Abstract
Purpose
Overactive bladder (OAB) is a clinical syndrome that is currently treated initially with anticholinergics, although some other therapeutic alternatives exist, such as neuromodulation, botulinum toxin, and posterior tibial nerve stimulation (PTNS). The purpose of this study was to assess the efficacy of PTNS in patients with OAB refractory to anticholinergics.
Materials and Methods
We present a cohort study of 14 women with OAB to whom we applied PTNS. We assessed (before and after the treatment) the diurnal micturitional frequency, the nocturnal micturitional frequency, urgency episodes, and urge incontinence episodes. Results were analyzed by using the Wilcoxon test for nonparametric samples.
Results
We observed statistically significant improvement in the diurnal micturitional frequency (p=0.05), in episodes of micturitional urgency (p=0.03), and in episodes of urge incontinence (p=0.004). A total of 50% of the patients felt subjective improvement from their pathology.
Conclusions
PTNS is a valid, minimally invasive treatment option with minimum morbidity for patients with OAB refractory to treatment with anticholinergics.
Keywords: Cholinergics antagonist, Tibial nerve, Urinary bladder overactive
INTRODUCTION
In 2002, overactive bladder syndrome (OAB) was defined by the International Continence Society as "denoting urgency with or without urge incontinence, usually with frequency and nocturia in the absence of urinary infection or another identifiable disease" [1]. The prevalence of this syndrome in adults varies between 12% and 17% and rises with age. In Europe, the prevalence ranges from 12 to 22%, in the United States the prevalence is around 16%, and in Spain it is 21% [2-4]. OAB negatively impacts the patients' quality of life, especially when they present with symptoms of bladder filling, such as micturitional urgency and its associated incontinence. Treatment with anticholinergic drugs acting on the detrusor muscarinic receptors are, in most cases, the first-choice treatment for this type of patient, although a significant percentage of patients do not respond to this treatment owing either to lack of adherence or lack of efficacy or tolerability [5,6]. When treatment with antimuscarinic drugs is not effective, other treatment alternatives include the following: conduction therapies, the use of drugs such as uroselective alpha-blockers, neuromodulation, intravesical botulinum toxin, and even posterior tibial nerve stimulation (PTNS) [7,8].
The purpose of our study was to assess in an objective way both the efficacy and the results of PTNS in patients with OAB in which treatment with anticholinergics (antimuscarinics) was not clinically effective.
MATERIALS AND METHODS
We designed a unique cohort study of 14 patients (before and after study) to assess the efficacy of treatment with PTNS in patients with OAB in which treatment with anticholinergics was not effective for 6 months previously.
Fourteen female patients were diagnosed with OAB according to the definitions set out by the International Continence Society in 2002.
Before stimulation, all patients had been treated with maximum-dose anticholinergics (solifenacin or fesoterodine) and presented with no clinical improvement according to their pre- and post-treatment micturitional diary in which diurnal micturitional frequency, nocturnal micturitional frequency, urgency episodes, and urge incontinence episodes were recorded and compared.
After a minimum 2-month washout period after the oral anticholinergic treatment, the PTNS treatment was started.
The treatment protocol consisted of 14 sessions per patient conducted as follows: 8 weekly sessions, 4 sessions every 15 days, and 2 monthly sessions.
Before starting treatment, a urodynamic study was performed and a 48-hour micturitional calendar was provided to assess diurnal micturitional frequency, nocturnal micturitional frequency, urgency episodes, and urge incontinence episodes. After 14 PTNS sessions, every patient turned in this 48-hour micturitional calendar in which the same aforementioned variables were assessed. We also assessed adherence to treatment, satisfaction or subjective feeling of improvement, and collateral side effects and complications of the treatment.
We performed statistical study of the results by applying the Wilcoxon test for nonparametric sample comparison, with statistical significance established as p≤0.05. A graphic representation of the results was also carried out. For the statistical analysis, we used SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
The Hospital Ethics Committee authorized the use of PTNS in OAB and approved this work.
RESULTS
A total of 14 women diagnosed with OAB with a mean age of 60.8 years were treated. Every patient underwent 14 sessions of PTNS, although two patients did not complete the treatment owing to a lack of subjective improvement.
Of the 14 patients included in this study, only 1 presented with OAB with a known cause (multiple sclerosis). The remaining patients had idiopathic OAB.
We conducted urodynamic study in all the patients before starting the PTNS treatment, and nine of the patients presented with detrusor hyperactivity waves. In the five remaining patients, the results of the urodynamic study were normal.
