Abstract
We performed a pooled analysis of data on self-reported history of infections in relation to the risk of non-Hodgkin lymphoma (NHL) from 17 case-control studies that included 12,585 cases and 15,416 controls aged 16–96 years at recruitment. Pooled odds ratios (OR) and 95% confidence intervals (95% CI) were estimated in two-stage random-effect or joint fixed-effect models, adjusting for age, sex and study centre. Data from the two years prior to diagnosis (or date of interview for controls) were excluded. A self-reported history of infectious mononucleosis (IM) was associated with an excess risk of NHL (OR=1.26, 95% CI=1.01–1.57 based on data from 16 studies); study-specific results indicate significant (I2=51%, p=0.01) heterogeneity. A self-reported history of measles or whooping cough was associated with an approximate 15% reduction in risk. History of other infection was not associated with NHL. We find little clear evidence of an association between NHL risk and infection although the limitations of data based on self-reported medical history (particularly of childhood illness reported by older people) are well recognised.
Introduction
Consistent associations between immune suppression and an increased risk of NHL have been identified in many studies and have led to the suggestion of an underlying infectious cause.1, 2 Some rare subtypes of NHL have been associated with specific infections although for the majority, evidence is uncertain.3 Here we report results from an international pooled analysis of case-control studies that examined data on self-reported history of infections in relation to the risk of NHL among adults.
Material and methods
Study population
We performed a pooled analysis of data from 17 case-control studies identified through the InterLymph Consortium (www.epi.grants.cancer.gov/InterLymph). Participating studies (Table 1) met the following eligibility criteria: cases diagnosed with incident histologically confirmed NHL as adults (age 16–96 years); collection of personal history of infections; and electronic data set available in March 2007. The pooled analysis was approved by the University of New South Wales Human Research Ethics Committee. Each participating study obtained local institutional ethical approval and informed consent from participants and, provided a de-identified data set with individual information for their study participants.
Table 1.
Characteristics of case-control studies participating in the pooled analysis of infections and risk of NHL
| Study acronym |
Location (reference) |
Year | Age (years) |
Matching variables |
Cases | Controls | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Rate1 (%) |
n | Rate1 (%) |
Source | |||||
| Aviano-N (18) | Aviano; Napoli, Italy | 1999– 2002 |
18–84 | None | 225 | 97 | 504 | 91 | Patients admitted to hospital for non-neoplastic, non-immunological conditions |
| BC (19) | British Columbia, Canada | 2000– 2003 |
20–80 | Age, sex, region | 828 | 79 | 845 | 46 | Random selection from client registry of Ministry of Health |
| Connecticut (20) | Connecticut, USA | 1995– 2001 |
21–84 | Age | 597 | 72 | 716 | 47–69 | <65y: RDD2; ≥65y: random selection from CMMS3 |
| EpiLymph (4) | Spain | 1998– 2003 |
17–96 | Age, sex, region |
435 | 82 | 630 | 96 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic or respiratory conditions |
| Germany | 1999– 2002 |
18–82 | Age, sex, region |
518 | 87 | 518 | 44 | Random selection from population registries | |
| Ireland | 1998– 2004 |
19–85 | Age, sex, center |
142 | 90 | 208 | 75 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic or respiratory conditions | |
| Czech Republic | 2001– 2003 |
19–82 | Age, sex, region |
199 | 90 | 199 | 60 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic and respiratory conditions | |
| France | 2000– 2003 |
18–82 | Age, sex, region |
217 | 91 | 272 | 74 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic and respiratory conditions | |
| Italy (Sardinia) | 1998– 2004 |
25–81 | Age, sex, region |
219 | 93 | 336 | 66 | Random selection from population registries | |
| Italy (6) | Turin; Novara; Forli; Vercelli; Varese; Verona; Florence; Siena; Latina; Ragusa; Imperia |
1990– 1993 |
20–74 | Age, sex, region |
1,640 | 82 | 1,771 | 74 | Random selection from demographic or National Health Service files |
| Mayo (20) | Minnesota, Iowa, and Wisconsin, USA |
2002– 2005 |
