Abstract
Background
Thyroid hormone receptors (TRs) are ligand-dependent transcription factors that mediate the actions of the thyroid hormone (T3) in development, growth, and differentiation. The THRA and THRB genes encode several TR isoforms that express in a tissue- and development-dependent manner. In the past decades, a significant advance has been made in the understanding of TR actions in maintaining normal cellular functions. However, the roles of TRs in human cancer are less well understood. The reduced expression of TRs because of hypermethylation, or deletion of TR genes found in human cancers suggests that TRs could function as tumor suppressors. A close association of somatic mutations of TRs with human cancers further supports the notion that the loss of normal functions of TR could lead to uncontrolled growth and loss of cell differentiation.
Scope of Review
In line with the findings from association studies in human cancers, mice deficient in total functional TRs (Thra1−/−Thrb−/− mice) or with a targeted homozygous mutation of the Thrb gene (denoted PV; ThrbPV/PV mice) spontaneous develop metastatic thyroid carcinoma. This review will examine the evidence learned from these genetically engineered mice that provided strong evidence to support the critical role of TRs in human cancer.
Major Conclusions
Loss of normal functions of TR by deletion or by mutations could contribute to cancer development, progression and metastasis.
General Significance
Novel mechanistic insights are revealed in how aberrant TR activities lead to carcinogenesis. Mouse models of thyroid cancer provide opportunities to identify molecular targets as potential treatment modalities.
Keywords: Thyroid hormone receptor mutant, Thyroid cancer, Phosphatidylinositol 3 kinase, Src kinase, β-catenin, Mouse models
1. Introduction
Molecular cloning of thyroid hormone receptors (TRs) cDNA in 1986 ushered in an exciting era in the understanding of the structure, expression, functions, and transcription regulation of TRs [1, 2]. Two human TR genes, THRA and THRB, located on different chromosomes, encode thyroid hormone (T3) binding TR isoforms (TRα1, β β2, and β3). Similar to other members of the nuclear receptor superfamily, these TR isoforms have an amino-terminal A/B domain, a central DNA-binding domain, and a carboxyl-terminal ligand-binding domain. These TR isoforms share extensive sequence homology in the DNA and ligand-binding domains, but differ in the length and amino acid sequence at the amino terminal A/B domain. Comparison of X-ray crystallographic structures of rat TRα and human TRβ ligand binding domains shows a close structural similarity [3, 4]. However, there are subtype-dependent differences in the ligand-binding pocket that allow selective recognition of certain ligands [4].
The transcriptional activity of TRs is regulated by multiple mechanisms including the type of thyroid hormone response elements (TREs) located on the promoters of T3 target genes, the tissue- and development-dependent expression of TR isoforms, and a host of nuclear co-repressors and co-activators [5]. In the absence of T3, TRs recruit corepressor proteins, such as nuclear receptor co-repressor 1 (NCOR1) and silencing mediator of retinoid and thyroid hormone receptors (SMRT), and repress the transcription of target genes. In the presence of T3, TRs undergo a conformational change and that results in the exchange of co-repressors for co-activators, such as p160/ steroid receptor co-activator-1 (SRC-1) family, to activate transcription of target genes. In addition to transcriptional stimulation, TRs also repress gene expression in a T3-dependent manner by binding to negative TREs [5]. However, recent advances have expanded this T3-dependent corepressor-coactivator exchange model and shown that NCOR1 and SMRT play a role in determining T3-sensitivity in vivo, suggesting that corepressors can be recruited to TR in the presence of T3 [6–8].
In spite of significant progress in understanding the molecular mechanisms by which TR functions in maintaining normal physiological T3-mediated homeostasis, the roles of TR in human cancers are less well understood. Early evidence to suggest that mutated TR could be involved in carcinogenesis came from the discovery that TRα1 is the cellular counterpart of the retroviral v-ERBA involved in the neoplastic transformation leading to acute erythroleukemia and sarcomas [2, 9]. It is a highly mutated chicken TRα1 that does not bind T3 and loses the ability to activate gene transcription. V-ERBA competes with TR for binding to TREs and interferes with the normal transcriptional activity of liganded-TR on several promoters [10, 11]. Early direct evidence that the v-ERBA oncoprotein can promote neoplasia in mammals through its dominant negative activity was provided by the finding that male transgenic mice overexpressing v-ERBA develop hepatocellular carcinomas [12].
The notion that the loss of TR functions could be involved in the development of human cancers gained further support by association studies. Loss in the expression of the THRB gene because of the truncation/deletion of chromosome 3p where the THRB gene is located was reported in many malignancies including lung, melanoma, breast, head and neck, renal cell, uterine cervical, ovarian, and testicular tumors [13–18]. The THRA locus undergoes frequent loss of heterozygosity (LOH) in sporadic breast cancer, and rearrangement of the THRA gene has also been reported in leukemia, breast, and stomach cancer [19–21]. Somatic mutations of TRs have been found in human hepatocellular carcinoma [22], renal clear cell carcinoma [23, 24], breast cancer [25], pituitary tumor [26, 27], and thyroid cancer [28] (Table 1). Many of these TR mutants have lost T3 binding activity and transcription capacity, and some exhibit dominant negative activity [23, 28] (Table 1).
Table 1.
Somatic mutations of thyroid hormone receptor genes in human cancers
| Type of cancer | Gene | Mutation Sites | Impaired activity
|
Dominant negative activity | Reference | |
|---|---|---|---|---|---|---|
| T3 binding | DNA binding | |||||
| Hepatocellular carcinoma | THRB | 234G insertion | ND | ND | ND | 22 |
| D211N | No | No | ||||
| R153L | No | No | ||||
| R194G | ND | ND | ||||
| M27I, C102R, T363N | Yes | Yes | ||||
| K258E | No | No | ||||
| S38L, C441R | ND | ND | ||||
| M308I | Yes | No | ||||
| H400Y, F434N | Yes | No | ||||
| K108N, T324P | Yes | No | ||||
| S38P, I54T, P273S, P273L, E306G | Yes | No | ||||
| K23E, I187V | Yes | Yes | ||||
|
| ||||||
| THRA | A225G, T227N | ND | ND | |||
| A225G, D246N, G350K | Yes | No | ||||
| S40T, K136R, L251P, V390A | Yes | No | ||||
| K74E, A264V | Yes | Yes | ||||
| K74R, M150T, E159K | Yes | No | ||||
| N179I | ND | ND | ||||
| S38Q, Q108K, F112L, I299V | Yes | Yes | ||||
| C110Y, C254A | Yes | Yes | ||||
| G24E, M256V, E343A, P269L | Yes | No | ||||
|
| ||||||
| Renal cell carcinoma | THRB | S99R, W129L, F451I | Yes | No | Yes | 23, 24 |
| Y321H | Yes | No | No | |||
| F451S | Yes | No | No | |||
| Q252R, A387P, F417L | Yes | No | No | |||
| K155E, K411E | No | Yes | No | |||
| Δ1–26, S380F | Yes | No | No | |||
| E299K, H412R, L456S | Yes | No | No | |||
|
| ||||||
| THRA | S183N, H184Q, R228H, K288E | Yes | Yes | No | ||
| I116N, M388I | Yes | Yes | No | |||
| I116N, A225T, M388I | Yes | Yes | No | |||
|
| ||||||
| Breast cancer | THRB | deletion (123–242) | ND | ND | ND | 25 |
| deletion (319–366) | ||||||
| truncated at 167 | ||||||
| deletion (166–402) | ||||||
| deletion (181–382) | ||||||
|
| ||||||
| Pituitary tumor | THRB | R438H | Yes | No | ND | 26, 27 |
| H450Y | Yes | No | Yes | |||
|
| ||||||
| Thyroid cancer | THRB | V109A, I431T | Yes | Yes | 28 | |
| R185K, T273A, L456S | Yes | Yes | ||||
| M32V | No | No | ||||
| E34G, P141L | Yes | Yes | ||||
| A318D, F451I | Yes | Yes | ||||
| N76D, S81L, I135V, Q136H, R201X | Yes | Yes | ||||
| F403L. C446R | Yes | Yes | ||||
| K91R, K289M | Yes | Yes | ||||
| THRB | Q235X, M379T, D427G | Yes | Yes | |||
| K411E | Yes | Yes | ||||
| Q205L | Yes | Yes | ||||
| K103R | Yes | Yes | ||||
| M32T, L373P | Yes | Yes | ||||
| K411E, H435R | Yes | No | ||||
| S99R | Yes | Yes | ||||
|
| ||||||
| THRA | T80I, L109P | Yes | Yes | |||
| E213D | Yes | Yes | ||||
| S305P, K337R | Yes | Yes | ||||
| G57E | Yes | Yes | ||||
| K29T, C97X | Yes | No | ||||
| Y352C | Yes | Yes | ||||
| S183N, H184Q, Q187X, R228H, E245V, K288E | Yes | Yes | ||||
| S183N, H184Q, R228H, M369V | Yes | Yes | ||||
| S183N, H184Q, R228H | Yes | Yes | ||||
| S271I | Yes | Yes | ||||
ND. Not determined.