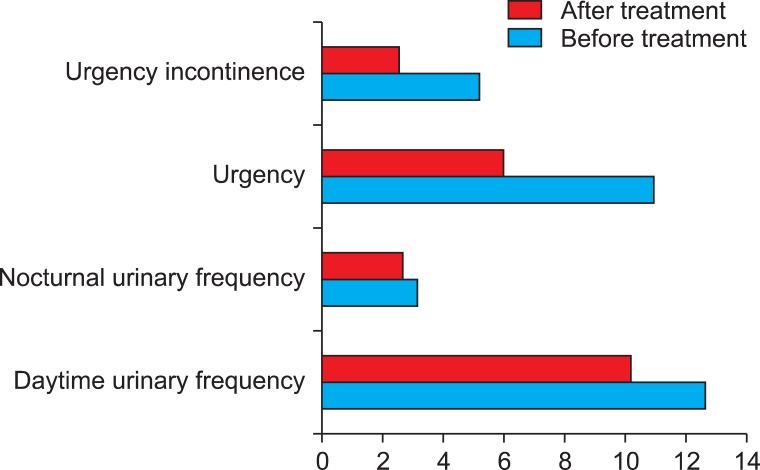
The 48-hour micturitional calendar provided by the patients before starting the PTNS treatment showed the following means: diurnal micturitional frequency, 12.6 times; nocturnal micturitional frequency, 3.1 times; urgency episodes, 10.9 times; and urge incontinence episodes, 5.2 times. At the completion of treatment (14 sessions), the patients were again requested to submit their 48-hour micturitional diary, which documented the following: diurnal micturitional frequency, 10.2 episodes; nocturnal micturitional frequency, 2.7 episodes; urgency, 6 episodes, and urge incontinence, 2.5 episodes (Fig. 1). After applying the Wilcoxon test for paired samples and analyzing these four variables, we observed statistically significant improvement in the diurnal micturitional frequency (p=0.05), urgency episodes (p=0.003), and urge incontinence episodes (p=0.004) but no significant improvement in the nocturnal micturitional frequency (p=0.472) (Table 1).
FIG. 1.
Changes in the principal parameters studied.
TABLE 1.
Relationship between the 4 variables measured in patients during 48 hours before and after the treatment with posterior tibial nerve stimulation
When we analyzed the patients' subjective feelings of improvement regarding the treatment, we found that 50% reported improvement and another 50% reporting feeling no difference. When we compared this result with the anticholinergic treatment in these patients previously (for which 100% of patients felt no subjective difference), the results were statistically significant (p<0.05).
Of the two patients who did not complete all 14 sessions, one showed improvement when we considered the micturitional calendar filled out after the 8th session (the last one for this patient). The other patient did not show improvement after filling out the calendar at the 6th session, which was the last one applied.
Ten patients reported pain in the electrode application zone during the treatment that was not an obstacle to completing the treatment. No complications or side effects were reported after PTNS in these 14 patients with OAB.
After a mean of 8.5 months of follow-up, the patients' symptoms had stabilized and no progression of symptoms was shown.
DISCUSSION
Treatment of OAB with anticholinergics (antimuscarinic) can have a lack of benefit in up to 60% of cases depending on the kind of patient and the sex of the patient. Accordingly, many patients express a wish to change their treatment to another anticholinergic drug or another type of therapeutic [9]. Generally, the most common reasons for a change of the medication are a lack of therapeutic response, new medications, side effects, and the acceptance, by some patients, that their pathology is not going to improve no matter which treatment they receive [10]. Analyzing the symptoms experienced by these patients, which result in often drastic measures taken in an attempt to lead a relatively normal life, brings to light the enormity of the problems that arise from this syndrome and explains why these patients often demand a solution to their problem or feel there is unacceptable improvement of their symptoms with the prescribed treatment [11]. Hence, it is necessary to give these patients other therapeutic alternatives with the intention of improving and controlling their symptoms. It is within this diagnostic-therapeutic framework that PTNS was born as a possible alternative for the treatment of OAB.
PTNS via surface electrodes was proposed in 1983 and, later, in 1987, in animal experimentation, it was proven that it could inhibit detrusor hyperactivity as well as control urge incontinence [12,13]. PTNS is the electrostimulation of the sacral roots (S2-S4) that produce an activation of the sacral plexus that controls the visceral organs and the pelvic floor muscles, thereby improving bladder control [12-14].
Since then, some studies have been conducted to prove the efficacy of PTNS versus other treatments and placebo for the treatment of OAB. Bellette et al. [14] conducted a randomized clinical trial comparing PTNS with placebo in 37 women and reported significant improvement regarding frequency and nocturia after the stimulation, although both quality of life, as assessed by questionnaire, and micturitional urgency improved in both groups without significant differences. Amarenco et al. [15] carried out a study with 44 patients with OAB treated with PTNS, without a control group, and reported improvement in urodynamic parameters, both in the maximum cystometric capacity and in the time of onset of the first involuntary detrusor contraction. Peters et al. [16] in 2009 published a clinical trial in which they compared tolterodine with PTNS in 100 patients with OAB. PTNS showed clinical improvement in 79.5% of the patients versus improvement in 54.8% of patients who took tolterodine 4 mg. In a group of 43 patients with OAB that did not respond to medical treatment with anticholinergics, Yoong et al. [17] tested a protocol of PTNS for 6 weeks (6 sessions) and saw a positive response in 69.7% of the patients. In those who responded to the treatment, significant decreases in nocturia and micturitional urgency were observed and quality of life improved, although the therapy duration in this study needs to be clearly defined, a point that is currently not clear. Vandoninck et al. [18] analyzed the urodynamic changes in OAB patients treated with PTNS and found an objective improvement rate of 56% of the patients, with an increase in bladder capacity. In a small percentage of cases, bladder hyperactivity decreased, leading to the conclusion that PTNS treatment may cause an increase in cystometric capacity as other clinical studies seem to suggest. However, it is not useful to abolish the detrusor hyperactivity. Finazzi-Agro et al. [19] in 2010 proved, in a controlled clinical trial with placebo, that PTNS leads to improvement in 71% of patients versus 0% in a control group, and there was also improvement in the treatment group for the number of incontinence episodes, micturitional volume, and quality of life compared with the placebo.
