Abstract
The study was to explore the effects of BMP-2 gene modified canine bone marrow stromal cells (bMSCs) mediated by a nonviral PEI derivative (GenEscort™ II) in promoting bone formation in vitro and in vivo. Canine bMSCs were cultured and transfected with plasmids containing bone morphogenetic protein-2 gene (pBMP-2) or enhanced green fluorescent protein gene (pEGFP). Gene transfection conditions were initially optimized by varying GenEscort™ II/plasmid ratios. Osteogenic differentiation of gene modified bMSCs was investigated via alkaline phosphatase (ALP) activity analysis and real-time quantitative PCR (RT-qPCR) analysis in vitro. The bone formation ability of pBMP-2 transfected bMSCs combined with apatite-coated silk scaffolds (mSS) was explored and compared with pEGFP transfected bMSCs/mSS or untreated bMSCs/mSS at 8, 12 weeks after operation. Results showed that gene transfection efficiency reached up to 36.67 ± 4.12% as demonstrated by EGFP expression. ALP staining and activity assay were stronger with pBMP-2 gene transfection, and the mRNA expression of BMP-2, bone sialoprotein (BSP), Runt-related transcription factor 2 (Runx-2) and osteopontin (OPN) up-regulated in bMSCs 3, 6, 9 days in pBMP-2 group. Besides, the tissue-engineered bone complex with pBMP-2 modified bMSCs achieved significantly increased de novo bone formation compared with control groups (P<0.01). We conclude that pBMP-2 transfection mediated by GenEscort™ II could enhance the osteogenic differentiation of canine bMSCs and promote the ectopic new bone formation in nude mice. GenEscort™ II mediated pBMP-2 gene transfer appears to be a safe and effective nonviral method for gene enhanced bone tissue engineering.
Keywords: nonviral gene therapy, polyethylenimine (PEI), bone marrow stromal cells (bMSCs), bone morphogenetic protein-2 (BMP-2), tissue engineering
INTRODUCTION
Bone grafting is often adopted after surgeries in cases involving trauma, tumor section or congenital malformations. Autologous bone grafting and artificial bone grafting belong to the most commonly used procedures. However, these procedures have their own limitations. For example, the former is restricted by the volume of bone grafts, potential donor-site morbidity and difficulties in preparing anatomically shaped grafts, while the latter is usually constrained by the deficiency of sufficient osteoinduction. To circumvent these limitations, a viable alternative strategy - bone tissue engineering is being investigated, which could regenerate osseous tissues through creating a biological microenvironment that induce transplanted cells residing in a biomedical three-dimensional scaffold to produce a desired extracellular matrix 1.
Bone morphogenetic proteins (BMPs), particularly for BMP-2, demonstrated with powerful ability to induce orthotopic and ecotopic de novo bone formation which had successfully been applied in clinical trials. As BMPs protein therapy has certain demerits, such as high cost, large dose requirements, short half-life and poor distribution, regional gene therapy has been a widely used alternative method for BMP delivery 2. Many vectors have been developed with the purpose of delivering genes efficiently into target cells. Although generally viral vectors have the advantages over nonviral vectors in terms of gene transfer efficiency, the nonviral vectors have their own obvious merits in their low immunogenicity, and absence of the risk of endogenous virus recombination 3, which are continuously being explored and optimized.
Among nonviral gene delivery systems, PEI is a versatile cationic polymer vector used to transfer gene or oligonucleotide into cells in vitro or in vivo 4–6. PEI becomes one of the most popularly employed cationic gene vectors with its superior transfection effects in many different types of cells resulting from the so-called “proton sponge” effect by allowing endosomal escape and transfer of DNA to the nucleus 4, 7. PEI and its derivatives have successfully been used for in vitro and in vivo gene delivery of DNA and RNA. GenEscort™ II, is a commercially available novel biodegradable PEI derivative with ultra-high branching structure. Although there are a few studies reported about PEI-mediated reporter gene transfection on bMSCs in vitro 5, 8–10, to the best of our knowledge, PEI and its derivatives have not been used to mediate BMP-2 gene transfection on bMSCs for a bone tissue engineering application in the previous literature.
Various scaffolds have been prepared for use in bone tissue engineering. Apatite-coated silk, which combined the osteoconductive properties of bioceramics with the mechanical resilience of polymers to have good biocompatibility, biodegradability, and mechanical properties, had been demonstrated to promote cellular attachment and proliferation in vitro and new bone formation in vivo 11, 12. bMSCs, which mainly reside in bone marrow (BM), can be easily harvested and expanded in vitro and differentiate into osteoblasts under osteogenic medium or growth factors like BMP-2. They have served as host cells for BMP gene therapy in many previous studies 11, 13. Bone defect of large animal, such as canine, was developed to verify the practicability of tissue engineering approaches closer to the real clinical situations 14. However, before using BMP-2 gene modified canine bMSCs mediated by nonviral vector in large animal models, ectopic subcutaneous implantation in immunodeficiency mice is a well-established model to initially evaluate their bone formation ability.
In present study, we explored the effects of BMP-2 gene modified canine bMSCs for the first time mediated by a novel nonviral PEI derivative(GenEscort™ II) in promoting bone formation in vitro and in nude mice. The study might provide with a safe and efficient nonviral method for gene enhanced bone tissue engineering.
