Abstract
The laminin-binding integrin α6β4 plays key roles in both normal epithelial and endothelial cells and during tumor cell progression, metastasis, and angiogenesis. Previous cysteine mutagenesis studies have suggested that palmitoylation of α6β4 protein supports a few integrin-dependent functions and molecular associations. Here we took another approach and obtained strikingly different results. We used overexpression and RNAi knockdown in multiple cell types to identify protein acyl transferase DHHC3 as the enzyme responsible for integrin β4 and α6 palmitoylation. Ablation of DHHC3 markedly diminished integrin-dependent cellular cable formation on Matrigel, integrin signaling through Src, and β4 phosphorylation on key diagnostic amino acids (S1356 and 1424). However, unexpectedly, and in sharp contrast to prior α6β4 mutagenesis results, knockdown of DHHC3 accelerated the degradation of α6β4, likely due to an increase in endosomal exposure to cathepsin D. When proteolytic degradation was inhibited (by Pepstatin A), rescued α6β4 accumulated intracellularly, but was unable to reach the cell surface. DHHC3 ablation effects were strongly selective for α6β4. Cell-surface levels of ~10 other proteins (including α3β1) were not diminished, and the appearance of hundreds of other palmitoylated proteins was not altered. Results obtained here demonstrate a new substrate for the DHHC3 enzyme and provide novel opportunities for modulating α6β4 expression, distribution, and function.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-0924-6) contains supplementary material, which is available to authorized users.
Keywords: Integrin, α6β4, DHHC3, Palmitoylation
Introduction
Integrins α6β4 and α6β1 mediate cell adhesion to various isoforms of laminin [1]. The α6β4 integrin, widely expressed in many epithelial cells, is a major component of hemidesmosomes [2] and plays a key role during skin development [3, 4]. The α6β1 integrin is present on platelets [5] and on multiple types of leukocytes, where it can contribute to immune functions [6, 7]. Integrins α6β1 and α6β4 also may play central roles in human papilloma virus binding to cells [8]. In cancer, both integrins contribute to tumor growth, invasion, metastasis, angiogenesis, epithelial–mesenchymal-transition (EMT), and drug resistance [9–12], and α6 is frequently used as a marker to help define stem cell/tumor initiating cell subpopulations [13, 14]. Consequently, these integrins have emerged as potential cancer drug targets.
The α6β4 and α6β1 integrins (together with laminin-binding integrins α3β1 and α7β1) share multiple features that distinguish them from all other integrins. First, they associate most closely with tetraspanin proteins, especially CD151 [15, 16], leading to residence within tetraspanin-enriched microdomains (TEMs) [17]. Second, they undergo post-translational palmitoylation at membrane-proximal cysteine sites [18, 19]. The β4 subunit contains seven potential sites, and there is one site each in the α6, α3, and α7 subunits. Mutation of β4 membrane-proximal cysteines prevented palmitoylation, but did not diminish α6β4 cell-surface expression [18, 19]. While on the cell surface, palmitoylation-deficient α6β4 mutants showed reduced signaling through p130Cas and Src family kinases, accompanied by diminished cell proliferation and spreading [18, 19]. These defects are likely linked to diminished physical and functional association of α6β4 mutants with tetraspanin proteins [18].
Mutagenesis is useful for study of protein palmitoylation, but is less than ideal for two reasons. First, removal of membrane-proximal cysteines could affect protein structure and function in a manner that goes beyond simply losing palmitoylation sites. Second, it is technically challenging to replace endogenous proteins with palmitoylation-deficient mutants in various cell types. Consequently, palmitoylation-deficient mutants have often been over-expressed in unnatural cellular environments, yielding potentially misleading results. As an alternative, we sought to identify and eliminate the key protein acyl transferase (PAT) responsible for integrin palmitoylation.
Protein acyl transferases within the DHHC family are responsible for many mammalian palmitoylation events. This family has 23 members, distributed among the Golgi, ER, and the plasma membrane [20–22]. They are polytypic membrane proteins, each with 4–6 transmembrane domains. There is a 50-amino-acid cysteine-rich domain (CRD) between transmembrane domains 2 and 3, on the cytosolic side of the molecule. This CRD domain (sometimes called the DHHC–CRD domain) contains a conserved Asp-His-His-Cys (DHHC) motif critical for catalytic activity [20]. For transmembrane proteins, palmitoylation of membrane-proximal cysteines can (a) regulate protein–protein interactions, e.g., for AMPA [23] and CD151 [24], (b) affect lipid bilayer positioning, e.g., for LRP6 [25], (c) regulate protein trafficking, e.g., for transferrin receptor [26], and (d) affect protein stability, e.g., for CD9, CD151, and CCR5 [24, 27]. Although there are many transmembrane proteins that undergo palmitoylation [28], in most cases the specific DHHC proteins involved have not been identified.