Decreased expression due to silencing of the THRB gene by promoter hyermethylation has been found in human cancer including breast, lung, and thyroid carcinoma [29–32]. A recent study has provided evidence that the expression of the THRB gene could also be repressed via micro RNAs regulatory mechanisms in papillary thyroid cancer [33]. The findings from these association studies raised the possibility that TRs could function as tumor suppressors in human cancers. However, this possibility could not be directly tested until genetically engineered mouse models became available. This article will briefly review the current mouse models of thyroid cancer caused by the loss of total functional TRs (Thra1−/−Thrb−/− mice) or a targeted homozygous mutation of the Thrb gene (denoted PV; ThrbPV/PV mice) and its derived strains. The altered molecular pathways leading to carcinogenesis due to the PV mutation or loss of TRs will be highlighted and the underlying mechanisms discussed.
2. Mouse models of thyroid cancer
To test the hypothesis that the loss of normal functions of TRβ contributes to cancer development and progression, several mutant mice have been developed, each of which was designed to explore a specific aspect of the roles TRs play in cancer.
2.1. The ThrbPV/PV mouse
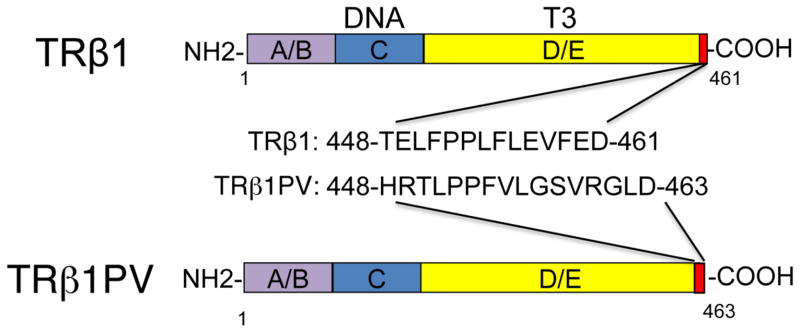
The ThrbPV/PV knockin mouse, created by targeting the PV mutation to the Thrb gene locus, was initially intended to study an inheritable disease with reduced tissue sensitivity to thyroid hormone known as resistance to thyroid hormone (RTH) [34]. The PV mutation was identified in an RTH patient with a frameshift mutation in the C-terminal 14 amino acids of TRß (Figure 1), resulting in a complete loss of T3 binding and transcriptional capacity [35]. This ThrbPV/PV mouse faithfully recapitulates human RTH with the dysregulation of the hypothalamus-pituitarythyroid axis, leading to elevated serum thyroid hormone accompanied by nonsuppressible high serum thyroid-stimulating hormone (TSH) [34]. As ThrbPV/PV mice age, they spontaneously develop follicular thyroid carcinoma, resembling the pathological progression of human thyroid cancer [36, 37]. Pathological changes progress from hyperplasia, capsular invasion, vascular invasion, and anaplasia to eventually distant metastasis (Figure 2). Metastasis occurs mainly in the lung and occasionally in the endocardium, but not in the local lymph nodes [36, 37]. The findings that ThrbPV/PV mice spontaneously develop follicular thyroid carcinoma similar to human cancer indicate that these mice could be used as a model to understand how the loss of normal functions of TRß could lead to cancer phenotypes and elucidate the molecular genetic changes underlying follicular thyroid carcinoma.
Figure 1. Schematic comparison of TRβ1 and TRβ1PV mutant.
The TRβ1PV mutation has a frame-shift mutation in the C-terminal 14 amino acids of TRβ1 (461 amino acids), ending with an addition of two amino acids (463 amino acids). TRβ1PV has completely lost of T3 binding activity and transcription capacity. The carboxyl-terminal sequences of the TRβ1 and TRβ1PV mutant are shown. The domains are also indicated.
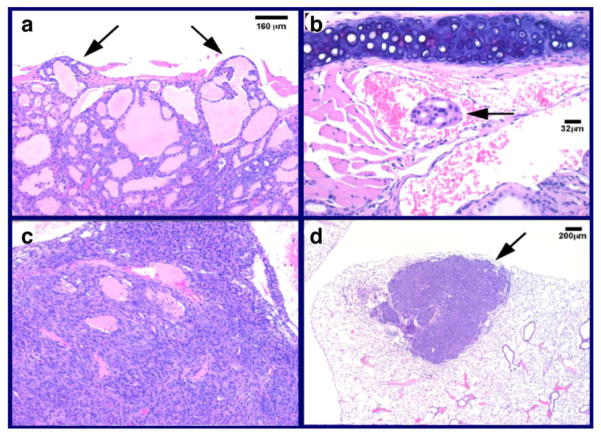
Figure 2. ThrbPV/PV mice spontaneously develop follicular thyroid carcinoma with pathological progression similar to human cancer.
Representative examples of pathological features in thyroid carcinogenesis of ThrbPV/PV mice: capsular invasion in the thyroid (a) (arrows), vascular invasion in the thyroid (b) (arrow), anaplasia in the thyroid (c, same magnification as panel a) and metastatic thyroid carcinoma lesions in the lung, often in a follicular pattern (d) (arrow) [36]. Magnifications are as indicated.
2.2. The ThrbPV/− mouse
The critical role of TRß in cancer is further illustrated by the generation of the ThrbPV/− mouse. This double mutant mouse was derived from the cross-breeding of heterozygous ThrbPV/+ mice with Thrb knockout mice [38]. Remarkably, in contrast to the heterozygous ThrbPV/+ mice, ThrbPV/− mice spontaneously develop follicular thyroid carcinoma with a pathological progression similar to that of ThrbPV/PV mice [38]. These findings indicate that one mutated Thrb allele in the absence of the other wild-type allele is sufficient to induce spontaneous thyroid carcinoma. Thyroid carcinoma occurs either when both Thrb alleles are mutated or when one allele is mutated and there is ablation of the other wild-type allele. Thus, this ThrbPV/− mouse model has provided direct in vivo evidence to indicate that the Thrb gene can function as a tumor suppressor and raises the possibility that the THRB gene could serve as a novel therapeutic target in thyroid cancer.