20–87 | Age, sex, region |
497 | 65 | 499 | 69 | Patients attending a pre-scheduled general medical exam |
| NCI-SEER (21) | Detroit; Iowa; Los Angeles; Seattle, USA |
1998– 2001 |
20–74 | Age, sex, region, race |
757 | 76 | 587 | 52 | <65y: RDD2; ≥65y: random selection from CMS3 |
| Northern Italy (5) | Aviano; Milan, Italy | 1983– 1992 |
17–85 | None | 429 | >97 | 1,157 | >97 | Patients admitted for non-neoplastic, non-immunological conditions in hospitals where cases diagnosed |
| NSW (9) | New South Wales, Australian Capital Territory, Australia |
2000– 2001 |
20–74 | Age, sex, region |
694 | 85 | 694 | 61 | Random selection from electoral rolls |
| SCALE (22) | Denmark; Sweden | 1999– 2002 |
18–74 | Age, sex, country |
3,055 | 81 | 3,187 | 71 | Random selection from population registries |
| UCSF (8) | San Francisco, CA, USA | 1988– 1993 |
21–74 | Age, sex, region |
1,305 | 72 | 2,404 | 78 | All ages: RDD2;plus for ≥65y: random selection from CMMS3 |
| UK (23) | Parts of north and southwest England |
1998– 2003 |
16–69 | Age, sex, region |
828 | 75 | 897 | 71 | Random selection from general practice lists |
Participation rate
RDD=Random digit dialing
CMMS=Centres for Medicare and Medicaid Service
Six studies reported response rates in cases of 65–79%, five of 81–87% and six of 90–97%. Response rates in controls were: 44–61% (six studies), 66–74% (five studies) and 75–99% (six studies). Seven of the studies used hospital controls and 10 were population-based. Information on ethnicity was available only in a minority of studies (although most participants were likely to be Caucasian). Some studies have previously reported associations by NHL subtype,4–8 or total NHL.9 Overall, the studies were conducted at 40 study sites or centres in 12 countries. Organ transplant recipients and individuals with HIV, as well as hospital controls admitted for infection (n=7), were excluded.
Exposure assessment
Past history of the conditions of interest was ascertained by structured questionnaires, either self-completed (two studies), by telephone (four studies) or in-person interview (11 studies); none of the studies allowed participation of proxies. All reports on occurrence of the conditions of interest within the two years prior to NHL diagnosis (cases) or interview (controls) were excluded from the pooled analyses, to avoid the possibility that onset of disease was responsible for an increased risk of infection. No attempt made to verify self-reported data with information obtained from examination of medical records.
Tumour classification
Most studies verified NHL diagnoses by histopathology review (report review in four, slide review in two, and a mixture of both in six studies). All WHO classification subtypes of NHL, except multiple myeloma, were included in the analysis, including the combined category of chronic lymphocytic leukemia/small lymphocytic lymphoma/prolymphocytic lymphoma/mantle-cell lymphoma (CLL/SLL/PLL/MCL), as recommended for epidemiological analyses.10 Cases originally classified using the WHO/ICD-O-3 scheme (seven studies) were categorized directly into the hierarchical groupings of the InterLymph nested classification. Cases classified using earlier schemes were converted where possible to WHO subtypes using the nested classification algorithms. Site of lymphoma, for cases with extranodal NHL, was recorded in six studies (7882 cases).
Statistical analysis
Study specific odds ratios (OR) and 95% confidence intervals (CI) were computed from unconditional logistic regression models, before using a two-stage random-effects model to estimate the pooled relative risk (hereafter called ‘risk’) of NHL, and a joint fixed-effects model to estimate risk by NHL subtype. All models were adjusted for the matching variables age, sex, and region/study centre. Analyses were conducted for men and women separately and showed no statistically significant differences between the sexes. The inclusion of a priori specified potential confounders - socioeconomic status, race, smoking history and sibship size - did not change the pooled estimates by more than 10%, and thus they were not retained in the final models. Furthermore, restriction to Caucasians only or stratification by birth order (only and 1st born, 2nd and 3rd born, 4th or higher order) did not reveal any systematic differences in the pooled risks across the conditions of interest.