Tetraspanin proteins are palmitoylated [29], and palmitoylation-deficient mutants show altered functions and reduced lateral associations with other proteins, including integrins [30–32]. DHHC2 was identified as the PAT most important for palmitoylation of tetraspanin proteins CD9 and CD151 [24]. Consistent with mutagenesis results, DHHC2 ablation also caused reduced tetraspanin associations with other proteins [24]. However, despite close proximity of α6β4 and α6β1 integrins to tetraspanins, integrin palmitoylation was not dependent on DHHC2 [24]. Here we identify DHHC3 as the DHHC family member most responsible for α6β4 and α6β1 palmitoylation. Previously DHHC3/GODZ was shown to affect palmitoylation of the γ2 subunit of GABA (A) receptors [23, 33] and to contribute (along with DHHC7) to palmitoylation of Gα proteins [34] and RGS4 [35]. Our study is now the first demonstration of DHHC3 affecting palmitoylation of widely expressed type I transmembrane proteins, such as the α6 and β4 integrin subunits.
Ablation of DHHC3 markedly diminished α6β4 and α6β1 palmitoylation and function, while not affecting palmitoylation of integrin α3β1 or a large number of other proteins. However, the consequence of reduced integrin palmitoylation due to DHHC3 ablation contrasts sharply with results obtained by β4 cysteine mutagenesis. Whereas β4 palmitoylation-deficient mutants were stable and readily expressed at the cell surface [18, 19], unpalmitoylated β4 in DHHC3-ablated cells shows enhanced susceptibility to proteolytic degradation and markedly reduced cell-surface expression. These results provide unexpected new insights into integrin palmitoylation, and provide a novel way to modulate α6β4 and α6β1 integrin functions, without directly targeting the α6 or β4 subunits themselves.
Materials and methods
Cells, antibodies, and reagents
Human Embryonic Kidney HEK 293 cells and MDA-MB-231 cells were obtained from ATCC and cultured in DMEM media with 10% FBS and antibiotics. Prostate cancer (PC3) cells were cultured in RPMI media. Monoclonal (mAb, MAB1964) and polyclonal (pAb, AB1922) antibodies to β4 integrin were from Chemicon. Polyclonal antibody to β4 S1424 [36] and S1356 [37] were described earlier. Other antibodies were anti-α6 integrin mAb GoH3 (BD Biosciences) and pAb H-87 (Santa Cruz), anti-DHHC3 pAb ab31387 (Abcam), anti-M2 Flag mAb (Sigma), anti-CD9 mAb MM2/57 (Millipore), anti-CD71 mAb 3B8 2A1 (Santa Cruz), anti-Src (Santa Cruz), anti-p-Src pAb (Cell Signaling). Antibodies to α3 (mAb A3X8), β1 (TS2/16), CD151 (5C11), and EWI-F (MX-1) have been described previously [18]. Pepstatin A (used at 100 μM), Leupeptin (100 μM), Bafilomycin A (150 nM) were obtained from Sigma, and E64 (100 μM) was from Calbiochem. [3H]-labeled palmitic acid was purchased from Perkin-Elmer.
Stable and transient transfection
DHHC3 stable knockdown was performed by infecting target cells with lentivirus expressing specific short-hairpin RNA (shRNA) cloned in pLKO.1, puromycin vector. The DHHC3-specific shRNA target sequence, 5′-CCGGGTATAGCATCATCAACGGAATCTCGAGATTCCGTTGATGATGCTATACTTTTTTG-3′ was from Sigma. For control knockdown, shRNA sequence from Open Biosystems (RHS4430-99138010) cloned in lentiviral vector was used for cell infection. For siRNA knockdown of DHHC3, a specific siRNA sequence (sense strand 5′-CGUUCUCAUGAAUGUUUAATT-3′) and control siRNA (Qiagen catalog no. 1027281) were used.
Metabolic labeling
For [3H]palmitate labeling, cells were starved for 1 h and then pulsed with [3H]palmitic acid (0.2 mCi/ml) in culture media with 5% dialyzed serum for 2 h. After labeling, cells were lysed in buffer (25 mM HEPES, 150 mM NaCl, 5 mM MgCl2, protease inhibitor PMSF) containing 1% NP40 or Brij96 detergent. After lysis, specific proteins were immunoprecipitated, separated, and detected for [3H] signal and by protein immunoblotting as described earlier [24]. Densitometry quantitation of protein after blotting was performed using Image Quant, version 5.2 software (GE Healthcare).