In relation to the aforementioned results, our study showed clinically significant improvements in diurnal micturitional frequency, micturitional urgency, and urge incontinence, but not in nocturia, which is one of the symptoms that most affects the patients' quality of life. Also, the subjective improvement of the patients was 50%, similar to previous studies in which the rate ranged between 56% and approximately 70%. However, in patients with no objective improvement, we did observe clinical improvement when the patients handed over their 48-hour micturitional calendar. It is possible that this improvement did not live up to their expectations.
One of the discussions about this therapy, apart from its clinical efficacy, is the application time and whether maintenance treatment must be applied. Regarding this, it seems that treatment with at least 12 continuous sessions leads to improvement of symptoms that can last for at least 12 months [20]. Some authors even recommend that if the PTNS treatment has been effective, it must be maintained, because it seems to stabilize the disease and improve the quality of life of these patients [21].
CONCLUSIONS
In conclusion, PTNS in patients with OAB refractory to treatment with anticholinergics is currently a valid and safe alternative, with an acceptable success rate, and does not increase the morbidity of the patient nor prevent the patient from undergoing another kind of treatment.
Footnotes
The authors have nothing to disclose.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–1314. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008;14:291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le NB, Kim JH. Expanding the role of neuromodulation for overactive bladder: new indications and alternatives to delivery. Curr Bladder Dysfunct Rep. 2011;6:25–30. doi: 10.1007/s11884-010-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia Matres MJ, Brenes Bermudez FJ. Diagnosis and management of patients with overactive bladder syndrome in urology clinics and general practitioner clinics in Spain. Arch Esp Urol. 2007;60:15–21. doi: 10.4321/s0004-06142007000100003. [DOI] [PubMed] [Google Scholar]
- 9.Castro D, Miranda P, Sanchez-Ballester F, Arumi D, Lizarraga I, Ebel C, et al. Assessment of reasons for overactive bladder treatment change. Actas Urol Esp. 2011;35:73–79. doi: 10.1016/j.acuro.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Agullo E, Ruiz-Cerda JL, Arlandis S, Rebollo P, Perez M, Chaves J, et al. Analysis of overactive bladder and urinary incontinence in working women aged between 25 and 64 years. EPICC study. Actas Urol Esp. 2010;34:618–624. [PubMed] [Google Scholar]
- 12.McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78–79. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 13.Stoller M. Afferent nerve stimulation for pelvic floor dysfunction. Eur Urol. 2000;37:33. [Google Scholar]
- 14.Bellette PO, Rodrigues-Palma PC, Hermann V, Riccetto C, Bigozzi M, Olivares JM. Posterior tibial nerve stimulation in the management of overactive bladder: a prospective and controlled study. Actas Urol Esp. 2009;33:58–63. doi: 10.1016/s0210-4806(09)74003-3. [DOI] [PubMed] [Google Scholar]
- 15.Amarenco G, Ismael SS, Even-Schneider A, Raibaut P, Demaille-Wlodyka S, Parratte B, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169:2210–2215. doi: 10.1097/01.ju.0000067446.17576.bd. [DOI] [PubMed] [Google Scholar]
- 16.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–1061. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Yoong W, Ridout AE, Damodaram M, Dadswell R. Neuromodulative treatment with percutaneous tibial nerve stimulation for intractable detrusor instability: outcomes following a shortened 6-week protocol. BJU Int. 2010;106:1673–1676. doi: 10.1111/j.1464-410X.2010.09461.x. [DOI] [PubMed] [Google Scholar]
- 18.Vandoninck V, van Balken MR, Finazzi Agro E, Petta F, Micali F, Heesakkers JP, et al. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn. 2003;22:227–232. doi: 10.1002/nau.10111. [DOI] [PubMed] [Google Scholar]
- 19.Finazzi-Agro E, Petta F, Sciobica F, Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol. 2010;184:2001–2006. doi: 10.1016/j.juro.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 20.MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183:234–240. doi: 10.1016/j.juro.2009.08.160. [DOI] [PubMed] [Google Scholar]
- 21.van der Pal F, van Balken MR, Heesakkers JP, Debruyne FM, Bemelmans BL. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU Int. 2006;97:547–550. doi: 10.1111/j.1464-410X.2006.06055.x. [DOI] [PubMed] [Google Scholar]