Heterogeneity among study-specific ORs was assessed using Cochrane´s Q statistic and the I2 statistic.11 In the presence of significant heterogeneity (p<0.10), forest plots and influence analyses were used to identify outlying studies. Also, sensitivity analyses were conducted to examine the potential effects of type of data collection (in-person interview, self-completed questionnaire, telephone interview); source of controls (hospital or population); case ascertainment (ascertainment of slides in at least in a 10% subset of cases); response rates in cases and controls and study region/continent. As there were no systematic differences found between studies or design factors, results have been presented for all studies combined. All statistical tests were two-sided, and the level of statistical significance for risks was set to α=0.05. Analyses were performed using STATA software version 10.0 (Stata Corporation, College Station, TX).
Results
The participating studies (Table 1) collectively contributed 12,585 cases and 15,416 controls to the analysis, from 12 countries across three continents. The distribution of pooled cases by age, sex and other characteristics broadly reflect known demographic patterns (Table 2).12 As expected, most tumours were B cell subtype.
Table 2.
Demographic and tumor characteristics of the pooled study participants
| Demographics | NHL Cases (%) |
Controls (%) |
Tumor characteristics | NHL Cases |
|---|---|---|---|---|
| Pooled total | 12,585 | 15,416 | Pooled total | 12,585 |
| Sex | B-cell NHL | 10,396 | ||
| Men | 6814 (54.1) | 8338 (54.1) | Follicular lymphoma | 2,582 |
| Women | 5771 (45.9) | 7078 (45.9) | Diffuse large B-cell lymphoma | 3,513 |
| Age (years)* | CLL / SLL / PLL / MCL | 2,142 | ||
| <20 | 25 (0.2) | 56 (0.4) | Marginal zone lymphoma | 707 |
| 20 – 29 | 356 (2.8) | 812 (5.3) | Burkitt’s lymphoma | 93 |
| 30 – 39 | 844 (6.7) | 1537 (10.0) | Lymphoplasmacytic lymphoma | 285 |
| 40 – 49 | 1657 (13.2) | 2233 (14.5) | Hairy cell leukemia | 118 |
| 50 – 59 | 3266 (26.0) | 3511 (22.8) | Precursor B-cell | 24 |
| 60 – 69 | 4028 (32.0) | 4428 (28.7) | B-cell NOS^ | 932 |
| 70 – 79 | 2262 (18.0) | 2671 (17.3) | T-cell NHL | 719 |
| 80+ | 147 (1.2) | 168 (1.1) | Mycosis fungoides / Sezary syndrome | 237 |
| Median (range) | 60 (17 – 89) | 58 (16 – 96) | Peripheral T-cell | 370 |
| Education / SES† | Adult T-cell leukemia / lymphoma | 2 | ||
| Low | 5007 (39.8) | 5488 (35.6) | NK/T-cell§ | 19 |
| Medium | 4180 (33.2) | 5484 (35.6) | T-cell large granular lymphocytic leukemia | 7 |
| High | 3293 (26.2) | 4344 (28.2) | T-cell prolymphocytic leukemia | 7 |
| Unknown / other | 105 (0.8) | 100 (0.6) | Precursor T-cell | 29 |
| Race‡ | T-cell NOS | 48 | ||
| White | 4788 (38.0) | 5809 (37.7) | NOS NHL | 1,470 |
| Black | 183 (1.5) | 305 (2.0) | Precursor NOS | 57 |
| Other / mixed | 497 (3.9) | 525 (3.4) | NOS | 1,413 |
| Missing | 7117 (56.6) | 8777 (56.9) |
The inclusion of Hodgkin lymphoma cases and matched controls and the removal of HIV-related cases in several studies led to an imbalance in the age distribution of controls compared to the non-Hodgkin lymphoma cases
Education / socioeconomic status (SES) groups in each study was based on the tertile distribution of years of education (10 studies) or SES levels obtained from census data (2 studies) in controls
Race was generally not recorded in the European studies.
NK/T-cell lymphoma nasal type/aggressive NK-cell leukemia
Not otherwise specified.