Immunofluorescence and flow cytometry
Control or DHHC3 stable knockdown cells were fixed with 4% paraformaldehyde and in some experiments were also permeabilized using 0.1% Triton X-100. After incubation for 1 h in 5% horse serum, fixed cells were incubated with specific antibodies overnight at 4°C. After washing with PBS, cells were incubated with respective secondary antibodies conjugated to fluorophores (Alexa Fluor 488 or Alexa Fluor 594) for 1 h at RT. Finally, after washing five times with PBS, slides were mounted with Prolong Gold antifade mounting media with DAPI (Invitrogen). Pictures were taken using a Leica SP5X laser scanning confocal microscope (Leica Microsystems, Chicago, IL) with a 63× magnification. Quantitation of staining intensity of individual cells was done using Scion Image software (Scion Corp., Frederick, MD). Flow cytometry was performed using a FACS Calibur (Becton–Dickinson, Bedford, MA) as previously described [24].
Cell signaling and cable formation
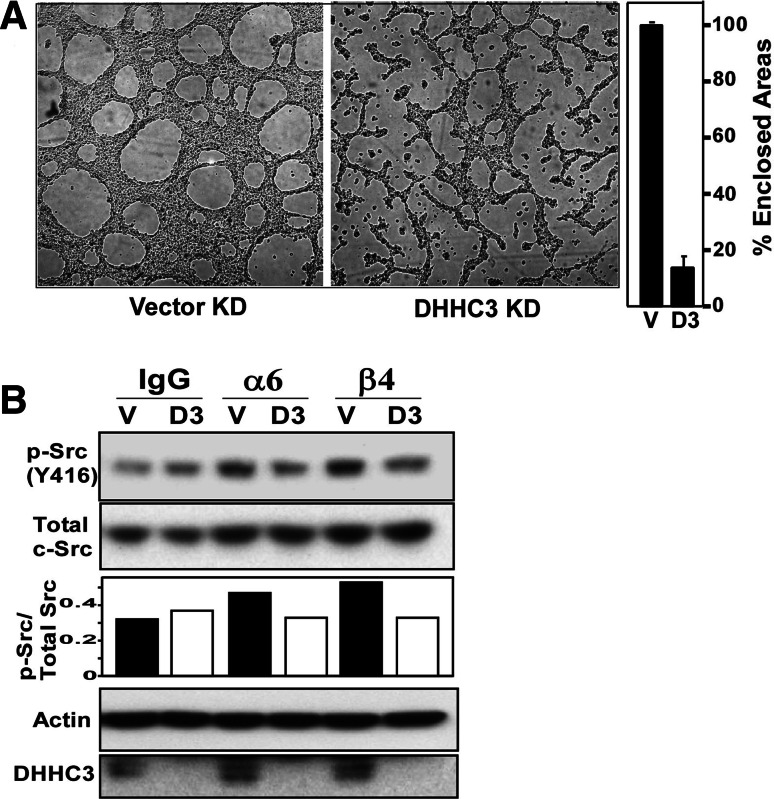
For integrin-triggered signaling, cells were starved overnight and suspended in culture media with no FBS. Equal numbers of cells from control or DHHC3 knockdown samples were incubated with IgG, α6 and β4 antibodies for 1 h at 4°C with shaking. After incubation, cells were plated on dishes precoated with anti-mouse or anti-rat IgG for 30 min at 37°C. Finally, cells were lysed and proteins were purified and separated on SDS-PAGE gel and then blotted for p-Src and total Src. For cable formation, cells were suspended in a media containing 5% FBS. Matrigel (300 μl) was added to a 24-well plate and allowed to solidify. Equal numbers of cells were plated on top of the Matrigel in triplicate for each condition. Plates were incubated at 37°C and photographed after 24 h [at 2× magnification, using monochrome charge-coupled device camera (RT SPOT, Diagnostic Instruments) on an Axiovert 135 inverted microscope (Zeiss Co.)]. For quantitation, numbers of closed areas were counted from different fields in each well for each condition. The data was compiled and plotted from three independent experiments each with three replicates.