2.3. The TR-double knockout (Thra1−/−Thrb−/−) mouse
Association studies show that the expression of TRs is frequently silenced in human cancers [23, 28, 30, 32, 33]. To further test the hypothesis that a lack of TRs is deleterious, Zhu et al. used Thra1−/−Thrb−/− mice [39–41] to delineate whether a total loss of all functional TRs could lead to cancer development. Remarkably, these mice spontaneously develop follicular thyroid cancer with pathological progression from hyperplasia to capsular invasion, vascular invasion, anaplasia, and metastasis, similar to human thyroid cancer [42]. These findings provided direct in vivo evidence to show that functional loss of both Thra1 and Thrb genes promotes thyroid tumor development and metastasis. Importantly, this mouse model provides an opportunity to identify the common pathways shared by the loss of TR functions due to mutations (ThrbPV/PV mice) or due to deletion of both TR genes (Thra1−/− Thrb−/− mice) in thyroid carcinogenesis. Moreover, by comparison of the gene expression profiles and the altered signaling pathways between these two mouse models of thyroid cancer, it would be possible to discern the different molecular actions of thyroid carcinogenesis resulting from TRβ mutation and deletion of total functional TRs.
3. Lessons learned from mouse models of thyroid cancer
The mouse models of thyroid cancer described above allowed elucidation of molecular actions of a mutated TRβ, as well as new insight into how deficiency of TR leads to thyroid carcinogenesis. cDNA microarray analyses indicate complex alterations of multiple pathways in thyroid carcinogenesis of ThrbPV/PV mice [43] suggesting PV could act as an oncogene via multiple mechanisms to promote cancer progression. The following sections will highlight what has been learned about the roles of TR in thyroid cancer via the loss-of-function approach by TRβ mutations and deletion of TRs.
3.1. Oncogenic actions of PV via transcription regulation
3.1.1. Down-regulation of the peroxisome proliferator-activated receptor γ (PPARγ )
PV was identified in an RTH patient and has been shown to mediate RTH phenotypes via its dominant negative activity by interfering with transcription activity of wild-type TRs (WT-TRs) [44, 45]. Thus, it was hypothesized that TR-positively regulated tumor suppressor genes in the thyroids would be repressed by PV. The repressed expression of the tumor suppressors in the thyroids would facilitate cancer progression. This PV-mediated action is exemplified by the regulation of PPARγ [46, 47]. The importance of PPARγ signaling in thyroid carcinogenesis was made clear by the identification of the PPARγ-paired box gene 8 (PAX8) fusion gene in approximately 35% of human follicular thyroid carcinomas [46]. This fusion gene loses its ability to stimulate PPARγ-ligand dependent transcription and inhibits PPARγ transcriptional activity [46]. These observations raised the possibility of PPARγ as a tumor suppressor in thyroid carcinogenesis.
Consistent with these findings, Pparγ mRNA levels are markedly decreased in thyroid carcinogenesis of ThrbPV/PV mice [37]. PPARγ protein levels are also reduced in thyroid tumors and are totally lost in lung metastases. In addition to its inhibition of expression, PV also acts to repress the ligand-dependent transcription activity of PPARγ [47]. Biochemical and cell-based studies indicate that similar to WT-TRβ, PV competes with PPARγ for binding to the peroxisome proliferator responsive element (PPRE) as homodimers or as heterodimers with PPARγ, thereby repressing the transcription activity of PPARγ (See “i” of Figure 3). PPRE–bound PV recruits the nuclear co-repressor NCOR1 to the promoter of PPARγ target genes, independent of T3. Such constitutive association of PV with co-repressors prevents the recruitment of the SRC-1 to the PPRE-bound PV/PPARγ complexes, in spite of the presence of PPARγ ligands (e.g., troglitazone). Thus, in the thyroids of ThrbPV/PV mice, decreased expression and repression of transcriptional activity of PPARγ lead to reduced expression of PPARγ downstream tumor suppressor target genes, thereby promoting the progression and development of thyroid cancer. A recent study further confirmed the tumor suppressor role of PPARγ in another mouse model in which the Pax8-Pparγ (PPFP) gene was targeted to the thyroid deficiency in PTEN (phosphatase and tensin homologue deleted from chromosome 10). Treatment of this mouse with another PPARγ ligand, pioglitazone, decreased thyroid growth and prevented metastatic disease [48].
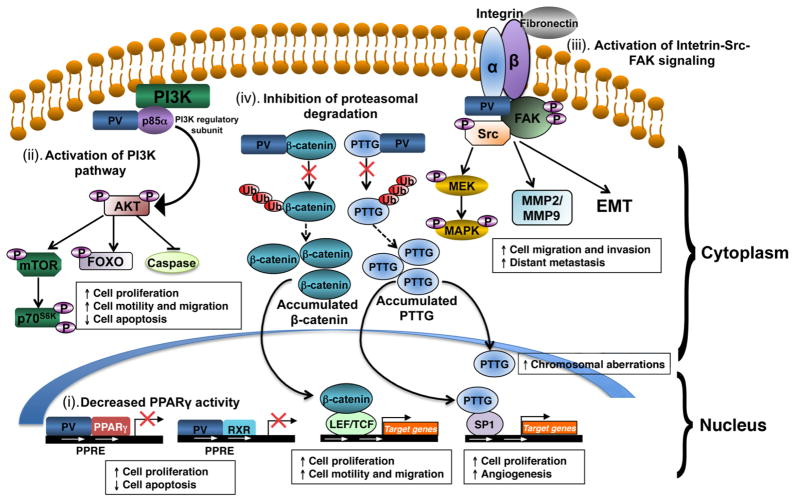
Figure 3. PV mediates oncogenic actions in thyroid carcinogenesis of ThrbPV/PV mice via multiple mechanisms.
(i). PV decreases peroxisome proliferator–activated receptor γ (PPARγ) activity by competition with PPARγ for binding to PPARγ response elements (PPRE) as heterodimers with PPARγ or the retinoid X receptor (RXR). Repressed transcriptional activity of PPARγ leads to increase cancer cell proliferation and decrease apoptosis in thyroids of ThrbPV/PV mice. (ii) PV activates phosphatidylinositol 3-kinase (PI3K) signaling pathway by physical interaction with the regulatory subunit of PI3K (p85α). Over-activation of PI3K-Akt signaling increases proliferation and migration of cancer cells and decreases apoptosis via the downstream pathways as shown. (iii) PV activates integrin-Src-focal adhesion kinase (FAK) signaling cascade. PV interacts with integrin and activates phosphorylation of Src and FAK. Activated Src-FAK signaling pathway induces epithelial-mesenchymal transition (EMT) and activation of matrix metalloproteases (MMPs) to increase cell migration, invasion and distant metastasis. Activated Src also potentiates mitogen-activated protein kinase (MAPK) signaling to increase cell proliferation and EMT. (iv) PV inhibits proteasomal degradation of the pituitary tumor transforming gene (PTTG) and β-catenin. PV binds to PTTG and prevents its degradation by proteasome, resulting in accumulation of PTTG. Elevated PTTG impedes mitotic progression, leading to chromosomal aberrations. Elevated PTTG, via transcription activation of its target genes, increases cell proliferation and augments angiogenesis. PV also increases the stability of β-catenin via direct protein-protein interaction, thereby inhibiting protesomal degradation of β-catenin. Elevated β-catenin protein levels increase transcription activation of its downstream target genes that affect cell proliferation and migration.