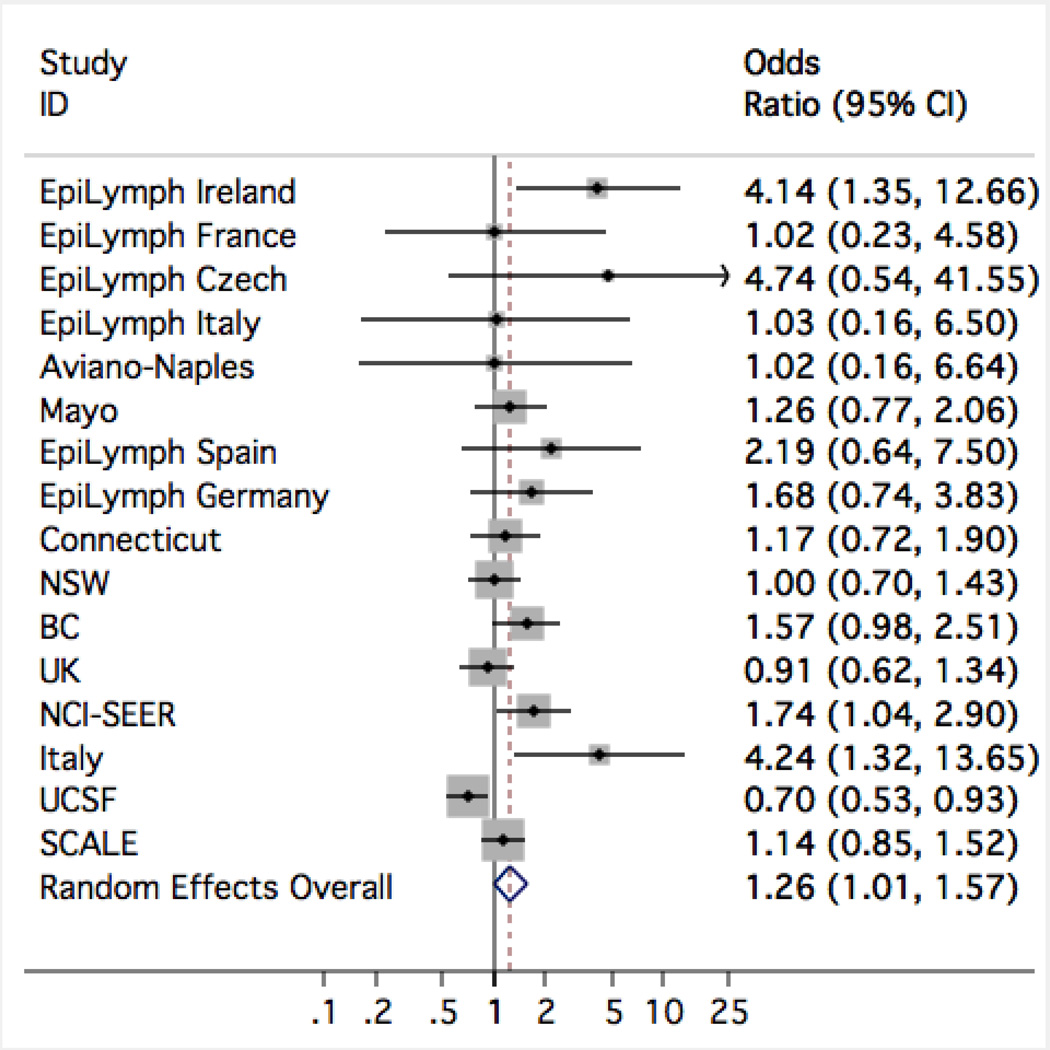
Table 3 shows the number of affected cases and controls, OR and 95% CI for a self-reported history of infections. Risk of NHL was elevated with a self-reported history of infectious mononucleosis (IM, OR=1.26, 95% CI=1.01–1.57), but was slightly lower for two other infections (measles: OR=0.84, 95% CI=0.76–0.93; whooping cough/pertussis: OR=0.85, 95% CI=0.78–0.93). Study-specific results for IM (Figure 1) showed significant heterogeneity (I2=51%, p=0.01); one study (UCSF) had a significantly decreased OR and, in general, the elevated ORs were found in studies using hospital controls rather than population-based controls. The risks of NHL subtypes in relation to a report of previous IM, is shown in Table 4: IM showed significantly elevated OR specifically for the CLL group (OR=1.71, 95% CI=1.30–2.25), and for T-cell NHL (OR=1.41, 95% CI=1.01–1.97), but not for other subtypes. Study-specific and NHL sub-type specific results for measles and whooping cough showed no significant heterogeneity.