Results
Screening of DHHC proteins for effects on integrin palmitoylation
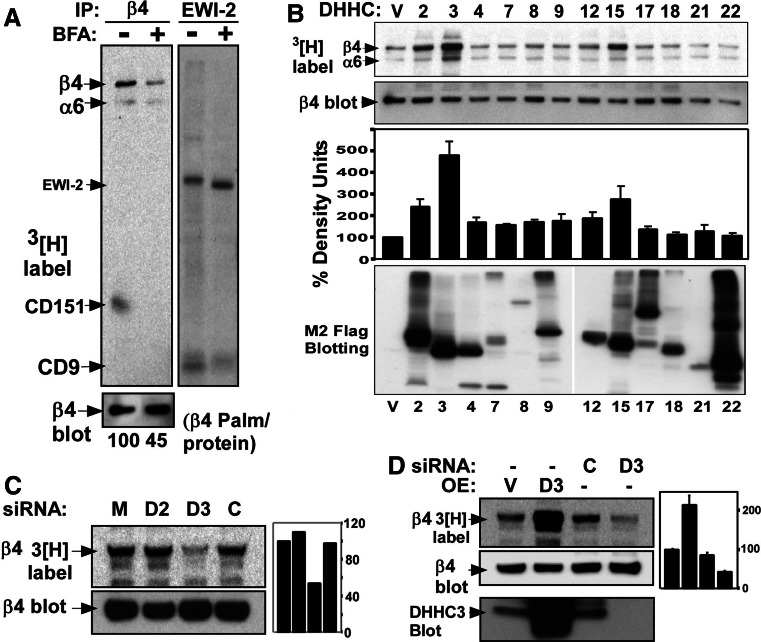
DHHC-mediated protein palmitoylation can occur in the Golgi, in the endoplasmic reticulum, and at the plasma membrane [22]. The Golgi-disrupting agent brefeldin A inhibited palmitoylation of the integrin β4 subunit (by 55%) as seen upon immunoprecipitation of endogenous β4 from [3H]palmitate-labeled MDA-MB-231 cells (Fig. 1a, lanes 1, 2). In control experiments, palmitoylation of tetraspanin CD9, which is known to occur in the Golgi [24], was substantially inhibited, whereas palmitoylation of CD9 partner protein EWI-2 was relatively unaffected by brefeldin A (Fig. 1a, lanes 3, 4). Hence, we focused attention on the 12 DHHC proteins known to at least partially reside in the Golgi [22, 38]. Among these, overexpression of DHHC3, and to a lesser extent DHHC15 and DHHC2, yielded elevated β4 palmitoylation in HEK293 cells (Fig. 1b, top panel). Also elevated is palmitoylation/protein (third panel), normalized for β4 protein expression (second panel). Palmitoylation of the integrin α6 subunit also appeared to be elevated in parallel with β4 (top panel). The bottom panel in Fig. 1b establishes the presence of each overexpressed DHHC protein in HEK293 cells.
Fig. 1.
Determination of DHHC proteins responsible for β4 integrin palmitoylation. a MDA-MB-231 cells were treated with 10 mg/ml of brefeldin A (Golgi inhibitor), 2 h prior to metabolic [3H]palmitate labeling. After cell lysis in 1% Brij96 buffer, β4 integrin and EWI2 proteins were immunoprecipitated. Proteins were visualized by [3H]palmitate labeling (top panels) and by blotting (bottom panel). Numbers at the bottom represent the normalized ratio of β4 integrin palmitoylation/protein. b HEK293 cells were transiently co-transfected with cDNA coding for indicated DHHC proteins and for β4 integrin. After 36-h post-transfection, cells were metabolically labeled with [3H]palmitate for 2 h, lysed in 1% NP40 buffer, and β4 integrin was immunoprecipitated. Shown are [3H]-labeled β4 proteins (top panel), total β4 protein (second panel), normalized β4 integrin palmitoylation in density units (third panel), and expression levels for different DHHC proteins blotted using anti-M2 Flag Ab (bottom panel). c HEK 293 cells stably expressing integrin β4 subunit were transfected with mock, DHHC2 siRNA, DHHC3 siRNA and control siRNA. After 3 days, cells were metabolically labeled with [3H]palmitate, lysed in 1% NP40 buffer, and then β4 integrin was immunoprecipitated, and proteins were visualized by radioactivity (top panel) and blotting (bottom panel). Bar graphs represent normalized β4 integrin palmitoylation/protein. d HEK293 cells overexpressing (OE) vector or DHHC3 cDNA; or treated with control siRNA or DHHC3 siRNA were processed as in part c. Bottom panel depicts DHHC3 expression
In a follow-up experiment, siRNA knockdown of DHHC3 (but not DHHC2) caused a reduction in β4 palmitoylation (by ~47%) in HEK293 cells (Fig. 1c). This result is consistent with previous results [24] showing that DHHC2 does not affect β4 integrin palmitoylation. In a side-by-side comparison, β4 palmitoylation was increased (over twofold) upon overexpression of DHHC3, and decreased (by ~70%) upon knockdown of endogenous DHHC3 in HEK293 cells (Fig. 1d). DHHC15 was not considered further because it is expressed minimally or not at all in many cells in which β4 is highly palmitoylated. Knockdown of other Golgi-resident DHHC’s did not decrease β4 palmitoylation.