MATERIALS AND METHODS
Experimental materials and animals
The apatite-coated silk fibroin scaffolds were prepared according to our previously published procedures 15. 1 male 2-year-old beagle dog with an average weight of 12.5 kg and 6 male 6-week-old athymic nude mice with a weight of 20 ± 2 g were used in this study. And all animal procedures were approved by the Animal Research Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University, School of Medicine.
Culture of canine bMSCs
Cell culture followed what has been previously described 16. Briefly, cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA), containing 100 U/ml of penicillin, 100 U/ml of streptomycin and 2mM L-glutamine (L-glutamine, Sigma, USA), at 37°C in a humidified atmosphere of 95% air and 5% CO2. After the first passage, the following 3 supplements were added: 10−8 M dexamethasone, 50 μg/ml L-2-ascorbic acid and 10 mM β-glycerophosphate. Cells at passage 2–3 were used for following experiments.
Plasmid preparation and gene transfection
Plasmids containing human bone morphogenetic protein-2 gene (Fig. 1) or enhanced green fluorescent protein gene were prepared and purified as previously 17, 18. Briefly, plasmids were transformed into E.coli, and purified with a TIANprep Mini Plasmid Kit (TIANGEN BIOTECH, Beijing, China).
FIGURE 1.
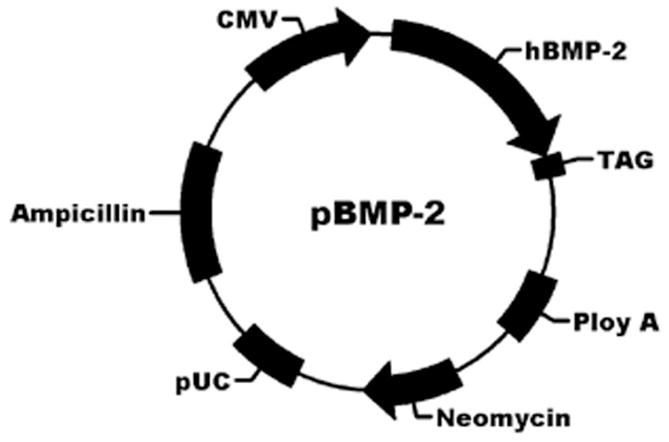
Structure of a recombinant transfer vector, pBMP-2.
Concerning to the gene transfection, taking 24-well plate for example, canine bMSCs were seeded at 50,000 cells per well 18 hours before transfection. GenEscortTM II (hyperbranched PEI, Wisegen Biotechnology Corp., Nanjing, China) mediated transient transfection was performed according to the protocol given by the supplier, and varying transfection reagent volumes from 0.5 μl to 6 μl with the constant amount of plasmid DNA (pDNA) 1 μg was initially used to optimize the gene transfection condition. The morphology of bMSCs was monitored under a phase contrast microscope. After 48 hours of transfection, EGFP-positive cells were detected under fluorescent microscopy (Leica TCS SP2, Heidelberg, Germany) and quantified using a Flow Cytometer (BD FACscanTM Flow Cytometer, BD Biosciences, CA, USA). The cell number and viability were evaluated with a Coulter Counter (BECKMAN COULTER, Zz Coulter Particle Count and Size Analyzer, USA). Subsequent experiments were carried out by using an optimized transfection condition, and each transfection was carried out in triplicate for in vitro studies.
ALP staining and ALP activity assay
ALP staining and ALP activity assay were investigated 4, 7, and 14 days after gene transfection. Cells in 12-well culture plates were evaluated for ALP staining as per the manufacturer’s instructions (ALP kit, Hongqiao, Shanghai, China). Briefly, the cells were fixed for 10 min at 4°C and incubated with a mixture of naphthol AS-MX phosphate and fast blue BB salt 19. Areas that stained purple were designated as positive.
ALP activity was measured as previously20. Briefly, cells were harvested in 200μl lysis buffer, incubated for 4 hours at 37°C. A 100μl sample was mixed with 100μl p-nitrophenyl phosphate (pNPP, Sigma, Saint Louis, USA) (1mg/ml) in 1M diethanolamine buffer at 37°C for 30 min. The reaction was stopped by the addition of 50μl of 0.2 M NaOH. Total protein content was determined by Bio-Rad Protein Assay (Kit II, Bio-Rad, USA). ALP levels were normalized to the total protein content at the end of the experiment. Each sample was assessed in triplicate.
RNA extraction and RT-qPCR analysis
Total RNA was isolated from cells using TRIzol Plus RNA purification kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA was further purified by utilizing a TURBO DNA-free kit (Ambion, Austin, TX, USA) to remove contaminating DNA. Reverse transcription reactions was conducted on 1 μg of total RNA using a PrimeScript RT reagent kit (Takara Bio, Shiga, Japan) according to manufacturer’s instructions. The primers for RT-qPCR were designed as follows: BMP-2 (forward, 5’-TGA ACA CAG CTG GTC TCA GG-3’; reverse, 5’-CTG GAC TTA AGA CGC TTC CG-3’), BSP (forward, 5’-CGA CGC TGA GAA CTC TAC CC-3’; reverse, 5’-GTT GCT GCT GGT GCT GTT TA-3’), Runx-2 (forward, 5’-ACG ATC TGA GAT TTG TGG GC-3’; reverse, 5’-CGT CTC CAA TAG GAA GGC AA-3’), OPN (forward, 5’-TTG CAG TGA TTT GCT TTT GC-3’; reverse, 5’-CAT CGT CAT GGC TTT CAT TG-3’), and the calibrator reference gene, GAPDH (forward, 5’-CGG GCG TTG ATG ACA AGT TTC CCG-3’; reverse, 5’-CTA CCC ACG GCA AAT TCC AC-3’). All RT-qPCR of these genes were performed with a Bio-Rad iQ5 real-time PCR system. Cycling conditions included an initial denaturation step of 3 min at 95°C followed by 40 cycles of 10 s at 95°C, 30 s at 60°C, 10 s at 72°C. CT (threshold cycle) values were calculated using the Applied Biosystems software, and mRNA expression level was analyzed by 2−ΔΔCt method. Analysis was based on calculating the relative expression level of selected genes of pBMP-2 transfected or pEGFP transfected bMSCs compared to that of untransfected bMSCs on day 3, 6 and 9, all values were normalized to GAPDH. Each sample was assessed in triplicate.
