Abstract
Aging (senescence) has long been a difficult issue to be experimentally analyzed because of stochastic processes, which contrast with the programmed events during early development. However, we have recently started to learn the molecular mechanisms that control aging. Studies of the mutant mouse, klotho, showing premature aging, raise a possibility that mammals have an “anti-aging hormone.” A decrease of cell proliferation ability caused by the telomeres is also tightly linked to senescence. Frontier experimental studies of aging at the molecular level are leading to fascinating hypotheses that aging is the price we had to pay for the evolution of the sexual reproduction system that produces a variety of genetic information and complex body structures.
Despite rapidly advancing technology, we still have no way to defeat our own aging. Moreover, nobody can predict when and where senescence starts to be realized in the body. Thus, the aging process is stochastic, and it contrasts with the programmed processes undertaken during early development. On the other hand, the fact that no one in the history of the human being has ever lived beyond 130 years old suggests an absolute (or maximum) life span. It also tells us that there are extrinsic and intrinsic factors acting on senescence. Recent efforts made by researchers from diverse fields have merged into a line of frontier sciences to search for the “absolute” mechanisms of aging. In the session, Aging Mechanisms, two topics were discussed: first is aging at the organismal level, which is genetically determined, and the second focused on the mechanisms by which cells know when to stop their proliferation.
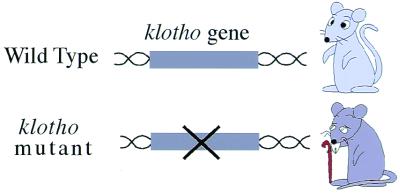
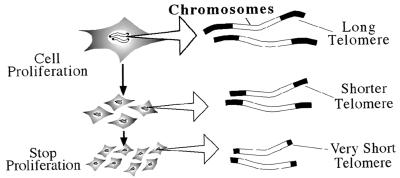
A family lineage exhibiting progeria (premature aging) indicates that senescence is, at least to some extent, controlled genetically. Accordingly, model animals in which a disruption of a single gene leads to the premature phenotype are useful and indispensable for aging studies. In this session, a novel mouse mutant, designated klotho (the name of the Greek goddess who spins the thread of life), was presented to exhibit premature aging similar to that in humans (Fig. 1). It is striking that aging seems to be controlled by humoral factors (see below). The second topic concerned the decrease of proliferative potential of cells in regard to senescence and longevity. The string-shaped chromosomes of eukaryotic cells, including mammalian cells, have a specialized structure, called the telomere, at both ends (Fig. 2). Every time normal cells (not cancer cells) divide, the telomere shortens, and eventually cells with very short telomeres stop their proliferation. This event is thought to be deeply relevant to the decrease of proliferative ability of the cells in an aged human (1, 2). In contrast, the germ cell that is transmitted to the next generation has a special device, telomerase, an enzyme that repairs shortened telomeres so that the fertilized egg can start its new life with longer telomeres (3). Why could a seemingly disadvantageous linear chromosome be evolved in eukaryotes? A conceptually new model was proposed in which animals evolved sexual proliferation by virtue of the telomeres at the expense of the life limit, aging (see below).
Figure 1.
A mutant model mouse is useful for studies of aging. The klotho phenotype (premature aging) is caused by a disruption of the single gene, klotho.
Figure 2.
The linear chromosomes of all of the eukaryote cells have the telomeres at both ends. As the cells continue to prolifereate, the length of the telomeres shortens and ultimately the cells stop dividing.
A Genetically Engineered Model Mouse Resembling Human Aging
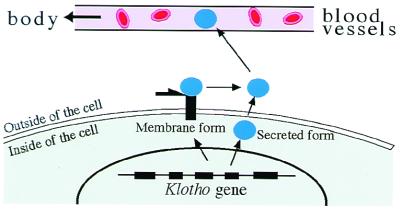
A new mouse mutant, termed klotho, was discovered that exhibits a syndrome resembling human aging (4), including a reduced life span, decreased activity, infertility, osteoporosis, arteriosclerosis, atrophy of the skin, etc. All these phenotypes were caused by the disruption of a single gene, klotho (Figs. 1 and 3) (4). The klotho gene encodes a novel protein that appears to function outside of the cells (secreted protein), and thus it is very different from that involved in previously described premature-aging syndromes and cell senescence, which function in the nucleus. In addition, the klotho gene exhibited a homology to β-glucosidase enzymes of both bacteria and plants. Evidence is accumulating that Klotho protein functions through a signaling pathway involving a circulating humoral factor(s) (Fig. 3) (4–6). (i) Despite the fact that klotho mutant mice show systemic aging phenotypes, only limited organs express the klotho gene endogenously. (ii) Klotho protein administered into limited organs rescued all of the systemic aging phenotypes of the klotho mutant. Thus, the possibility is raised that the Klotho protein functions as a humoral factor or “anti-aging hormone” (Fig. 3).
Figure 3.
A hypothesis that Klotho protein itself may be a humoral factor. A part of the extracellular domain of Klotho protein could be liberated into the extracellular space either through proteolytic cleavage or through alternative RNA splicing. Klotho protein may be secreted into the blood stream and function as an “anti-aging” hormone (4).