DHHC3 shows selectively for integrin α6 and β4 subunits
Stable ablation of DHHC3 in MDA-MB-231 cells did not affect palmitoylation of other prominent cell-surface proteins including tetraspanins CD9 and CD151 and the transferrin receptor (CD71) (Fig. 2; Supplemental Fig. S1). Furthermore, analysis of [3H]palmitate-labeled whole-cell lysates from PC3 and MDA-MB-231 cells revealed no detectable effects of DHHC3 ablation on hundreds of unidentified [3H]palmitate-labeled proteins (Fig. 2, right panel; Fig. 4a, right panel; Fig. 5c; Supplemental Fig. S1, right panel; and Supplemental Fig. S2, right panel).
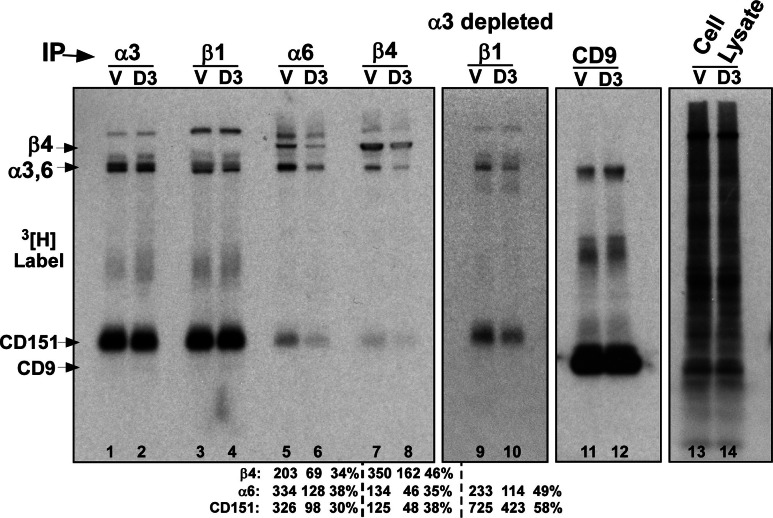
Fig. 2.
DHHC3 palmitoylation specificity among integrin subunits. PC3 cells with vector (V) and DHHC3 (D3) stable knockdown were labeled with [3H]palmitate for 2 h. Then α3, β1, α6, β4 integrin and CD9 proteins were immunoprecipitated and visualized by [3H]palmitate labeling (lanes 1–8, 11,12). In the second panel (lanes 9, 10), β1 immunoprecipitation was done after depletion of α3 from the lysates, leaving α6β1. The far right panel shows unidentified [3H]palmitate labeled proteins in the whole-cell lysate
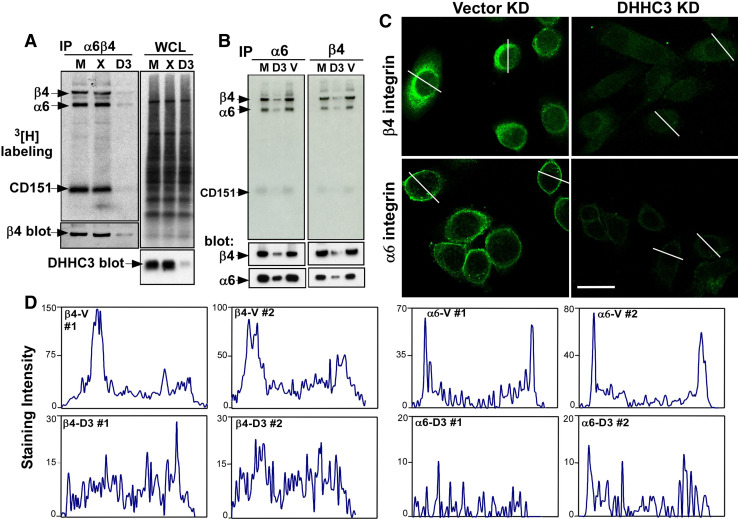
Fig. 4.