Ectopic bone formation in nude mice
3 days after gene transfection, pBMP-2 or pEGFP transfected bMSCs and bMSCs were separately trypsinized into cell suspension and loaded at identical concentrations of 2×107 cells/ml into porous mSS scaffolds (5 mm diameter, 4 mm height) to generate cell-scaffold constructs. 6 athymic nude mice were anesthetized by intramuscular injection of pentobarbital after light ether inhalation. Four subcutaneous pockets created on the back of each mouse by blunt dissection with a distance of more than 3 cm between each implant, which randomly received the following 4 groups of implants: (1) mSS with pBMP-2 transfected bMSCs; (2) mSS with pEGFP transfected bMSCs; (3) mSS with bMSCs; (4) mSS alone.
3 mice were sacrificed at 8 and 12 weeks after operation respectively. The specimens were harvested, fixed in 10% formalin solution (pH 7.4), decalcified in 10% EDTA, embedded in paraffin, and then sectioned along the maximum surfaces of the samples and stained with hematoxylin and eosin (H-E). New bone volume (NBV), the percentage of new bone area among the observation area 21, was calculated using the average value of the three randomly selected parallel slices with Image Pro 5.0 system (Media Cybernetics, Silver Springs, MD, USA). The mean value of the three measurements was calculated for each graft and was further used to calculate mean values for each group.
Statistical analysis
Results are reported as the mean ± standard deviation. Statistically significant differences (P<0.05) among the groups were determined using analysis of variance (ANOVA), followed by Student-Neuman-Keuls (SNK). All statistical analysis was carried out using an SAS 6.12 statistical software package (SAS, Cary, NC, USA).
RESULTS
Transfection condition optimization
To obtain a higher transfection efficiency while still maintaining a relative lower cytotoxicity, transfection conditions were optimized by varying ratio of GenEscortTM II over plasmid. The gene transfer efficiency was dose dependent from 0.5 to 2 μl/1 μg and a higher transfer efficiency could be achieved similarly under conditions with 2 μl/1 μg, 4 μl/1 μg and 6 μl/1 μg ratios, however, the viability of cells began to decrease obviously from 4 μl/1 μg to 6 μl/1 μg ratios (Fig. 2). Thus the optimal gene transfection ratio for PEI/pDNA ratio was determined as 2 μl/1 μg for 50,000 cells and used for subsequent experiments.
FIGURE 2.
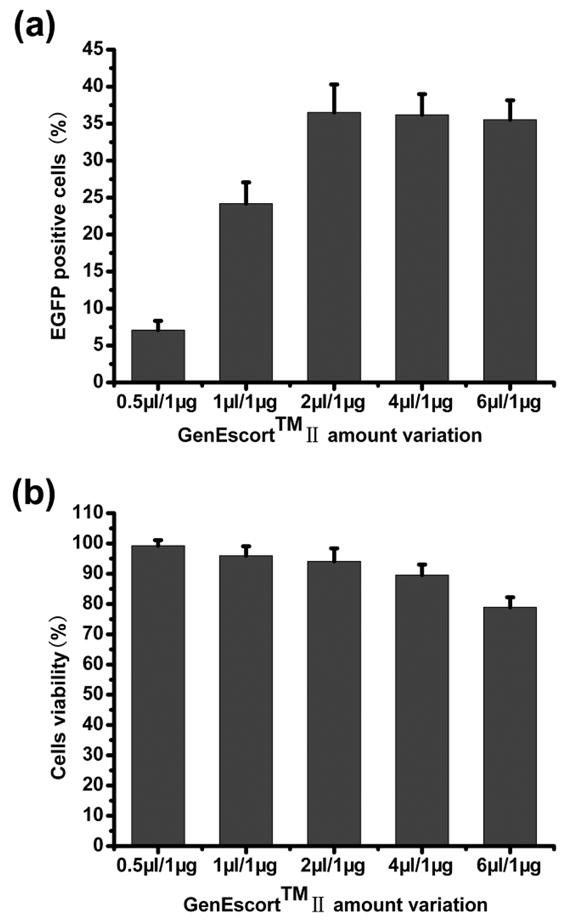
Transfection efficiency and bMSCs viability of various transfection mixtures to bMSCs. (a) transfection efficiency of various transfection mixtures, (b) bMSCs viability of various transfection mixtures at 48 hours after gene transfer.
Cell culture and gene transfection
Cellular morphology was similar 48 hours after pBMP-2 (Fig. 3a) or pEGFP (Fig. 3b) transfection when compared with untransfected control cells (Fig. 3c). Under the optimal condition, 36.67 ± 4.12% of cells were detected positive under flow cytometer, and bMSCs emitted bright and intense green fluorescence under fluorescent microscope (Figs. 3d and 3e).