Table 3.
Self reported childhood and adult infections, in relation to risk of NHL
| Ever during lifetime | ||||
|---|---|---|---|---|
| Condition | Cases | Controls | OR (95% CI) * | Heterogeneity |
| Ever / never | Ever / never | |||
| Childhood infection | ||||
| Measles | 4470 / 1016 | 6446 / 1483 | 0.84 (0.76 – 0.93) | 8 studies, p=0.48, I2=0.0% |
| Mumps | 3124 / 1829 | 4514 / 2769 | 0.99 (0.91 – 1.07) | 7 studies, p=0.75, I2=0.0% |
| Whooping cough / pertussis | 1513 / 2618 | 2294 / 4090 | 0.85 (0.78 – 0.93) | 6 studies, p=0.87, I2=0.0% |
| Chickenpox | 4851 / 1753 | 6406 / 2363 | 0.94 (0.87 – 1.03) | 10 studies, p=0.40, I2=4.8% |
| German measles / rubella | 1695 / 2599 | 2410 / 4076 | 0.95 (0.85 – 1.07) | 7 studies, p=0.14, I2=38.3% |
| Scarlet fever | 451 / 3241 | 698 / 5264 | 0.98 (0.77 – 1.24) | 6 studies, p=0.01, I2=65.4% |
| Diphtheria | 56 / 1256 | 56 / 1321 | 0.99 (0.60 – 1.62) | 2 studies, p=0.24, I2=26.8% |
| Adult infections | ||||
| Infectious mononucleosis | 521 / 11412 | 612 / 13484 | 1.26 (1.01 – 1.57) | 16 studies, p=0.01, I2=51.4% |
| Tuberculosis | 251 / 10305 | 244 / 12346 | 1.16 (0.96 – 1.39) | 13 studies, p=0.78, I2=0.0% |
| Diarrhoea | 169 / 2215 | 249 / 2559 | 0.88 (0.67 – 1.17) | 7 studies, p=0.13, I2=39.0% |
| Herpes simplex / cold sores | 2593 / 5490 | 2999 / 6738 | 1.05 (0.97 – 1.13) | 13 studies, p=0.26, I2=17.8% |
| Genital herpes | 85 / 4753 | 127 / 5781 | 0.96 (0.72 – 1.28) | 5 studies, p=0.96, I2=0.0% |
| Shingles | 552 / 5509 | 655 / 7876 | 1.05 (0.93 – 1.19) | 8 studies, p=0.43, I2=0.0% |
Odds Ratio (OR) and 95% confidence interval (CI) from a two-stage random-effects model, adjusted for age (in 5 year intervals), sex, and study centre.
Figure 1.
Study-specific results for a self-reported history of infectious mononucleosis in relation to risk of non-Hodgkin lymphoma.
Table 4.