DHHC3 knockdown affects endogenous β4 and α6 palmitoylation and expression. a MDA-MB-231 cells and b PC3 cells, each with mock, control and DHHC3 stable knockdown, were labeled with [3H]palmitate for 2 h. Cells were lysed in 1% Brij96 buffer and then endogenous β4 and α6 integrins were immunoprecipitated and detected from the [3H] radioactive signal (top panel) and by protein immunoblotting. In part a, the top right panel shows many unidentified palmitoylated proteins in the whole cell lysate, and the bottom right panel shows DHHC3 protein expression. c Vector and DHHC3 knockdown PC3 cells were permeabilized and stained with β4 and α6 antibodies, and visualized by confocal microscopy. V Vector control knockdown; D3 DHHC3 knockdown. Bar,20 μm. d Staining intensity (units from ImageJ program) is quantitated for two representative cells from each panel in part c
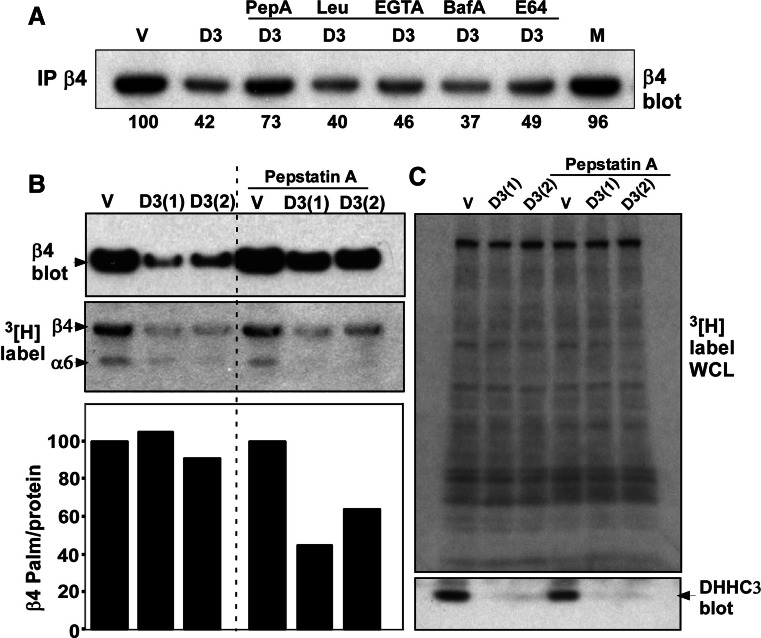
Fig. 5.
Pepstatin A restores β4 integrin expression after DHHC3 ablation. a PC3 cells with vector control (V) or DHHC3 stable knockdown (D3) were treated with the indicated protease inhibitors or mock-treated (M) for 24 h. After cell lysis, β4 integrin expression was detected by blotting. Numbers represent % of β4 integrin expression relative to vector knockdown cells. b PC3 cells with vector and DHHC3 stable knockdown (2 independent samples) were treated with or without Pepstatin A for 24 h before labeling with [3H]palmitate for 2 h. β4 integrin was immunoprecipitated and then expression of β4 (top panel) was detected by blotting, and by [3H] labeling (second panel). Normalized β4 palmitoylation/protein was determined (bottom panel). c Shown are many unidentified [3H]palmitate-labeled proteins from PC3 whole cell lysate (top panel) and immunoblotting for DHHC3 protein (bottom panel)
Among commonly expressed laminin-binding integrin subunits (α3, α6, β4), DHHC3 showed surprising selectivity for α6 and β4 but not α3. DHHC3 ablation did not affect appearance of either palmitoylated α3 or abundantly associated CD151, immunoprecipitated using either anti-α3 antibody (Fig. 2, lanes 1, 2), or anti-β1 antibody (lanes 3, 4). The abundance of α3 likely obscures possible changes in α6 levels in lanes 3 and 4. However, direct immunoprecipitation of α6 shows that DHHC3 ablation diminishes appearance of palmitoylated β4, α6, and associated CD151, each by 62–70% (Fig. 2, lanes 5, 6). Likewise, immunoprecipitation of β4 revealed decreased recovery (by 54–65%) of palmitoylated β4, α6, and CD151 (Fig. 2, lanes 7, 8). Lane 5 contains 2.5-fold more α6 and 42% less labeled β4 compared to lane 7. This indicates that (a) substantial α6β1 was present, and (b) there is decreased recovery of both α6β1 and α6β4 in lane 6. To analyze α6β1 in the absence of α3, α3β1 was immunodepleted from PC3 cell lysate, and then α6β1 was immunoprecipitated using anti-β1 antibody. Once again, recoveries of palmitoylated α6 and associated CD151 were substantially reduced (by 42–51%) due to DHHC3 ablation (Fig. 2, lanes 9, 10). Note that CD151 is not a substrate for DHHC3 [24] (Supplemental Fig. S2). An explanation for decreased CD151 palmitoylation is provided below (see Fig. 4).