FIGURE 3.
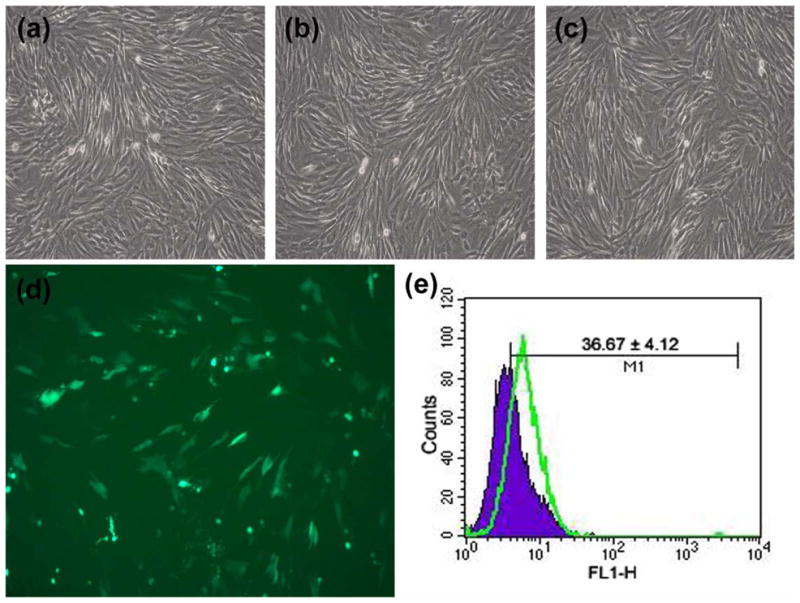
Gene transfection and the effects on canine bMSCs. The cellular morphology of (a) pBMP-2 transfected cells, (b) pEGFP transfected cells, (c) untransfected control cells, (d) bMSCs emitted bright and intense green fluorescence (×100), (e) flow cytometry profiles of untransfected bMSCs (blue) and bMSCs transfected with pEGFP (green) at 48 hours after gene transfer.
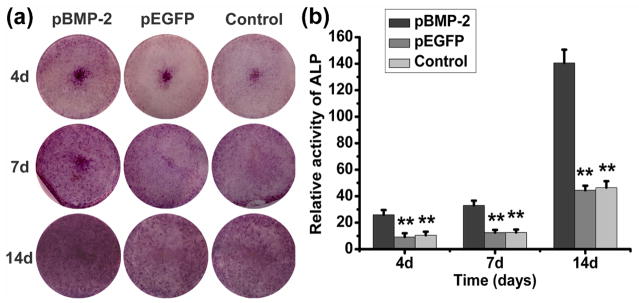
ALP staining and ALP activity assay
At any selected culture period of 4, 7 and 14 days after gene transfer, ALP staining was more pronounced in bMSCs transfected with pBMP-2 than that in pEGFP transfected or untransfected bMSCs (Fig.4a). As shown in Fig. 4b, bMSCs transfected with pBMP-2 presented the highest activity at any given time, with a significant statistical difference to control groups (P < 0.01).
FIGURE 4.
ALP staining and ALP activity assay. (a) ALP staining and (b) ALP activity of pBMP-2 transfected bMSCs, pEGFP transfected bMSCs and untransfected bMSCs 4, 7 and 14 days after gene transfection in vitro. The ALP activity was significantly higher in pBMP-2-transfected bMSCs compared to control groups (**P<0.01).
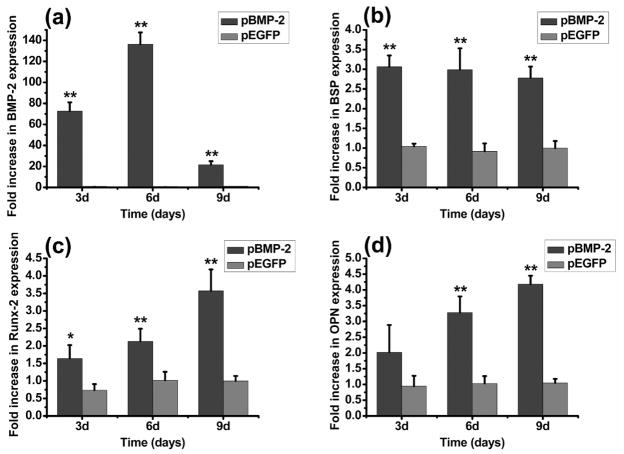
RT-qPCR analysis
RT-qPCR analysis of BMP-2 expression and markers for osteoblastic differentiation was done at 3, 6 and 9 days after gene transfection. Compared with untransfected bMSCs, pBMP-2 transfected bMSCs resulted in a BMP-2 significant up-regulation of 71.56-fold at 3 days, 136.32-fold at 6 days, and 21.57-fold at 9 days (P<0.01) (Fig. 5a). The expression level of key osteogenic gene mRNA transcripts also showed significant increase in response to pBMP-2 transfection compared to control groups (Fig. 5b, 5c, 5d), except OPN at 3 days. For example, at 9 days, BSP was increased up to 2.78-fold (Fig. 5b), Runx-2 showed a 3.57-fold increase (Fig. 5c) and OPN showed a 4.18-fold increase (Fig. 5d) in pBMP-2 transfected bMSCs.
FIGURE 5.