The impact of infectious mononucleosis on the risk of NHL, stratified by NHL subtype
| NHL subtype | Infectious mononucleosis | |
|---|---|---|
| Ever/never | OR (95% CI)* | |
| Controls | 612 / 13484 | 1.00 (ref) |
| B-cell | 442 / 9618 | 1.12 (0.98 – 1.28) |
| Follicular lymphoma | 141 / 2340 | 1.10 (0.90 – 1.34) |
| Diffuse large B-cell lymphoma | 161 / 3238 | 1.06 (0.89 – 1.28) |
| CLL / SLL / PLL / MCL | 74 / 2038 | 1.71 (1.30 – 2.25) |
| Marginal zone lymphoma | 27 / 661 | 0.97 (0.64 – 1.47) |
| Burkitt lymphoma | 6 / 85 | 1.09 (0.46 – 2.57) |
| Lymphoplasmacytic lymphoma | 9 / 270 | 1.14 (0.57 – 2.27) |
| Hairy cell leukemia | 2 / 114 | 0.53 (0.13 – 2.23) |
| Precursor B-cell | 0 / 24 | - |
| B-cell NOS | 22 / 848 | 0.98 (0.62 – 1.53) |
| T-cell | 43 / 660 | 1.41 (1.01 – 1.97) |
| Mycosis fungoides / Sezary syndrome | 10 / 222 | 1.03 (0.53 – 1.97) |
| Peripheral T-cell | 28 / 333 | 1.72 (1.14 – 2.59) |
| Precursor T-cell | 3 / 26 | 1.87 (0.54 – 6.44) |
| T-cell NOS | 2 / 46 | 1–32 (0.30 – 5.74) |
| NHL NOS | 36 / 1134 | 1.00 (0.70 – 1.42) |
| Precursor NOS | 2 / 44 | 1.00 (0.23 – 4.39) |
| NHL NOS | 34 / 1090 | 1.00 (0.70 – 1.45) |
Odds ratio (OR) and 95% confidence interval (CI) from a joint-fixed effects model, adjusted for age (in 5 year intervals), sex, and study centre
Discussion
In this pooled analysis of case-control study data on self-reported history of infections in relation to the risk of non-Hodgkin lymphoma (NHL) we found that a reported history of infectious mononucleosis (which is caused by infection with EBV in adolescence or later life) was associated with a moderate increase in risk of NHL, although there was significant heterogeneity across studies. However, the strength of the association varied by NHL subtype, being highest in those that have been previously associated with EBV (CLL and T-cell NHL).13 We cannot completely exclude the possibility of some diagnostic overlap with Hodgkin lymphoma, a proportion of which are caused by EBV and which has been linked with past history of IM in previous studies, although this is unlikely given the pathology review process.3, 14 Furthermore, the data on IM from all participating studies is based on self-report, rather than linkage with medical records – it is unclear if a formal laboratory-confirmed diagnosis of IM was ever made. It is possible, therefore, that the finding is unreliable, although it is also possible that a diagnosis of IM may be more reliably reported than certain other conditions. Indeed, a recent prospective study does not support the view that EBV is a major cause of NHL in apparently immunocompetent individuals.15 That said, significantly elevated odds ratios were specific to the CLL group and for T-cell NHL, lending more credence to a possible real association. Most cases of NHL occurred among people in later life and, where relevant data were available, IM occurred among children or young adults, years prior to diagnosis. No significant differences were identified, in the risk of NHL, by age at diagnosis of IM (data not shown).
In contrast, a self-reported history of measles or whooping cough was associated with a small reduction in risk of NHL. Since there is little other literature to support the view that certain infections reduce the risk of NHL, it is most likely that this finding is spurious and probably due to chance or systematic bias in reporting. These infections typically occur in childhood and have become less common with vaccination; furthermore, cases tend to be relatively elderly and recall of infections may not be accurate. No other report of infection was associated with NHL. The strengths of this pooled analysis include the large study size and the ability to stratify risk estimates by study design features and NHL subtypes. Thus the pooled data do not support a generalised effect of past infection on NHL risk, or risk of a specific NHL subtype.
In summary, therefore, we find little clear evidence of an association between risk of NHL as an adult and infection (as a child or adult) although the weakness of data based on self-report of medical history (particularly when older people are asked to report on childhood illness) is well recognised.16, 17 Indeed the principle limitation of this analysis is the reliability of self-reported exposure data on past medical history underpinning any associations. This problem is common to all of the individual participating studies and emphasises the need for primary source data on past infections (from medical records for example) in such investigations. Furthermore, the individual studies did not use a common pre-coded questionnaire and so data were collected in slightly different ways and for certain conditions – such as “diarrhoea”, the cause is ill-defined. Another problem common to some but not all of the participating studies is the lack of diagnostic detail recorded for the disease outcome (lymphomas) – ICD-O3 is the current gold-standard for classification of haematological malignancies, but was not universally applied across studies. Understanding of the epidemiology of non-Hodgkin lymphomas has been impaired by an inability to adequately measure relevant exposures and outcomes – it is critical that these issues are addressed in future studies.
