforced silk scaffolds in both dry and wet conditions was close to linear (R2=0.839, and R2=0.922, respectively). Even though the magnitude of the compressive properties was below the reported data for cancellous bone, the new methods developed here to fabricate fibrous protein-based scaffolds with tunable pore size, porosity, density, and mechanical robustness suggest new and important options for robust porous polymeric biomaterials for tissue repair for osteoregenerative applications, as the values are in the same order of magnitude.
FIGURE 7.
Plots of compressive modulus for the silk scaffolds against density measured at physiological (wet) and dry (dry) conditions. Highest and lowest Young’s modulus of human cancellous bone as a function of density were adapted from ref 18. Note the logarithmic scales. (N=4, Bars represent SD.)
Enzymatic degradation
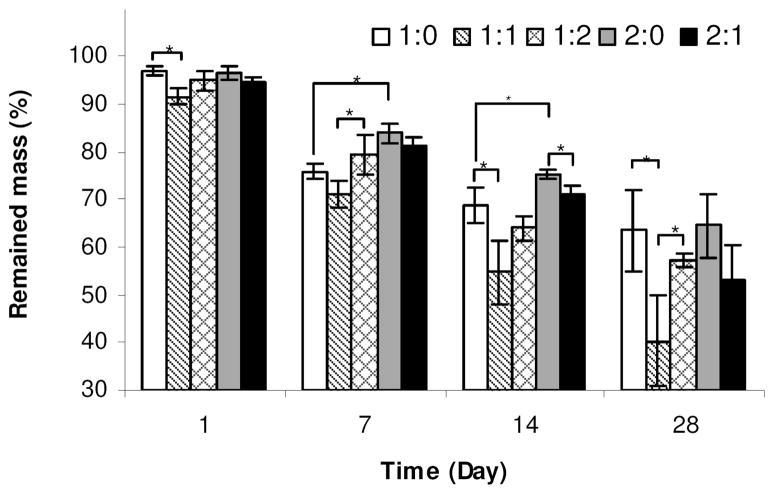
Enzymatic degradation of the silk scaffolds was studied by measuring the mass change of the scaffolds prepared from 16 or 32 w/v % silk fibroin with 0 ~ 32 w/v % silk particles with NaCl of 500–600 μm diameter particle sizes during a degradation period of 28 days by protease (5 U/ml) (Figure 8). Degradation rate is also an important factor in tissue engineering. The desired degradation rate of scaffold materials will depend on the specific application, related to differences in load bearing functions and rates of remodeling required.58 The rate of biomaterial degradation and new tissue formation can help with tissue regeneration and avoid loss of scaffold function, poor transport due to premature degradation, or barriers to transport if degradation is too slow.3,17 Although biomaterials from natural sources, such as chitosan,9 starch59 and collagen,7 have intrinsic biodegradability, their degradation rate is often less tunable unless chemical crosslinking is used, unlike synthetically designed degradable polymers.12 However, naturally derived silk-based biomaterials can offer different degradation kinetics due to the control of structural and morphological features via physical crosslinks related to the formation of crystalline beta sheets.31 Three dimensional porous silk scaffolds degraded in vivo from 2 months to beyond 1 year by altered processing methods.31 Usually, all-aqueous derived silk sponges have faster degradation rates than organic solvent-based (HFIP) derived versions 26. Related to the beta sheet features. Also, the original silk fibroin concentration and pore size have been shown to influence the degradation rate of silk sponges.26
FIGURE 8.
Mass of scaffold remaining over time. Scaffolds were prepared with NaCl of 500–600 μm diameter particle sizes. The concentration of protease XIV was 5 U/ml in PBS (pH 7.4).
All the HFIP-derived scaffolds prepared from 16~ 32 % silk fibroin and 0 ~ 32 % silk particles gradually degraded with time and the mass remaining ranged from 40.2 ± 9.5 % (the 1:1 scaffolds) to 64.5 ± 6.6 % (the 2:0 scaffolds) after 28 days; the rate was the 1:1 scaffolds > the 1:0 scaffolds ≅ the 1:2 scaffolds ≅ the 2:1 scaffolds > the 2:0 scaffolds. The presence of 16% of silk particles slightly reduced the degradation rate in both cases with 16 w/v % and 32 w/v % silk solution, overall, the differences of silk solution concentrations or silk particle content did not significantly affect the degradation patterns of the scaffolds.
CONCLUSIONS
Mechanically reinforced porous silk scaffolds were synthesized by increasing silk concentration and by adding silk particles. The porous silk scaffolds showed controllable pore sizes, pore wall roughness, density, porosity, enzymatic degradability and dramatically enhanced mechanical properties. Functional and morphological properties of these new reinforced protein-protein composite scaffolds were controlled by the concentration of the silk fibroin solution used, the content of silk particles, and the particle size of NaCl used in the process. From the systematic comparison of the properties of the silk scaffolds we found a strong relation between the density of the scaffolds and the compressive modulus. These new composite silk scaffolds provide improved mechanical properties which should provide new options for their use in load bearing applications related to osteoregenerative needs.