RT-qPCR analysis. Gene expression of (a) BMP-2, and osteogenic markers of (b) BSP, (c) Runx-2, and (d) OPN in pBMP-2 transfected and pEGFP transfected bMSCs relative to the expression of untransfected bMSCs, all values were normalized to GAPDH. *P<0.05 and **P<0.01 indicate significant difference between pBMP-2-transfected bMSCs and untransfected bMSCs.
Histological and histomorphological analysis
During the whole course of observation, new bone formation increased over time in all groups, except that there was no new bone formation in mSS alone group (Figs. 6g, 6h, 7g and 7h), which was filled with abundant fibrous connective tissue instead.
FIGURE 6.
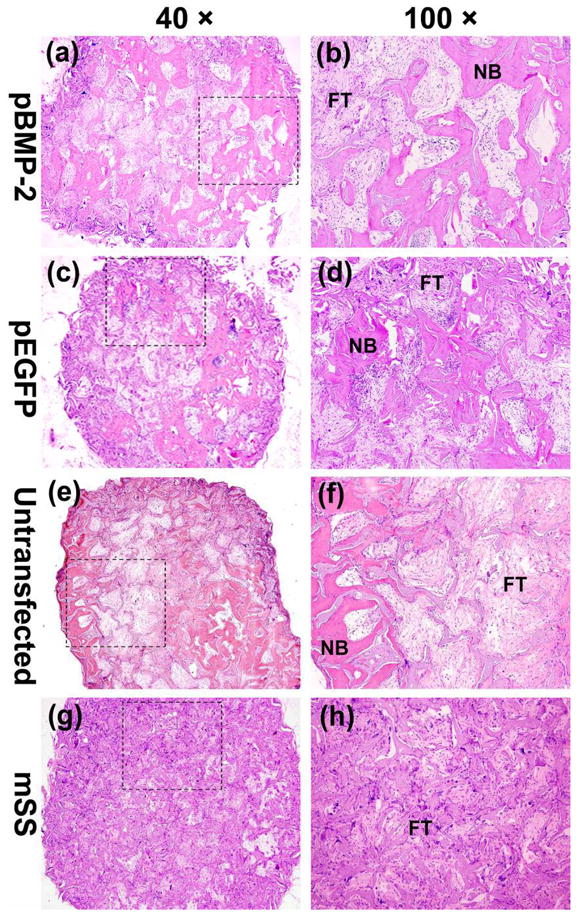
The whole and local photomicrograph of the histologic images of the implants among four groups 8 weeks postoperatively. The whole images of representative slices in (a) pBMP-2 group, (c) pEGFP group, (e) untransfected group and (g) mSS alone group (a, c, e, g, 40×). The local magnified photomicrographs in (b) pBMP-2 group, (d) pEGFP group, (f) untransfected group and (h) mSS alone group (b, d, f, h, 100×). NB: new bone; FT: fibrous tissue.
FIGURE 7.
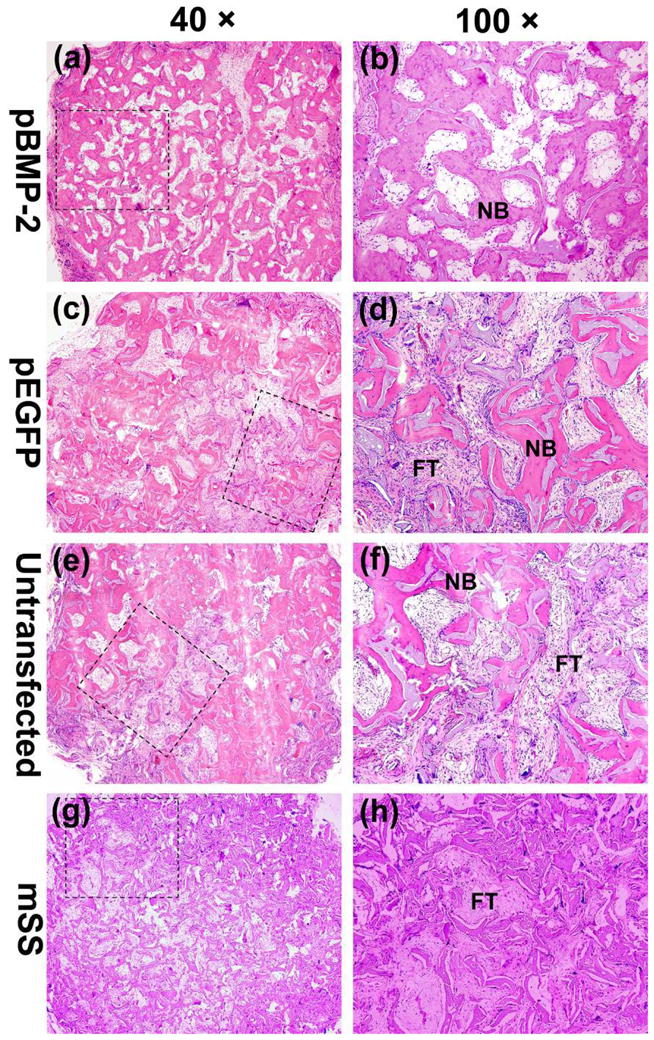
The whole and local photomicrograph of the histologic images of the implants among four groups 12 weeks postoperatively. The whole images of representative slices in (a) pBMP-2 group, (c) pEGFP group, (e) untransfected group and (g) mSS alone group (a, c, e, g, 40×). The local magnified photomicrographs in (b) pBMP-2 group, (d) pEGFP group, (f) untransfected group and (h) mSS alone group (b, d, f, h, 100×). NB: new bone; FT: fibrous tissue.