3.2. Oncogenic actions of PV via novel extra-nuclear actions
Recent studies have indicated that TRs could also mediate T3 biological activities beyond TRE-mediated gene transcription. These extra-nuclear actions were reported to regulate ion channels, glucose transporters, protein kinases, and phospholipid metabolism that affect cellular functions [5]. The extra-nuclear TR actions could initiate signal transduction via direct protein-protein interaction. One prominent example is TR-mediated activation of phosphatidylinositol 3-kinase (PI3K) activity in human endothelial cells reported by Simoncini et al. [49]. This activation is through direct physical interaction of TR with the p85α subunit of PI3K, leading to the phosphorylation and activation of Akt and endothelial nitric oxidase synthase [50]. This TR-mediated PI3K activation has also been demonstrated in other cell types such as human fibroblasts, neonatal rat cardiomyocytes, and human and rat insulinoma cell lines [51–55]. These studies suggest that non-TRE-dependent effects of TR via protein-protein interactions could be an important mode of TR actions. Likewise, mutations of TR that perturb the normal protein-protein interactions in vivo could lead to deleterious consequences as exemplified by the aberrant interaction of PV with several key regulators critical for cell proliferation, apoptosis, motility, and invasion in the thyroids of ThrbPV/PV mice. The key regulators that aberrantly interact with PV and their impact on cellular signaling are highlighted below.
3.2.1. Sustained activation of signaling pathways via direct interaction of PV with key cellular regulators
3.2.1.1. Phosphatidylinositol 3-kinase signaling
The PI3K signaling pathway controls a wide variety of cellular processes including cell death and survival, cell migration, protein synthesis, and metabolism. Aberrant PI3K-dependent signaling, mediated by Akt kinase, is well-known to contribute to abnormal cell growth and cellular transformation in a variety of neoplasms, including thyroid cancer [56, 57]. PI3Ks consist of a catalytic subunit with a molecular weight of about 110 kD (p110) and a tightly associated regulatory subunit (p85α, p85β, or p55γ). The regulatory subunits do not possess an intrinsic enzymatic activity, but are responsible for the activation and subcellular localization of the catalytic subunits. These molecules contain rho-GTPase-activating protein homology domains, Src homology (SH) 2 domains, SH3 domains, and proline-rich motifs [58]. Upon activation by membrane receptors, PI3K phosphorylates phosphatidylinositol-4,5 biphosphate (PIP2) to form phosphatidylinositol-3,4,5-triphosphate (PIP3). Through phosphatidylinositol-dependent kinases, the immediate downstream effectors of PI3K, Akt is phosphorylated and activated to further phosphorylate downstream protein substrates. The activity of PI3K is negatively regulated by PTEN, a protein phosphatase that dephosphorylates PIP3 to form PIP2 [58].
Prompted by reports that the Akt activity is over-activated in thyroid cancer in humans and in ThrbPV/PV mice [56, 57, 59], Furuya et al. examined whether the increased Akt activity was mediated by the increased kinase activity of PI3K. Indeed, the activity of PI3K in the thyroids of ThrbPV/PV mice is increased up to 10-fold that in normal thyroids [60]. This increase is due to the direct physical interaction of the hormone binding domain of PV with the C-terminal SH2 domain of p85α subunit [60, 61] (see “ii” of Figure 3). The signal of PV-mediated PI3K activation is relayed via phosphorylation cascades of the downstream effectors, Akt, integrin-linked kinase, mammalian target of rapamycin (mTOR), and p70S6K to increase cell proliferation, migration, and invasion to promote thyroid carcinogenesis [60] (“ii” of Figure 3). That this novel oncogenic action of PV via direct protein-protein interaction with the p85α subunit is critical in thyroid carcinogenesis was further illustrated by the delay of cancer progression and the blockage of distant metastasis when PI3K activity was inhibited by treatment of ThrbPV/PV mice with a specific inhibitor, LY294002 [62]. Moreover, when the PI3K negative regulator, PTEN, is haplo-deficient in ThrbPV/PVPten+/− mice, thyroid tumor progression is greatly accelerated by further activation of PI3K-Akt signaling leading to shortening of survival and increased tumor size [63].
3.2.1.2. Integrin-Src-FAK signaling pathway
Invasion of the basement membrane (BM) and migration through the extracellular membrane (ECM) are critical processes in cancer progression and metastasis. These processes are mediated mainly by interactions of integrin receptors and BM/ECM components with subsequent degradation of ECM by metalloproteases (MMPs) to help the cancer cell invasion through the membrane barriers. After binding to their ligands, integrin receptors undergo conformational changes and activate intracellular signaling molecules such as Src kinase and focal adhesion kinase (FAK) [64, 65]. Aberrant expression of integrins has been reported in many human cancers including thyroid cancer [66, 67]. Overactivation of Src and its downstream effector, FAK, are frequent in human cancer and associated with invasive potential in thyroid cancer [68–70]. Recent studies showed increased protein abundance of integrins α5, αV, β1, and β3 and their ligand fibronectin in the thyroid of in ThrbPV/PV mice [71] (see “iii” of Figure 3). Importantly, PV was found to physically complex with integrins α5 and β1. PV also activated Src kinase pathway by increasing its phosphorylation, which activates FAK by increasing phosphorylation on several tyrosine sites [71]. Interestingly, PV also physically interacts with FAK, thus forming a large complex of PV-integrins-FAK-PI3K, leading to activation of downstream signaling of p38 mitogen-activated protein kinase (MAPK) via increased phosphorylation cascades to stimulate the expression of MMP-9 at the mRNA and protein levels (“iii” of Figure 3). The activation of cSrc-FAK is known to remodel the actin cytoskeleton in cancer cells that could underlie aberrant cell migration and invasion [72–74]. Lu et al. also found an increased protein abundance of β-actin and erzin, which links the cytoskeletal structure and the plasma membrane. The findings that PV also complexes with β-actin, as well as with erzin, uncover a novel mechanism by which PV could change cytoskeletal organization to promote cell migration and invasion (“iii” of Figure 3).
That PV-induced activation of Src-FAK-MAPK signaling via complex formation is critical in thyroid carcinogenesis was shown in a recent study in which Src-FAK signaling was also found to be highly activated in a more aggressive mouse model of ThrbPV/PVPten+/− mice [75]. Treatment of ThrbPV/PVPten+/− mice with a Src-specific inhibitor, SKI-606 (bosutinib), prevents tumor growth, blocks distant metastasis, and regains the differentiation state of thyroid follicular cells. These responses were accompanied by down-regulation of MAPK pathways and inhibition of epithelial-mesenchymal transition (EMT; “iii” of Figure 3). These findings suggest that one of the mechanisms by which PV act as an oncogene is via complexing with key cellular regulators in multiple signaling pathways to affect expression and activity of ECM, adhesion, migration, EMT, and differentiation of cancer cells in thyroid carcinogenesis [75].