Acknowledgments
The pooled analysis was funded by the Leukaemia Foundation of Australia (LFA GIA 24). Individual studies were supported by the Italian Association for Cancer Research and the Italian League Against Cancer (Avi-N, Northern Italy); the Canadian Cancer Society and the Canadian Institutes for Health Research (British Columbia); National Cancer Institute (CA62006) (Connecticut); European Commission (QLK4-CT-2000-00422) (EpiLymph); Association pour la Recherche contre le Cancer (5111) and Fondation de France (1999 008471) (EpiLymph-France); Compagnia di San Paolo di Torino, Programma Oncologia 2001 (EpiLymph-Italy); Health Research Board (EpiLymph-Ireland); Spanish Ministry of Health FISS (PI040091) and CIBERESP (06/06/0073) (EpiLymph-Spain); German Federal Office for Radiation Protection (StSch4261 and StSch4420) (EpiLymph-Germany); National Institutes of Health (CA51086), the European Community and the Italian League against Cancer (Italy); National Cancer Institute (CA92153) (Mayo); National Cancer Institute (PC65064, PC67008, PC67009, PC67010, PC71105) (NCI-SEER); American Institute for Cancer Research (99B083) (Nebraska); National Health and Medical Research Council of Australia (990920) (NSW); National Institutes of Health (CA69269-02) and the Swedish Cancer Society (04 0458) (SCALE); National Institutes of Health (CA45614, CA89745, CA87014, CA104682) (UCSF); and the Leukaemia Research Fund of Great Britain (UK). The funders did not participate in the design, data collection or analyses of the individual studies, or in the interpretation and writing of manuscripts.
References
- 1.Beral V, Newton R. Overview of the epidemiology of immunodeficiency-associated cancers. J Natl Cancer Inst Monogr. 1998;(23):1–6. doi: 10.1093/oxfordjournals.jncimonographs.a024164. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007 Mar;16(3):405–408. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El GF, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009 Apr;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.Becker N, Fortuny J, Alvaro T, Nieters A, Maynadie M, Foretova L, Staines A, Brennan P, Boffetta P, Cocco PL, De SS. Medical history and risk of lymphoma: results of a European case-control study (EPILYMPH) J Cancer Res Clin Oncol. 2009 Aug;135(8):1099–1107. doi: 10.1007/s00432-009-0551-2. [DOI] [PubMed] [Google Scholar]
- 5.Tavani A, La VC, Franceschi S, Serraino D, Carbone A. Medical history and risk of Hodgkin's and non-Hodgkin's lymphomas. Eur J Cancer Prev. 2000 Feb;9(1):59–64. doi: 10.1097/00008469-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Vineis P, Crosignani P, Sacerdote C, Fontana A, Masala G, Miligi L, Nanni O, Ramazzotti V, Rodella S, Stagnaro E, Tumino R, Vigano C, et al. Haematopoietic cancer and medical history: a multicentre case control study. J Epidemiol Community Health. 2000 Jun;54(6):431–436. doi: 10.1136/jech.54.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, Zhang B, Zou K, Flynn S, Tallini G, Owens PH, Zheng T. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004 May;15(4):419–428. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 8.Holly EA, Bracci PM. Population-based study of non-Hodgkin lymphoma, histology, and medical history among human immunodeficiency virus-negative participants in San Francisco. Am J Epidemiol. 2003 Aug 15;158(4):316–327. doi: 10.1093/aje/kwg145. [DOI] [PubMed] [Google Scholar]
- 9.Vajdic CM, Grulich AE, Kaldor JM, Fritschi L, Benke G, Hughes AM, Kricker A, Turner JJ, Milliken S, Armstrong BK. Specific infections, infection-related behavior, and risk of non-Hodgkin lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2006 Jun;15(6):1102–1108. doi: 10.1158/1055-9965.EPI-06-0078. [DOI] [PubMed] [Google Scholar]