8 weeks after surgery, substantial bone formation characterized by the typical trabeculae structure was observed in pBMP-2 transfected bMSCs /mSS group (Figs. 6a and 6b), while less bone found in pEGFP transfected bMSCs /mSS (Figs. 6c and 6d) or untransfected bMSCs /mSS group (Figs. 6e and 6f). In aforementioned three groups, irregularly arranged woven bone tissue with bone lacuna was observed in the scaffold pores, and lots of multinucleated giant cells were present in the surface of mSS which might be associated with scaffold degradation.
12 weeks after surgery, pBMP-2 group exhibited more mature bone formation, with a large amount of mineralized bone tissue and immature bone marrow observed, as well as the reducing area of fibrous connective tissues (Figs. 7a and 7b). Meanwhile, irregularly arranged woven bone tissue was shown in the scaffold pores in pEGFP transfected bMSCs (Figs. 7c and 7d) or untransfected bMSCs group (Figs. 7e and 7f).
Histomorphological analysis demonstrated that pBMP-2 group achieved the highest NBV level among three groups (P <0.05) at both 8 weeks and 12 weeks postoperatively (Fig. 8).
FIGURE 8.
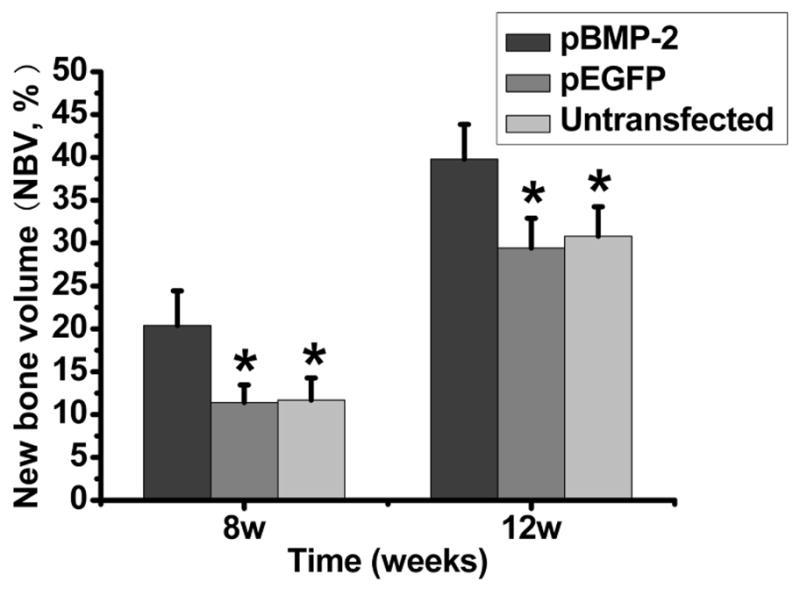
Histomorphometric data summarizing NBV within the specimens for the different groups. The extent of new bone formation was significantly higher in pBMP-2 group compared to all other groups (P<0.01).
DISCUSSION
In the present study, for the first time, we investigated the effects of BMP-2 gene modified canine bMSCs mediated by a novel nonviral PEI derivative (GenEscort™ II). Results have demonstrated the enhanced bone formation ability of the gene modified bMSCs both in vitro and in vivo.
Generally speaking, preclinical translational testing in bone tissue engineering should be performed in large skeletally mature animals, rather than rabbits or rodents. Dog is one of the most utilized species 22, 23, which could provide a skeletal biological environment that is not undergoing ongoing growth or modeling. In this study, we investigated the effects of BMP-2 gene modified canine bMSCs mediated by PEI derivative. We opted for an immunodeficiency mouse model with ectopic implantation because it is well established for the initial evaluation of bone formation ability.
As one of the most powerful and versatile families of synthetic cationic polymers carriers, PEI is often considered as the “gold standard” due to its unique transfection efficiencies in vitro and in vivo 24–26. A trail of PEI derivatives and numerous physical characterizations of polyplexes including size, surface charge, and shape have been performed to enhance transfection efficiency, cell viability and cellular uptake 24–26. GenEscort™ II is a novel biodegradable hyperbranched PEI derivative. An advantage of this kind of degradable polymer is its low cytotoxicity, a result of easier elimination from the cells and body 27. Another virtue is its improved transfection ability attributing to its hyperbranched structure 26.