Effects of DHHC3 ablation on α6β4-dependent functions
Having established that DHHC3 affects integrin α6β4 palmitoylation, we next looked at effects of DHHC3 ablation on functions involving α6 and/or β4. First, we analyzed Matrigel cable formation, which is a model for α6 integrin-dependent extracellular matrix remodeling [39]. DHHC3-ablated PC3 prostate tumor cells and control knockdown cells differed significantly in ability to form cables on Matrigel (Fig. 3a). Second, we looked for differences in antibody-triggered integrin signaling in PC3 cells. Activation of p-Src (Y416) was increased by 47% upon α6 stimulation and 72% upon β4 stimulation, compared to control IgG stimulation in vector control cells. On the other hand, DHHC3 KD cells showed an 8% decrease in p-Src (Y416) activation after α6 and β4 stimulation as compared to control IgG stimulation.
Fig. 3.
DHHC3 affects integrin-dependent cable formation and signaling in PC3 cells. a PC3 cells with vector (vector KD) and DHHC3 (DHHC3 KD) knockdown were plated on polymerized Matrigel in complete growth medium in 24-well plates. After 24 h, photographs were taken and mean % cable formation was quantitated (n = 3). b PC3 cells with vector knockdown (V) and DHHC3 knockdown (D3) were incubated with control mouse IgG, with rat anti-α6 mAb, or with mouse anti-β4 mAb for 1 h at 4°C. Cells coated with primary antibody were then plated in wells coated with goat-anti-mouse or goat-anti-rat IgG, and incubated for 30 min at 37°C, to trigger integrin signaling. After cell lysis, proteins were separated and blotted for the indicated signaling molecules. Density units in the bar graph represent p-Src(416)/Total c-Src (from top two panels). The bottom two panels show an actin loading control and confirm DHHC3 knockdown
DHHC3 effects on endogenous integrin palmitoylation, distribution, and protein expression
To understand further the basis for the functional differences observed in Fig. 3, we analyzed stable DHHC3 knockdown effects on endogenous α6β4 palmitoylation, expression, and localization in representative prostate (PC3) and breast (MDA-MB-231) carcinoma cell lines. Stable ablation of DHHC3 not only reduced recovery of [3H]-labeled β4 and α6 in these cells (Fig. 4a, b) but also caused diminished expression of both β4 and α6 proteins as seen by immunoblotting (Fig. 4a, b, bottom panels). Diminished expression of β4 and α6 was confirmed by immunofluorescence staining of PC3 cells (Fig. 4c) and by cell-surface flow cytometry (Supplemental Fig. S3). Cell-surface expression of nine other proteins was not diminished (Supplemental Fig. S3). Targeting of DHHC3 (by siRNA) at a different nucleotide region confirmed that ablation of endogenous DHHC3 causes reductions in both [3H] palmitoylation and expression of α6β4, in both MDA-MB-231 and PC3 cells (Supplemental Fig. S2, left panels). Reduced α6 and β4 protein expression explains why there is also a decrease in appearance of integrin-associated, [3H]-labeled CD151 (Figs. 2, 4, S2), even though CD151 is not a substrate for DHHC3. Expression of β4 and α6 was not only diminished upon DHHC3 ablation but also became more evenly distributed throughout the area of permeabilized cells (Fig. 4c, d). These results are consistent with loss of cell-surface expression.
Rescue of protein expression after DHHC3 ablation
Results in Fig. 4 and Fig. S2 suggest that DHHC3 ablation causes loss of palmitoylation, which leads to protein destabilization. To test this hypothesis, it was necessary to inhibit, at least partially, the loss of protein expression. Among inhibitors tested (Leupeptin, E64, bafilomycin A, Pepstatin A, ALLM, lactacystin), the one best able to rescue β4 integrin protein expression in DHHC3-ablated PC3 tumor cells (from 37–42% back up to 73%; Fig. 5a) was Pepstatin A (a cathepsin D protease inhibitor). With β4 protein expression partially stabilized (~twofold increased in the presence of Pepstatin A), the palmitoylation/protein ratio for endogenous β4 integrin was reduced by ~50% in DHHC3-ablated cells as compared to control knockdown cells (Fig. 5b). In the whole cell lysate (Fig. 5c), palmitoylation was unchanged for many unidentified [3H]-labeled proteins. The bottom panel in Fig. 5c confirms the loss of DHHC3 expression in two replicate samples.