3.2.2. Aberrant activation of signaling pathways via PV-mediated stabilization of key cellular regulators
3.2.2.1. Pituitary tumor-transforming gene (PTTG)
PTTG, also known as securin, is a critical mitotic checkpoint protein that helps hold sister chromatids together before entering anaphase [76]. It was originally isolated from GH4 pituitary cells and shown to cause in vitro cell transformation and to induce tumor formation in vivo [77] and genetic instability in a variety of cells including thyroid cells [78–80]. Although overexpression of PTTG is evident in thyroid carcinomas [81, 82], how the over-expressed PTTG acts in thyroid carcinogenesis was not clear. The finding that the PTTG is over-expressed in the thyroid tumors of ThrbPV/PV mice provided a tool to probe its role in thyroid carcinogenesis [43]. The link of the over-expressed PTTG to chromosomal aberrations was directly demonstrated in cell lines derived from thyroid tumors of ThrbPV/PV mice [83]. Spectral karyotyping analysis (SKY) of seven tumor cell lines showed abnormal karyotypes, and also exhibited a variety of structural chromosomal aberrations, including common recurrent translocations and deletions [83]. This SKY analysis suggested that the development and progression of follicular thyroid carcinoma in ThrbPV/PV mice comprise recurrent structural and numerical genomic changes, some of which mimic those described in human thyroid cancer. Ying et al. elucidated the molecular basis of PTTG-induced chromosomal instability during thyroid carcinogenesis of ThrbPV/PV mice [84]. PTTG protein was found to physically associate with TRβ as well as PV. Concomitant with T3-induced degradation of TRβ [85], PTTG proteins are degraded by the proteasomal machinery, but no such degradation occurs when PTTG is associated with PV (see “iv” of Figure 3). The degradation of the PTTG-TRβ complex is activated by the direct interaction of the liganded TRβ with the steroid receptor coactivator-3 (SRC-3) that recruits a proteasome activator (PA28γ) [84]. PV that does not bind T3, thus cannot recruit SRC-3-PA28γ complex to activate proteasome-mediated degradation, and as a result PTTG protein level is increased (“iv” of Figure 3). The accumulated aberrantly elevated PTTG impedes mitotic progression in cells expressing PV [84]. The PV-induced stabilization of PTTG protein, a critical regulator of chromosomal integrity, serves as a novel extra-nuclear action to contribute to thyroid carcinogenesis.
3.2.2.2. β-catenin
β-catenin is key regulator of the Wnt signaling pathway, which plays important roles not only in normal physiological processes in adults, but also in embryogenesis and carcinogenesis [86]. In the canonical pathway, stabilized β-catenin translocates from cytoplasm into the nucleus and interacts with T cell factor/lymphoid enhancer factor (TCF/LEF) family transcription factors to stimulate the expression of genes critical for normal cellular functions. The cellular level of β-catenin is regulated by two distinct proteolytic pathways involving axin/glycogensynthase kinase 3β (GSK-3β)/adenomotous polyposis coli (APC) complex and p53 inducible pathway [87, 88]. Abnormally accumulated β-catenin has been reported in many human cancers, including thyroid cancer [89].
The aberrantly elevated β-catenin in thyroid tumors of ThrbPV/PV mice led to the discovery that the cellular stability of β-catenin is down-regulated by the liganded TRβ, but elevated by PV [90] (see “iv” of Figure 3). Cell-based studies showed that T3 induces the degradation of β-catenin in cells expressing TRβ via proteasomal pathways. In contrast, since PV does not bind T3, this T3-mediated regulatory mechanism is lost in PV, leading to accumulated β-catenin in PV-expressing cells (“iv” of Figure 3). Further analyses showed that β-catenin physically associates with the unliganded TRβ or PV. However, in the presence of T3, β-catenin is dissociated from TRβ/β-catenin complexes, but not from PV/β-catenin complexes. Thus, β-catenin signaling is repressed by T3 in TRβ-expressing cells through decreasing β-catenin-mediated transcription activity and target gene expression, whereas sustained β-catenin signaling is observed in PV-expressing cells [90] (“iv” of Figure 3). Importantly, an increased β-catenin level in thyroid tumors of ThrbPV/PV mice is associated with an increase in phosphorylated β-catenin (serine 552), accompanied by increased expression of β-catenin downstream target genes, cyclin D1, c-myc, and matrix metalloprotease (MMP)-1. Altogether, these studies show that β-catenin signaling is constitutively activated in the thyroids of ThrbPV/PV mice (“iv” of Figure 3). The stabilization of β-catenin, via association with a mutated TRβ, represents a novel activating mechanism of the oncogenic β-catenin that could contribute to thyroid carcinogenesis in ThrbPV/PV mice.
3.3. Oncogenic actions of PV via “Gain-of-function”
A critical issue in understanding the oncogenic actions of a mutated TRβ (i.e., PV) is whether PV acts simply via loss of the WT-TR tumor suppressor function or also results from gain-of-function activities. As indicated in Section 2.3., Thra1−/−Thrb−/− mice spontaneously develop follicular thyroid carcinoma [42]. The ThrbPV/PV and Thra1−/−Thrb−/− mice exhibit similarly elevated serum levels of TSH and thyroid hormones [91], but intriguingly the Thra1−/−Thrb−/− mice develop follicular thyroid carcinoma with a slower progression and a less aggressive malignant phenotype [36, 42]. These observations raised the possibility that in addition to the loss of normal tumor suppressor functions of WT-TRβ, PV could acquire additional oncogenic activity via gain-of-function through mutation. This possibility was tested by using cDNA microarrays to compare the gene expression profiles of laser-captured microdissected thyroid tumor cells of ThrbPV/PV mice and Thra1−/−Thrb−/− mice [92]. Analysis of the cDNA microarray data between tumor cells of these two mutant mice showed contrasting global gene expression profiles. With stringent selection using 2.5-fold change in cDNA microarray analysis, 241 genes with altered gene expression were identified. Nearly half of the genes (n=113: 49% of total) with altered gene expression in thyroid tumor cells of ThrbPV/PV mice were associated with tumorigenesis and metastasis; some of these genes function as oncogenes in human thyroid cancers. The remaining genes were found to function in transcriptional regulation, RNA processing, cell proliferation, apoptosis, angiogenesis, and cytoskeleton modification. These results indicate that the more aggressive thyroid tumor progression in ThrbPV/PV mice was not due simply to the loss of tumor suppressor functions of TR via mutation but also, importantly, was a result of gain-of-function in the oncogenic activities of PV to drive thyroid carcinogenesis. Thus, the mechanisms by which PV acts as an oncogene are more diverse than previously envisioned in that as a mutated TRβ can evolve with an oncogenic advantage to promote thyroid carcinogenesis.
However, it also important to point out that there are common altered pathways shared in Thra1−/−Thrb−/− and ThrbPV/PV mice during thyroid carcinognesis [42, 93–95]. Aberrant activation of Akt-mTOR-p70S6K pathway, vascular growth factor and its receptors were observed in the thyroid tumors of both Thra1−/−Thrb−/− and ThrbPV/PV mice [42, 59]. The over-expression of known tumor promoters such as the Pttg1 gene and the suppression of tumor suppressors such as the PPARγ also were similarly detected in thyroid carcinogenesis in both mutant mice [42, 96]. These findings clearly show that functional loss of both Thra and Thrb genes by deletion or through mutation in PV promote thyroid tumor development and metastasis via some common pathways.