References
- 1.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.LeGeros RZ. Properties of osteoconductive biomaterials: Calcium phosphates. Clin Orthop Relat R. 2002:81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YF, Chen FL, Ho ST, Woodruff MA, Lim TM, Hutmacher DW. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 2007;28:814–824. doi: 10.1016/j.biomaterials.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24:2363–2378. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 6.Vitale-Brovarone C, Baino F, Verne E. High strength bioactive glass-ceramic scaffolds for bone regeneration. J Mater Sci-Mater M. 2009;20:643–653. doi: 10.1007/s10856-008-3605-0. [DOI] [PubMed] [Google Scholar]
- 7.Dawson JI, Wahl DA, Lanham SA, Kanczler JM, Czernuszka JT, Oreffo ROC. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2008;29:3105–3116. doi: 10.1016/j.biomaterials.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Pek YS, Gao SJ, Arshad MSM, Leck KJ, Ying JY. Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials. 2008;29:4300–4305. doi: 10.1016/j.biomaterials.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira JM, Rodrigues MT, Silva SS, Malafaya PB, Gomes ME, Viegas CA, Dias IR, Azevedo JT, Mano JF, Reis RL. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27:6123–6137. doi: 10.1016/j.biomaterials.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Le Nihouannen D, Le Guehennec L, Rouillon T, Pilet P, Bilban M, Layrolle P, Daculsi G. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006;27:2716–2722. doi: 10.1016/j.biomaterials.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 11.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 12.Ochi K, Chen GP, Ushida T, Gojo S, Segawa K, Tai H, Ueno K, Ohkawa H, Mori T, Yamaguchi A, et al. Use of isolated mature osteoblasts in abundance acts as desired-shaped bone regeneration in combination with a modified poly-DL-lactic-Co-glycolic acid (PLGA)-collagen sponge. J Cell Physiol. 2003;194:45–53. doi: 10.1002/jcp.10185. [DOI] [PubMed] [Google Scholar]
- 13.Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001;55:203–216. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Behravesh E, Timmer MD, Lemoine JJ, Liebschner MAK, Mikos AG. Evaluation of the in vitro degradation of macroporous hydrogels using gravimetry, confined compression testing, and microcomputed tomography. Biomacromolecules. 2002;3:1263–1270. doi: 10.1021/bm020067+. [DOI] [PubMed] [Google Scholar]
- 15.Yunoki S, Marukawa E, Ikoma T, Sotome S, Fan HS, Zhang XD, Shinomiya K, Tanaka J. Effect of collagen fibril formation on bioresorbability of hydroxyapatite/collagen composites. J Mater Sci-Mater M. 2007;18:2179–2183. doi: 10.1007/s10856-007-3011-z. [DOI] [PubMed] [Google Scholar]
- 16.Yang XBB, Bhatnagar RS, Li S, Oreffo ROC. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10:1148–1159. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Qian H, Young WG, Bartold PM. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Eng. 2003;9:1167–1177. doi: 10.1089/10763270360728071. [DOI] [PubMed] [Google Scholar]
- 18.Hodgskinson R, Currey JD. Young Modulus, Density and Material Properties in Cancellous Bone over a Large Density Range. J Mater Sci-Mater M. 1992;3:377–381. [Google Scholar]
- 19.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 20.Cullinane DM, Einhorn TA. Biomechanics of bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. Vol. 1. San Diego, CA: Academic Press; 2001. pp. 17–32. [Google Scholar]
- 21.Del Gaudio C, Filippini P, Construsciere V, Di Federico E, Bianco A, Grigioni M. Assessment of electrospun PCL scaffold for tissue engineering. Int J Artif Organs. 2006;29:537–537. [Google Scholar]
- 22.Izquierdo R, Garcia-Giralt N, Rodriguez MT, Caceres E, Garcia SJ, Ribelles JLG, Monleon M, Monllau JC, Suay J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J Biomed Mater Res A. 2008;85A:25–35. doi: 10.1002/jbm.a.31396. [DOI] [PubMed] [Google Scholar]
- 23.Liaoa J, Guoa X, Nelsona DFKK, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. 2010;6:2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 25.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442–4452. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 29.Marolt D, Augst A, Freed LE, Vepari C, Fajardo R, Patel N, Gray M, Farley M, Kaplan D, Vunjak-Novakovic G. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–6149. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kim UJ, Leisk GG, Bayan C, Georgakoudi I, Kaplan DL. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci. 2007;7:643–655. doi: 10.1002/mabi.200700030. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim UJ, Kim HS, Li CM, Wada M, Leisk GG, Kaplan DL. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42:1226–1234. doi: 10.1016/j.bone.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk implants for the healing of critical size bone defects (vol 37, pg 688, 2005) Bone. 2008;43:1122–1122. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li CM, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71A:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 35.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 36.Collins AM, Skaer NJV, Cheysens T, Knight D, Bertram C, Roach HI, Oreffo ROC, Von-Aulock S, Baris T, Skinner J, et al. Bone-like Resorbable Silk-based Scaffolds for Load-bearing Osteoregenerative Applications. Adv Mater. 2009;21:75–78. [Google Scholar]