Transfection efficiency is considered as one of the main thresholds for nonviral system for bone tissue engineering, because a considerable numbers of transfected cells are necessary to achieve an enhance osteogenic differentiation and bone formation. Besides, for most polymeric vectors, dose dependant higher transfection efficiency would usually be associated with higher cellular toxicity 28. However, there were little literatures available on the transfection parameters of cationic polymer PEI in primary bMSCs 5, 8–10, despite its relatively long history of use for modification of immortal cells. Thus, in the present study, we initially investigated a wide range of GenEscort™ II/pDNA ratios (0.5–6 μl/1 μg) to optimize the transfection condition by balancing a high transfection efficiency and an acceptable cell viability. As expected, our results showed that, lower ratios (0.5 μl/1 μg, 1 μl/1 μg) were less efficient, whereas high ratios (6 μl /1 μg) caused an increased cytopathic effect as revealed by decreased cell viability. By using an optimized transfection condition of 2 μl/1 μg, an efficiency up to 36.67 ± 4.12% was achieved, higher than that in other studies for bMSCs, from 5.18% to 17.1% 5, 9, 10. With the following RT-qPCR analysis, the fact that BMP-2 transcripts reached 136.32 fold at 6 days after gene transfer, and lasted till 9 days with a 21.57 folds up-regulation further confirmed the success of pBMP-2 gene transfer. As known, gene expression mediated by conventional nonviral vectors, which include polymers, liposomal formulations and basic proteins, belongs to transient expression 29, that is to say, the duration of BMP-2 over expression by nonviral gene transfer is normally limited to a relatively short period of time, for example, approximate 2 weeks 30. Meanwhile, during the process of bMSCs culture (ALP staining and ALP activity assay) till 14 days, we did not find obvious cytotoxicity after gene transfer compared with untransfected bMSCs. Therefore, we considered that the dramatic decrease in BMP-2 expression at 9 days is the result of transient expression, but not the later-on toxic effects of gene transfection.
As for osteogenic differentiation of pBMP-2 modified canine bMSCs, ALP, a membrane bound enzyme that is abundant in early bone formation, showed stronger expression when compared with pEGFP and untransfected cells at 4, 7, 14 days with both staining and activity analysis. Osteogenic markers including BSP, Runx-2 and OPN, which are associated with osteoblasts maturation and matrix mineralization, also showed an up-regulation of mRNA transcript in pBMP-2 transfected bMSCs compared to other two groups, demonstrating the enhancement of osteogenic differentiation of bMSCs after BMP-2 gene transfer. Nevertheless, the mRNA transcript of osteogenic markers in pEGFP transfected bMSCs remained at basal levels over the whole observation period.
The combination of gene therapy and tissue engineering results in a novel technology to promote tissue regeneration 31. In this study, we transfected canine bMSCs with pBMP-2 and fabricated gene enhanced tissue engineering bone with mSS. Data showed that the tissue-engineered bone complex of both pEGFP transfected bMSCs and untransfected bMSCs achieved similar new bone formation at both 8, 12 weeks postoperatively, while there was no bone formation in mSS alone group, which suggested that the bMSCs seeded into the scaffolds might have contributed to this new bone formation. Although in this study, we have no direct evidence to label the original implanted cells. Our previous maxillary sinus floor elevation study has demonstrated that green fluorescence in newly formed bone was observed for implanted bMSCs transduced with EGFP, which suggested that those implanted cells have contributed to the new bone formation 32, 33. Moreover, more mature and increased volume of newly formed bone were found in pBMP-2 group than that in pEGFP group or untransfected group, which indicated that this cell-mediated BMP-2 transient expression was successful and might be sufficient to initiate a BMP cascade reaction to promote new bone formation by autocrine and paracrine mechanism 30. With the nature of this transient gene expression system, we expect that many bMSCs implanted should have lost their ability to express EGFP at 8 weeks in vivo. Thus, future experiments would be necessary to trace the fate of all implanted bMSCs by using long-term expressing systems like lentivirus or retrovirus.
In the present study, gene transfection efficiency reached up to 36.67 ± 4.12%. The success of pBMP-2 gene transfer encouraged in vitro ALP activity and the mRNA expression of BMP-2, BSP, Runx-2 and OPN in pBMP-2 group. Furthermore, the tissue-engineered bone complex with pBMP-2 modified bMSCs achieved significantly increased new bone formation compared with control groups. Taken the above results, we conclude that pBMP-2 transfection mediated by GenEscort™ II could enhance the osteogenic differentiation of canine bMSCs and promote the ectopic new bone formation in nude mice. GenEscort™ II mediated pBMP-2 gene transfer appears to be a safe and effective nonviral method for gene enhanced bone tissue engineering.
Acknowledgments
The authors thank Carmen Preda for fabricating the silk scaffolds. This work was supported by National Natural Science Foundation of China 30772431, 30973342; Science and Technology Commission of Shanghai Municipality 08DZ2271100, 0852nm02900, 0952nm04000, 10430710900, 10dz2211600, 1052nm04300, 10JC1408600, 1052nm04300, and 10JC1408600.