4. Summary and future directions
Studies of the oncogenic actions of mutant PV in thyroid carcinogenesis of ThrbPV/PV mice have provided direct in vivo evidence to show that TRβ mutations can lead to cancer development. Importantly, novel molecular mechanisms of TRβ mutations in carcinogenesis have been uncovered. PV acts as an oncogene via multiple molecular mechanisms. It can function by interfering with the transcription activity of WT-TR by abnormal repression in the expression of tumor promoters (e.g., PPARγ). PV can also act at the transcription level independent of TR, via “gain-of-function.” Importantly, PV can also function via extra-nuclear sites, for example by initiating the actions via direct protein-protein interaction with key cellular regulators such as PI3K, integrins, FAK, β-actin, and erzin. Via phosphorylation cascades, the signals initiated by the interaction of PV with these regulators are transduced to activate downstream pathways, such as PI3KAKT-mTOR signaling and MAPK signaling, to promote cancer cell proliferation, apoptosis, migration, and invasion. Moreover, new regulatory mechanisms of protein stability of key cellular regulators by PV have also been elucidated, such as the PV induced stabilization of PTTG, leading to genomic abnormalities. The discovery of these complex actions of PV revealed that thyroid carcinogenesis resulted from alterations of multiple signaling pathways. The identification of these altered cellular pathways provides new opportunities for potential molecular targets for diagnosis and treatments.
Although the ThrbPV/PV mouse has provided a valuable tool to advance our understanding of how a mutated TRβ can lead to thyroid carcinogenesis, many challenging issues are yet to be elucidated. 1). At present, no thyroid cancer has been detected in heterozygous ThrbPV/+ mice. Studies show that PV is oncogenic via collaboration with other key regulators in homozygous ThrbPV/PV mice to bring about thyroid cancer. It is important to identify the factors with which PV collaborates to invoke its oncogenic activity in heterozygous ThrbPV/+ mice. Addressing this issue will shed new light on why RTH patients who are mostly heterozygous in the mutation of one single allele of the THRB gene have no increased propensity to develop thyroid cancer. 2). Another issue is whether the oncogenic action of a mutated TRβ is limited to a PV-specific mutated sequence or also extends to other mutations in the helix 11 and 12 of TRβ that can invoke oncogenic activity. Clarification of this central question would further advance our understanding of the role of TRβ mutations in thyroid cancer. 3). In addition to thyroid cancer, PV has been shown to play key roles in the development of pituitary tumors [91] and breast cancer [97], suggesting that the oncogenic actions of PV are not restricted to just the thyroid. Further identification of other target organs that are affected by the expression of PV could shed new light on the understanding of the somatic mutations in human cancers such as hepatocellular carcinomas [22] and renal carcinomas reported previously by others [24]. Addressing these challenges and others that may emerge subsequently will certainly lead to recognition and appreciation of the important roles of TR in cancer biology.
Highlights.
Mutations of thyroid hormone receptors (TR) are associated with human cancers
Loss of TR normal functions by deletion or mutations contribute to cancer development
Mice harboring a homozygous mutation of TRβ spontaneously develop thyroid cancer
Nuclear and extra-nuclear actions of a TRβ mutant mediate thyroid carcinogenesis
Mouse models of thyroid cancer allow uncovering novel molecular targets for treatment
Acknowledgments
We regret any reference omissions due to length limitation. We wish to thank all colleagues and collaborators who have contributed to the work described in this review. The research described in this review by the authors and their colleagues at National Cancer Institute was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- BM
basement membrane
- ECM
extracellular membrane
- EMT
epitherlial mesenchymal transition
- FAK
focal adhesion kinase
- MAPK
mitogenactivated protein kinase
- LOH
loss of heterozygosity
- NCOR1
nuclear receptor corepressor 1
- MMP
metalloprotease
- mTOR
mammalian target of rapamycin
- PAX8
paired box gene 8
- PIP2
phosphatidylinositol-4,5-biphosphate
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- PI3K
phosphatidylinositol 3-kinase
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
peroxisome proliferator responsive element
- PPTG
pituitary tumor-transforming gene
- PTEN
phosphatase and tensin homologue delated from chromosone 10
- RTH
resistance to thyroid hormone
- RXRα
retinoid X receptor α
- SH
Src homology
- SKY
spectral karyotyping analysis
- SMRT
silencing mediator of retinoid and thyroid hormone receptors
- SRC
steroid receptor coactivator
- TREs
thyroid hormone response elements
- TRs
thyroid hormone nuclear receptors
- T3
triiodothyronine
- TSH
thyroid stimulating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Published by Elsevier B.V.
References
- 1.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 2.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 3.Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 4.Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. Hormone selectivity in thyroid hormone receptors. Mol Endocrinol. 2001;15:398–410. doi: 10.1210/mend.15.3.0608. [DOI] [PubMed] [Google Scholar]
- 5.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bucan M, Ahima RS, Kaestner KH, Lazar MA. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456:997–1000. doi: 10.1038/nature07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci U S A. 2008;105:19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astapova I, Vella KR, Ramadoss P, Holtz KA, Rodwin BA, Liao XH, Weiss RE, Rosenberg MA, Rosenzweig A, Hollenberg AN. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25:212–224. doi: 10.1210/me.2010-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thormeyer D, Baniahmad A. The v-erbA oncogene (review) Int J Mol Med. 1999;4:351–358. [PubMed] [Google Scholar]
- 10.Yen PM, Ikeda M, Brubaker JH, Forgione M, Sugawara A, Chin WW. Roles of v-erbA homodimers and heterodimers in mediating dominant negative activity by v-erbA. J Biol Chem. 1994;269:903–909. [PubMed] [Google Scholar]
- 11.Chen HW, Privalsky ML. The erbA oncogene represses the actions of both retinoid X and retinoid A receptors but does so by distinct mechanisms. Mol Cell Biol. 1993;13:5970–5980. doi: 10.1128/mcb.13.10.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow C, Meister B, Lardelli M, Lendahl U, Vennstrom B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 1994;13:4241–4250. doi: 10.1002/j.1460-2075.1994.tb06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leduc F, Brauch H, Hajj C, Dobrovic A, Kaye F, Gazdar A, Harbour JW, Pettengill OS, Sorenson GD, van den Berg A, et al. Loss of heterozygosity in a gene coding for a thyroid hormone receptor in lung cancers. Am J Hum Genet. 1989;44:282–287. [PMC free article] [PubMed] [Google Scholar]
- 14.Sisley K, Curtis D, Rennie IG, Rees RC. Loss of heterozygosity of the thyroid hormone receptor B in posterior uveal melanoma. Melanoma Res. 1993;3:457–461. doi: 10.1097/00008390-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Chen LC, Matsumura K, Deng G, Kurisu W, Ljung BM, Lerman MI, Waldman FM, Smith HS. Deletion of two separate regions on chromosome 3p in breast cancers. Cancer Res. 1994;54:3021–3024. [PubMed] [Google Scholar]