The klotho mutant mouse, the first laboratory animal model established, is expected to bring new insights into human aging mechanisms and also to validate the novel concept that a humoral factor(s) can regulate aging.
Aging and Sexual Reproduction
The inability of conventional DNA polymerases to replicate the very ends of a linear DNA (end replication problem) leads to the gradual shortening of telomeres during cell proliferation (Fig. 2) (7). Accordingly, somatic cells stop dividing after a limited number of cell divisions because of the telomere attrition (1, 2). Shortened telomeres are also involved in cancer development (8). These observations illustrate that linear chromosomes are accompanied by a number of troubles. Why do we have linear chromosomes paying extra costs, such as telomerase, and suffering from the burdens of aging and cancers? Is there any benefit in choosing this expensive strategy, instead of cheap circular chromosomes? Recent studies using yeast have shown that, indeed, there is a benefit, and we eukaryotes enjoy highly adaptive complex bodies thanks to the linearity of our chromosomes.
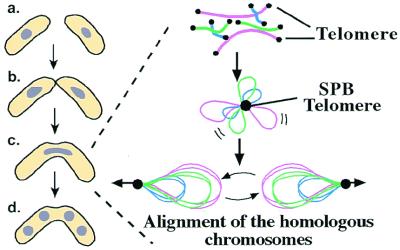
One direct strategy to address these issues is to examine what biological feature will be lost in eukaryotic cells having exclusively circular chromosomes. Recently, fission yeast cells maintaining chromosomes exclusively in circular configuration have been reported (9, 10). It has long been proposed that telomeres are important for meiosis, the most essential step for sexual reproduction, and recently Hiraoka proposed a striking explanation for the role of telomeres in this step (11). Meiosis is a process in which two chromosomes derived from a father and mother (homologous chromosomes) are paired, genetically recombined, and segregated to four gametes by two successive meiotic cell divisions (Fig. 4). How do the partners pair correctly? Hiraoka's group found that, at the premeiotic stage, all telomeres are colocalized at the spindle pole body (SPB), an equivalent of centrosome in higher eukaryotes. Subsequently, SPB shows a dynamic oscillating movement in a cell, during which chromosomes looping out from SPB also oscillate, lagging behind the SPB (11, 12) (Fig. 4). Hiraoka's group proposed that this oscillating movement helps the homologous chromosomes to be aligned and paired correctly. Indeed, this proposal was found to be true by the findings that mating between fission yeast cells engineered to have circular chromosomes instead of the linear did give rise to virtually no viable spores (aborted meiosis) (9). Therefore, having linear chromosomes is closely coupled with the ability to accomplish meiosis. In other words, the aging caused by telomeres and the wealth of genetic information in eukaryotes that have been evolved through sexual reproduction may be the opposite sides of the same coin (Fig. 5).
Figure 4.
Telomeres and meiosis. Two fission yeast cells undergo mating and meiosis by a series of steps: a, mating; b, kissing; c, cell and nuclear fusion followed by oscillating movement of the nucleus; and d, spore formation. At step c, two homologous chromosomes need to be paired. This pairing is accomplished is accomplished by colocalization of all of the telomeres at SPB, and dynamic movement of chromosomes led by SPB (11).
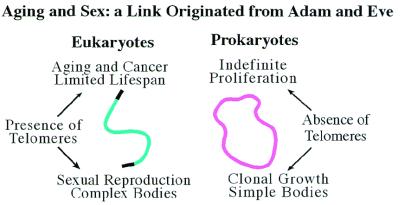
Figure 5.
Comparison between the eukaryotes that have the linear chromosomes and telomeres and the prokaryotes that have the circular chromosome in respect of aging, sex, and complexity of the organisms.
Perspective
Gerontology has entered a new era where aging mechanisms are studied at the molecular and cellular levels. Molecular genetics of different model organisms including mice and yeast connect aging events between the cellular and organismal levels. Knowledge of intrinsic factors controlling senescence will lead to understanding extrinsic factors that seem to affect individuals in divergent ways. The discoveries of the telomere/telomerase and also useful model animals for accelerated aging syndrome of human urge researchers from a variety of fields toward a journey of seeking the eternal life.
Abbreviation
- SPB
spindle pole body
Footnotes
This paper is a summary of a session presented at the second annual Japanese–American Frontiers of Science symposium, held October 1–3, 1999, at the International Conference Center, Tsukuba, Japan.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210382097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210382097
References
- 1.Hayflick L. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1988;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn E H. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Nature (London) 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 6.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 7.Watson J D. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 8.Chin L, Artandi S E, Shen Q, Tam A, Lee S L, Gottlieb G J, Greider C W, DePinho R A. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 9.Naito T, Matsuura A, Ishikawa F. Nat Genet. 1998;20:203–206. doi: 10.1038/2517. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T M, Cooper J P, Cech T R. Science. 1998;282:493–496. doi: 10.1126/science.282.5388.493. [DOI] [PubMed] [Google Scholar]
- 11.Hiraoka Y. Genes Cells. 1998;3:405–413. doi: 10.1046/j.1365-2443.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 12.Chikashige Y, Ding D Q, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]