- 37.Rajkhowa R, Gil ES, Kluge J, Numata K, Wang L, Wang X, Kaplan DL. Reinforcing silk scaffolds with silk particles. 2010;10:599–611. doi: 10.1002/mabi.200900358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Kaplan DL. Water-stable silk films with reduced beta-sheet content. Adv Funct Mater. 2005;15:1241–1247. [Google Scholar]
- 39.Rajkhowa R, Wang L, Kanwar J, Wang X. Fabrication of ultrafine powder from eri silk through attritor and jet milling. 191:155–163. [Google Scholar]
- 40.Hu X, Kaplan D, Cebe P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules. 2006;39:6161–6170. [Google Scholar]
- 41.Wu LB, Zhang H, Zhang JC, Ding JD. Fabrication of three-dimensional porous scaffolds of complicated shape for tissue engineering. I. Compression molding based on flexible-rigid combined mold. Tissue Eng. 2005;11:1105–1114. doi: 10.1089/ten.2005.11.1105. [DOI] [PubMed] [Google Scholar]
- 42.Nazarov R, Jin H-J, Kaplan DL. Porous 3-D Scaffolds from Regenerated Silk Fibroin. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 43.Park SJ, Lee KY, Ha WS, Park SY. Structural changes and their effect on mechanical properties of silk fibroin/chitosan blends. J Appl Polym Sci. 1999;74:2571–2575. [Google Scholar]
- 44.Banse X, Devogelaer JP, Munting E, Delloye C, Cornu O, Grynpas M. Inhomogeneity of human vertebral cancellous bone: Systematic density and structure patterns inside the vertebral body. Bone. 2001;28:563–571. doi: 10.1016/s8756-3282(01)00425-2. [DOI] [PubMed] [Google Scholar]
- 45.Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, Ruegsegger P. Morphometric analysis of human bone biopsies: A quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998;23:59–66. doi: 10.1016/s8756-3282(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 46.Itala AI, Ylanen HO, Ekholm C, Karlsson KH, Aro HT. Pore diameter of more than 100 mu m is not requisite for bone ingrowth in rabbits. J Biomed Mater Res. 2001;58:679–683. doi: 10.1002/jbm.1069. [DOI] [PubMed] [Google Scholar]
- 47.Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 48.Yang SF, Leong KF, Du ZH, Chua CK. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 49.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10:S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewandrowski KU, Bondre S, Hile DD, Thompson BMJ, Wise DL, Tomford WW, Trantolo DJ. Porous poly(propylene fumarate) foam coating of orthotopic cortical bone grafts for improved osteoconduction. Tissue Eng. 2002;8:1017–1027. doi: 10.1089/107632702320934119. [DOI] [PubMed] [Google Scholar]
- 51.Lewandrowski KU, Bondre SP, Gresser JD, Wise DL, Tomford WW, Trantolo DJ. Improved osteoconduction of cortical bone grafts by biodegradable foam coating. Bio-Med Mater Eng. 1999;9:265–275. [PubMed] [Google Scholar]
- 52.Lin-Gibson S, Cooper JA, Landis FA, Cicerone MT. Systematic investigation of porogen size and content on scaffold morphometric parameters and properties. Biomacromolecules. 2007;8:1511–1518. doi: 10.1021/bm061139q. [DOI] [PubMed] [Google Scholar]
- 53.Borden M, El-Amin SF, Attawia M, Laurencin CT. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials. 2003;24:597–609. doi: 10.1016/s0142-9612(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 54.Shutov FA. Foamed Polymers - Cellular Structure and Properties. Adv Polym Sci. 1983;51:155–225. [Google Scholar]
- 55.Lau KT, Gu C, Hui D. A critical review on nanotube and nanotube/nanoclay related polymer composite materials. Compos Part B-Eng. 2006;37:425–436. [Google Scholar]
- 56.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–1224. [Google Scholar]
- 57.Shi F. Miscibility and rheology of in-situ composite materials of thermoplastics with thermotropic liquid crystalline polymers II. Int J Polym Mater. 1995;28:1–10. [Google Scholar]
- 58.Dellinger JG, Wojtowicz AM, Jamison RD. Effects of degradation and porosity on the load bearing properties of model hydroxyapatite bone scaffolds. J Biomed Mater Res A. 2006;77A:563–571. doi: 10.1002/jbm.a.30658. [DOI] [PubMed] [Google Scholar]
- 59.Alves CM, Yang Y, Carnes DL, Ong JL, Sylvia VL, Dean DD, Agrawal CM, Reis RL. Modulating bone cells response onto starch-based biomaterials by surface plasma treatment and protein adsorption. Biomaterials. 2007;28:307–315. doi: 10.1016/j.biomaterials.2006.09.010. [DOI] [PubMed] [Google Scholar]