- 16.Gonzalez-Sancho JM, Garcia V, Bonilla F, Munoz A. Thyroid hormone receptors/THR genes in human cancer. Cancer Lett. 2003;192:121–132. doi: 10.1016/s0304-3835(02)00614-6. [DOI] [PubMed] [Google Scholar]
- 17.Huber-Gieseke T, Pernin A, Huber O, Burger AG, Meier CA. Lack of loss of heterozygosity at the c-erbA beta locus in gastrointestinal tumors. Oncology. 1997;54:214–219. doi: 10.1159/000227691. [DOI] [PubMed] [Google Scholar]
- 18.Ali IU, Lidereau R, Callahan R. Presence of two members of c-erbA receptor gene family (c-erbA beta and c-erbA2) in smallest region of somatic homozygosity on chromosome 3p21–p25 in human breast carcinoma. J Natl Cancer Inst. 1989;81:1815–1820. doi: 10.1093/jnci/81.23.1815. [DOI] [PubMed] [Google Scholar]
- 19.Futreal PA, Soderkvist P, Marks JR, Iglehart JD, Cochran C, Barrett JC, Wiseman RW. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992;52:2624–2627. [PubMed] [Google Scholar]
- 20.Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283–287. [PubMed] [Google Scholar]
- 21.Dayton AI, Selden JR, Laws G, Dorney DJ, Finan J, Tripputi P, Emanuel BS, Rovera G, Nowell PC, Croce CM. A human c-erbA oncogene homologue is closely proximal to the chromosome 17 breakpoint in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1984;81:4495–4499. doi: 10.1073/pnas.81.14.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KH, Shieh HY, Chen SL, Hsu HC. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog. 1999;26:53–61. doi: 10.1002/(sici)1098-2744(199909)26:1<53::aid-mc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Puzianowska-Kuznicka M, Nauman A, Madej A, Tanski Z, Cheng S, Nauman J. Expression of thyroid hormone receptors is disturbed in human renal clear cell carcinoma. Cancer Lett. 2000;155:145–152. doi: 10.1016/s0304-3835(00)00416-x. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya Y, Puzianowska-Kuznicka M, McPhie P, Nauman J, Cheng SY, Nauman A. Expression of mutant thyroid hormone nuclear receptors is associated with human renal clear cell carcinoma. Carcinogenesis. 2002;23:25–33. doi: 10.1093/carcin/23.1.25. [DOI] [PubMed] [Google Scholar]
- 25.Silva JM, Dominguez G, Gonzalez-Sancho JM, Garcia JM, Silva J, Garcia-Andrade C, Navarro A, Munoz A, Bonilla F. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21:4307–4316. doi: 10.1038/sj.onc.1205534. [DOI] [PubMed] [Google Scholar]
- 26.Safer JD, Colan SD, Fraser LM, Wondisford FE. A pituitary tumor in a patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid. 2001;11:281–291. doi: 10.1089/105072501750159750. [DOI] [PubMed] [Google Scholar]
- 27.Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab. 2001;86:5572–5576. doi: 10.1210/jcem.86.11.7984. [DOI] [PubMed] [Google Scholar]
- 28.Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–1128. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- 29.Joseph B, Ji M, Liu D, Hou P, Xing M. Lack of mutations in the thyroid hormone receptor (TR) alpha and beta genes but frequent hypermethylation of the TRbeta gene in differentiated thyroid tumors. J Clin Endocrinol Metab. 2007;92:4766–4770. doi: 10.1210/jc.2007-0812. [DOI] [PubMed] [Google Scholar]
- 30.Ling Y, Xu X, Hao J, Ling X, Du X, Liu X, Zhao X. Aberrant methylation of the THRB gene in tissue and plasma of breast cancer patients. Cancer Genet Cytogenet. 2010;196:140–145. doi: 10.1016/j.cancergencyto.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki Y, Sunaga N, Tomizawa Y, Imai H, Iijima H, Yanagitani N, Horiguchi K, Yamada M, Mori M. Epigenetic inactivation of the thyroid hormone receptor beta1 gene at 3p24.2 in lung cancer. Ann Surg Oncol. 2010;17:2222–2228. doi: 10.1245/s10434-010-0956-9. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Meng ZH, Chandrasekaran R, Kuo WL, Collins CC, Gray JW, Dairkee SH. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002;62:1939–1943. [PubMed] [Google Scholar]
- 33.Jazdzewski K, Boguslawska J, Jendrzejewski J, Liyanarachchi S, Pachucki J, Wardyn KA, Nauman A, de la Chapelle A. Thyroid hormone receptor beta (THRB) is a major target gene for microRNAs deregulated in papillary thyroid carcinoma (PTC) J Clin Endocrinol Metab. 2011;96:E546–553. doi: 10.1210/jc.2010-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest. 1991;88:2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 37.Ying H, Suzuki H, Zhao L, Willingham MC, Meltzer P, Cheng SY. Mutant thyroid hormone receptor beta represses the expression and transcriptional activity of peroxisome proliferator-activated receptor gamma during thyroid carcinogenesis. Cancer Res. 2003;63:5274–5280. [PubMed] [Google Scholar]
- 38.Kato Y, Ying H, Willingham MC, Cheng SY. A tumor suppressor role for thyroid hormone beta receptor in a mouse model of thyroid carcinogenesis. Endocrinology. 2004;145:4430–4438. doi: 10.1210/en.2004-0612. [DOI] [PubMed] [Google Scholar]
- 39.Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- 40.Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrest D, Vennstrom B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52. doi: 10.1089/thy.2000.10.41. [DOI] [PubMed] [Google Scholar]
- 42.Zhu XG, Zhao L, Willingham MC, Cheng SY. Thyroid hormone receptors are tumor suppressors in a mouse model of metastatic follicular thyroid carcinoma. Oncogene. 2010;29:1909–1919. doi: 10.1038/onc.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC, Cheng SY. Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis. 2003;24:1467–1479. doi: 10.1093/carcin/bgg111. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SY. Multi-factorial regulation of in vivo action of TR beta mutants. Lessons learned from RTH mice with a targeted mutation in the TR beta gene. In: Mannavola SE, editor. Syndromes of Hormone Resistance in the Hypothalamic-Pituitary-Thyroid Axis. Kluwer; The Netherlands: 2004. pp. 137–148. [Google Scholar]
- 45.Cheng SY. New Developments in Thyroid Hormone Resistance. Hot Thyroidology. 2004 Janunary;(1) ( www.hotthyroidology.com)
- 46.Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected] Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 47.Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene. 2006;25:2736–2747. doi: 10.1038/sj.onc.1209299. [DOI] [PubMed] [Google Scholar]
- 48.Dobson ME, Diallo-Krou E, Grachtchouk V, Yu J, Colby LA, Wilkinson JE, Giordano TJ, Koenig RJ. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8-PPARgamma fusion protein thyroid carcinoma. Endocrinology. 2011;152:4455–4465. doi: 10.1210/en.2011-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–112. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- 52.Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281:20666–20672. doi: 10.1074/jbc.M512671200. [DOI] [PubMed] [Google Scholar]
- 53.Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL. Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:5197–5201. doi: 10.1073/pnas.0600089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verga Falzacappa C, Panacchia L, Bucci B, Stigliano A, Cavallo MG, Brunetti E, Toscano V, Misiti S. 3,5,3’-triiodothyronine (T3) is a survival factor for pancreatic beta-cells undergoing apoptosis. J Cell Physiol. 2006;206:309–321. doi: 10.1002/jcp.20460. [DOI] [PubMed] [Google Scholar]