References
- 1.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Baltzer AW, Lieberman JR. Regional gene therapy to enhance bone repair. Gene Ther. 2004;11:344–350. doi: 10.1038/sj.gt.3302195. [DOI] [PubMed] [Google Scholar]
- 3.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Boussif O, Lezoualc'h F, Zanta MA, Mergny M, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SW, Ogawa T, Tabata Y, Nishimura I. Efficacy and cytotoxicity of cationic-agent-mediated nonviral gene transfer into osteoblasts. J Biomed Mater Res A. 2004;71A:308–315. doi: 10.1002/jbm.a.30160. [DOI] [PubMed] [Google Scholar]
- 6.Yamano S, Dai J, Moursi AM. Comparison of transfection efficiency of nonviral gene transfer reagents. Mol Biotechnol. 2010;46:287–300. doi: 10.1007/s12033-010-9302-5. [DOI] [PubMed] [Google Scholar]
- 7.Merdan T, Kunath K, Fischer D, Kopecek J, Kissel T. Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experiments. Pharmaceutical Research. 2002;19:140–146. doi: 10.1023/a:1014212630566. [DOI] [PubMed] [Google Scholar]
- 8.Farrell LL, Pepin J, Kucharski C, Lin X, Xu Z, Uludag H. A comparison of the effectiveness of cationic polymers poly-L-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC) Eur J Pharm Biopharm. 2007;65:388–397. doi: 10.1016/j.ejpb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Peng L, Liu M, Xue YN, Huang SW, Zhuo RX. Transfection and intracellular trafficking characteristics for poly(amidoamine)s with pendant primary amine in the delivery of plasmid DNA to bone marrow stromal cells. Biomaterials. 2009;30:5825–5833. doi: 10.1016/j.biomaterials.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Ahn HH, Lee MS, Cho MH, Shin YN, Lee JH, Kim KS. DNA/PEI nano-particles for gene delivery of rat bone marrow stem cells. Colloids Surf A Physicochem Eng Asp. 2008;313:116–120. [Google Scholar]
- 11.Jiang X, Zhao J, Wang SY, Sun XJ, Zhang XL, Chen J, Kaplan DL. Mandibular repair in rats with premineralized silk scaffolds and BMP-2-modified bMSCs. Biomaterials. 2009;30:4522–4532. doi: 10.1016/j.biomaterials.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John GH, Thomas RS. Composite materials based on silk proteins. Prog Polym Sci. 2010;35:1093–1115. [Google Scholar]
- 13.Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, Krenek L. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240–4250. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim UJ, Kim HS, Li C, Wada M, Leisk GG, Kaplan DL. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42:1226–1234. doi: 10.1016/j.bone.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Zhang ZY, Wang SY, Sun XJ, Zhang XL, Chen J, Kaplan DL. Apatite-coated silk fibroin scaffolds to healing mandibular border defects in canines. Bone. 2009;45:517–527. doi: 10.1016/j.bone.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang XQ, Chen JG, Gittens S, Chen CJ, Zhang XL, Zhang ZY. The ectopic study of tissue-engineered bone with hBMP-4 gene modified bone marrow stromal cells in rabbits. Chin Med J (Engl) 2005;118:281–288. [PubMed] [Google Scholar]
- 18.Kawai M, Bessho K, Kaihara S, Sonobe J, Oda K, Iizuka T, Maruyama H. Ectopic bone formation by human bone morphogenetic protein-2 gene transfer to skeletal muscle using transcutaneous electroporation. Hum Gene Ther. 2003;14:1547–1556. doi: 10.1089/104303403322495052. [DOI] [PubMed] [Google Scholar]
- 19.Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone (TM) and bMSCs in rabbits. Clin Oral Implants Res. 2008;19:804–813. doi: 10.1111/j.1600-0501.2008.01577.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu WQ, Jiang XQ, Zhang FQ, Xu L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J Biomed Mater Res A. 2010;94:1012–1022. doi: 10.1002/jbm.a.32687. [DOI] [PubMed] [Google Scholar]
- 21.Lu JX, Gallur A, Flautre B, Anselme K, Descamps M, Thierry B, Hardouin P. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J Biomed Mater Res. 1998;42:357–367. doi: 10.1002/(sici)1097-4636(19981205)42:3<357::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.O'Loughlin PF, Morr S, Bogunovic L, Kim AD, Park B, Lane JM. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am. 2008;90(Suppl 1):79–84. doi: 10.2106/JBJS.G.01585. [DOI] [PubMed] [Google Scholar]
- 23.Muschler GF, V, Raut P, Patterson TE, Wenke JC, Hollinger JO. The design and use of animal models for translational research in bone tissue engineering and regenerative Medicine. Tissue Eng Part B-Rev. 2010;16:123–145. doi: 10.1089/ten.TEB.2009.0658. [DOI] [PubMed] [Google Scholar]
- 24.Boussif O, Zanta MA, Behr JP. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000–fold. Gene Ther. 1996;3:1074–1080. [PubMed] [Google Scholar]
- 25.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: Polyethylenimine. Hum Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 26.Fischer W, Calderón M, Haag R. Hyperbranched Polyamines for Transfection. Top Curr Chem. 2010 doi: 10.1007/128_2010_64. [DOI] [PubMed] [Google Scholar]
- 27.Jere D, Jiang HL, Arote R, Kim YK, Choi YJ, Cho MH, Akaike T. Degradable polyethylenimines as DNA and small interfering RNA carriers. Expert Opin Drug Deliv. 2009;6:827–834. doi: 10.1517/17425240903029183. [DOI] [PubMed] [Google Scholar]
- 28.Huh SH, Do HJ, Lim HY, Kim DK, Choi SJ, Song H, Kim NH. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals. 2007;35:165–171. doi: 10.1016/j.biologicals.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 30.Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, Neukam FW. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 31.Betz VM, Betz OB, Harris MB, Vrahas MS, Evans CH. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13:833–841. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- 32.Jiang XQ, Sun XJ, Lai HC, Zhao J, Wang SY, Zhang ZY. Maxillary sinus floor elevation using a tissue-engineered bone complex with beta-TCP and BMP-2 gene-modified bMSCs in rabbits. Clin Oral Implants Res. 2009;20:1333–1340. doi: 10.1111/j.1600-0501.2009.01755.x. [DOI] [PubMed] [Google Scholar]
- 33.Sun XJ, Xia LG, Chou LL, Zhong W, Zhang XL, Wang SY. Maxillary sinus floor elevation using a tissue engineered bone complex with BMP-2 gene modified bMSCs and a novel porous ceramic scaffold in rabbits. Arch Oral Biol. 2010;55:195–202. doi: 10.1016/j.archoralbio.2010.01.006. [DOI] [PubMed] [Google Scholar]