- 10.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Costantini AS, Rudiger T, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007 Jul 15;110(2):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Smith A, Roman E, Howell D, Jones R, Patmore R, Jack A. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010 Mar;148(5):739–753. doi: 10.1111/j.1365-2141.2009.08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Sanjose S, Bosch R, Schouten T, Verkuijlen S, Nieters A, Foretova L, Maynadie M, Cocco PL, Staines A, Becker N, Brennan P, Benavente Y, et al. Epstein-Barr virus infection and risk of lymphoma: immunoblot analysis of antibody responses against EBV-related proteins in a large series of lymphoma subjects and matched controls. Int J Cancer. 2007 Oct 15;121(8):1806–1812. doi: 10.1002/ijc.22857. [DOI] [PubMed] [Google Scholar]
- 14.Hjalgrim H, Askling J, Sorensen P, Madsen M, Rosdahl N, Storm HH, Hamilton-Dutoit S, Eriksen LS, Frisch M, Ekbom A, Melbye M. Risk of Hodgkin's disease and other cancers after infectious mononucleosis. J Natl Cancer Inst. 2000 Sep 20;92(18):1522–1528. doi: 10.1093/jnci/92.18.1522. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand KA, Birmann BM, Chang ET, Spiegelman D, Aster JC, Zhang SM, LAden F. A prospective study of Epstein-Barr virus antibodies and risk of non-Hodgkin lymphoma. Blood. 2010;116(18):3547–3553. doi: 10.1182/blood-2010-05-282715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JE, Gaboda D, Davis D. Early childhood chronic illness: comparability of maternal reports and medical records. Vital and Health Statistics. 2001;Series 2(131):1–10. [PubMed] [Google Scholar]
- 17.Tilley BC, Barnes AB, Bergstralh E, Labarthe D, Noller KL, Colton T, Adam E. A comparison of pregnancy history recall and medical records: implications for retrospective studies. American Journal of Epidemiology. 1985;121(2):269–281. doi: 10.1093/oxfordjournals.aje.a113997. [DOI] [PubMed] [Google Scholar]
- 18.Montella M, Maso LD, Crispos A, Talamini R, Bidoli E, Grimaldi M, Giudice A, Pinto A, Franceschi S. Do childhood diseases affect NHL and HL risk? A case-control study from northern and southern Italy. Leuk Res. 2006;30(8):917–922. doi: 10.1016/j.leukres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Spinelli JJ, Ng C, Weber JP, Connors JM, Gascoyne RD, Lai A, Brooks-Wilson A, Le ND, Berry B, Gallagher RP. Organochlorines and risk of non-Hodgkin lymphoma. International Journal of Cancer. 2007 Dec 15;121(12):2767–2775. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- 20.Cerhan JR, Ansell SM, Fredericksen ZS, Kay NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W, Macon WR, Jelinek D, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007 Dec 15;110(13):4455–4463. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cozen W, Cerhan JR, Martinez-Maza O, Ward MH, Linet M, Colt JS, Davis S, Severson RK, Hartge P, Bernstein L. The effect of atopy, childhood crowding, and other immune-related factors on non-Hodgkin lymphoma risk. Cancer Causes Control. 2007 Oct;18(8):821–831. doi: 10.1007/s10552-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 22.Melbye M, Smedby KE, Lehtinen T, Rostgaard K, Glimelius B, Munksgaard L, Schollkopf C, Sundstrom C, Chang ET, Koskela P, Adami HO, Hjalgrim H. Atopy and risk of non-Hodgkin lymphoma. J Natl Cancer Inst. 2007 Jan 17;99(2):158–166. doi: 10.1093/jnci/djk019. [DOI] [PubMed] [Google Scholar]
- 23.Willett EV, Smith AG, Dovey GJ, Morgan GJ, Parker J, Roman E. Tobacco and alcohol consumption and the risk of non-Hodgkin lymphoma. Cancer Causes Control. 2004 Oct;15(8):771–780. doi: 10.1023/B:CACO.0000043427.77739.60. [DOI] [PubMed] [Google Scholar]