- 55.Verga Falzacappa C, Petrucci E, Patriarca V, Michienzi S, Stigliano A, Brunetti E, Toscano V, Misiti S. Thyroid hormone receptor TRbeta1 mediates Akt activation by T3 in pancreatic beta cells. J Mol Endocrinol. 2007;38:221–233. doi: 10.1677/jme.1.02166. [DOI] [PubMed] [Google Scholar]
- 56.Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105–6111. [PubMed] [Google Scholar]
- 57.Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- 58.Mellor P, Furber LA, Nyarko JN, Anderson DH. Multiple roles for the p85alpha isoform in the regulation and function of PI3K signalling and receptor trafficking. Biochem J. 2012;441:23–37. doi: 10.1042/BJ20111164. [DOI] [PubMed] [Google Scholar]
- 59.Kim CS, Vasko VV, Kato Y, Kruhlak M, Saji M, Cheng SY, Ringel MD. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology. 2005;146:4456–4463. doi: 10.1210/en.2005-0172. [DOI] [PubMed] [Google Scholar]
- 60.Furuya F, Hanover JA, Cheng SY. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc Natl Acad Sci U S A. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furuya F, Guigon CJ, Zhao L, Lu C, Hanover JA, Cheng SY. Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol Cell Biol. 2007;27:6116–6126. doi: 10.1128/MCB.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuya F, Lu C, Willingham MC, Cheng SY. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis. 2007;28:2451–2458. doi: 10.1093/carcin/bgm174. [DOI] [PubMed] [Google Scholar]
- 63.Guigon CJ, Zhao L, Willingham MC, Cheng SY. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28:509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 66.Dahlman T, Grimelius L, Wallin G, Rubin K, Westermark K. Integrins in thyroid tissue: upregulation of alpha2beta1 in anaplastic thyroid carcinoma. Eur J Endocrinol. 1998;138:104–112. doi: 10.1530/eje.0.1380104. [DOI] [PubMed] [Google Scholar]
- 67.Ensinger C, Obrist P, Bacher-Stier C, Mikuz G, Moncayo R, Riccabona G. beta 1-Integrin expression in papillary thyroid carcinoma. Anticancer Res. 1998;18:33–40. [PubMed] [Google Scholar]
- 68.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol. 1996;3:100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 69.Kim SJ, Park JW, Yoon JS, Mok JO, Kim YJ, Park HK, Kim CH, Byun DW, Lee YJ, Jin SY, Suh KI, Yoo MH. Increased expression of focal adhesion kinase in thyroid cancer: immunohistochemical study. J Korean Med Sci. 2004;19:710–715. doi: 10.3346/jkms.2004.19.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michailidi C, Giaginis C, Stolakis V, Alexandrou P, Klijanienko J, Delladetsima I, Chatzizacharias N, Tsourouflis G, Theocharis S. Evaluation of FAK and Src expression in human benign and malignant thyroid lesions. Pathol Oncol Res. 2010;16:497–507. doi: 10.1007/s12253-010-9269-3. [DOI] [PubMed] [Google Scholar]
- 71.Lu C, Zhao L, Ying H, Willingham MC, Cheng SY. Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology. 2010;151:1929–1939. doi: 10.1210/en.2009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angers-Loustau A, Hering R, Werbowetski TE, Kaplan DR, Del Maestro RF. SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Mol Cancer Res. 2004;2:595–605. [PubMed] [Google Scholar]
- 73.Avizienyte E, Keppler M, Sandilands E, Brunton VG, Winder SJ, Ng T, Frame MC. An active Src kinase-beta-actin association is linked to actin dynamics at the periphery of colon cancer cells. Exp Cell Res. 2007;313:3175–3188. doi: 10.1016/j.yexcr.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 74.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 75.Kim WG, Guigon CJ, Fozzatti L, Park JW, Lu C, Willingham MC, Cheng SY. SKI-606, a Src inhibitor, reduces tumor growth, invasion, and distant metastasis in a mouse model of thyroid cancer. Clin Cancer Res. 2012 Jan 23; doi: 10.1158/1078-0432.CCR-11-2892. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu R, Melmed S. Oncogene activation in pituitary tumors. Brain Pathol. 2001;11:328–341. doi: 10.1111/j.1750-3639.2001.tb00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–441. doi: 10.1210/mend.11.4.9911. [DOI] [PubMed] [Google Scholar]
- 78.Yu R, Heaney AP, Lu W, Chen J, Melmed S. Pituitary tumor transforming gene causes aneuploidy and p53-dependent and p53-independent apoptosis. J Biol Chem. 2000;275:36502–36505. doi: 10.1074/jbc.C000546200. [DOI] [PubMed] [Google Scholar]
- 79.Yu R, Lu W, Chen J, McCabe CJ, Melmed S. Overexpressed pituitary tumortransforming gene causes aneuploidy in live human cells. Endocrinology. 2003;144:4991–4998. doi: 10.1210/en.2003-0305. [DOI] [PubMed] [Google Scholar]
- 80.Kim D, Pemberton H, Stratford AL, Buelaert K, Watkinson JC, Lopes V, Franklyn JA, McCabe CJ. Pituitary tumour transforming gene (PTTG) induces genetic instability in thyroid cells. Oncogene. 2005;24:4861–4866. doi: 10.1038/sj.onc.1208659. [DOI] [PubMed] [Google Scholar]
- 81.Heaney AP, Nelson V, Fernando M, Horwitz G. Transforming events in thyroid tumorigenesis and their association with follicular lesions. J Clin Endocrinol Metab. 2001;86:5025–5032. doi: 10.1210/jcem.86.10.7886. [DOI] [PubMed] [Google Scholar]
- 82.Kim DS, McCabe CJ, Buchanan MA, Watkinson JC. Oncogenes in thyroid cancer. Clin Otolaryngol Allied Sci. 2003;28:386–395. doi: 10.1046/j.1365-2273.2003.00732.x. [DOI] [PubMed] [Google Scholar]
- 83.Zimonjic DB, Kato Y, Ying H, Popescu NC, Cheng SY. Chromosomal aberrations in cell lines derived from thyroid tumors spontaneously developed in TRbetaPV/PV mice. Cancer Genet Cytogenet. 2005;161:104–109. doi: 10.1016/j.cancergencyto.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng SY. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J Clin Invest. 2006;116:2972–2984. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, Cheng S. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci U S A. 2000;97:8985–8990. doi: 10.1073/pnas.160257997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Polakis P. Casein kinase 1: a Wnt’er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 89.Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 90.Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28:4598–4608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25:124–135. doi: 10.1128/MCB.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu C, Mishra A, Zhu YJ, Meltzer P, Cheng SY. Global expression profiling reveals gain-of-function oncogenic activity of a mutated thyroid hormone receptor in thyroid carcinogenesis. Am J Cancer Res. 2011;1:168–191. [PMC free article] [PubMed] [Google Scholar]
- 93.Guigon CJ, Cheng SY. Novel oncogenic actions of TRbeta mutants in tumorigenesis. IUBMB Life. 2009;61:528–536. doi: 10.1002/iub.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guigon CJ, Cheng SY. Novel non-genomic signaling of thyroid hormone receptors in thyroid carcinogenesis. Mol Cell Endocrinol. 2009;308:63–69. doi: 10.1016/j.mce.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu C, Cheng SY. Extranuclear signaling of mutated thyroid hormone receptors in promoting metastatic spread in thyroid carcinogenesis. Steroids. 2011;76:885–891. doi: 10.1016/j.steroids.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim CS, Ying H, Willingham MC, Cheng SY. The pituitary tumor-transforming gene promotes angiogenesis in a mouse model of follicular thyroid cancer. Carcinogenesis. 2007;28:932–939. doi: 10.1093/carcin/bgl231. [DOI] [PubMed] [Google Scholar]
- 97.Guigon CJ, Kim DW, Willingham MC, Cheng SY. Mutation of thyroid hormone receptor-beta in mice predisposes to the development of mammary tumors. Oncogene. 2011;30:3381–3390. doi: 10.1038/onc.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]