Unpalmitoylated and palmitoylated β4 differ with respect to cell-surface expression
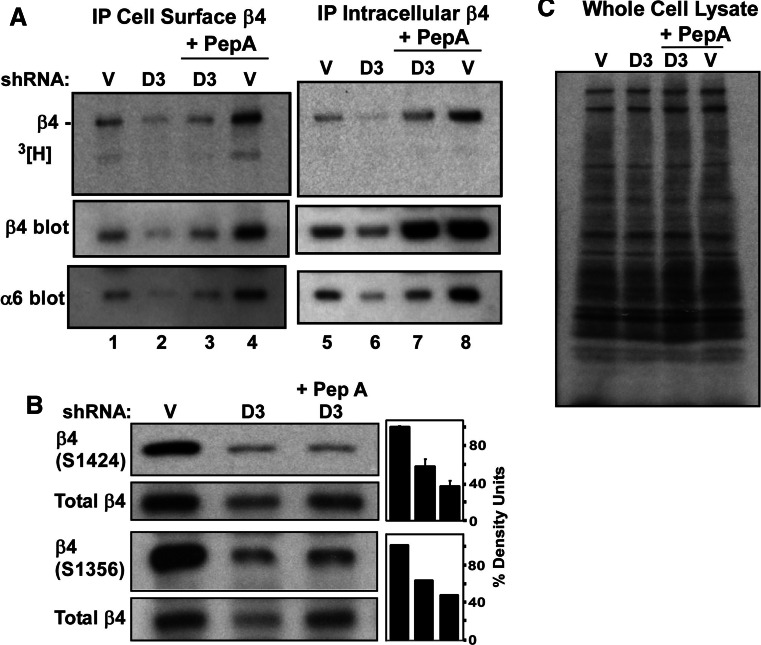
Ablation of DHHC3 in PC3 cells caused intracellular β4 expression to decrease by ~50% (Fig. 6a, lanes 5, 6). However, intracellular β4 expression was largely retained (only 15% decrease) if Pepstatin A was present (lanes 8, 7). By contrast, DHHC3 ablation caused a 64% loss of cell surface β4 protein (lanes 1, 2), which was not prevented by Pepstatin A (67% loss; lanes 4, 3). Densitometry numbers used to determine % changes in Fig. 6a are shown in Supplemental Table 1. Hence, blocking of cathepsin D may protect or rescue unpalmitoylated β4 protein intracellularly, but it does not appear to get to the cell surface. Consistent with the stabilization of intracellular unpalmitoylated β4, the palmitoylation/protein ratio decreased markedly (by 41%) when Pepstatin A was present during DHHC3 ablation. By contrast, DHHC3 ablation caused the palmitoylation/protein ratio to decrease by only 12.9% on the cell surface when Pepstatin was present (Supplemental Table 1). Hence, intracellular β4 is relatively unpalmitoylated, whereas the cell surface is enriched for the small amount of remaining palmitoylated β4. When Pepstatin A was absent, DHHC3 ablation had less effect on the β4 palmitoylation/protein ratio (decreased by 3% on the cell surface; 14.5% intracellularly; Supplemental Table 1).
Fig. 6.
Unpalmitoylated β4 fails to reach the cell surface. a PC3 cells expressing control vector or DHHC3 knockdown vector were treated with or without Pepstatin A (for 24 h) before labeling with [3H]palmitate for 2 h. Intact cells were then incubated with anti-β4 mAb, washed, and lysed. Cell-surface β4 complexes were then captured using protein G beads, and β4 was detected by [3H] labeling (top panel) and also β4 (middle panel), and α6 (bottom panel) were detected by immunoblotting (lanes 1–4). After removal of cell-surface proteins, the remaining lysate (containing intracellular β4) was immunoprecipitated using anti-β4 mAb, and intracellular proteins were visualized (lanes 4–8). b PC3 cells, with or without Pepstatin A treatment, with control or DHHC3 stable knockdown vectors, were lysed in 1% Triton X-100 buffer. Samples were then blotted for β4 phosphorylated at S1424 and S1356 and the same membranes were blotted for total β4 (2nd and 4th panels) after stripping. Density units in the bar graph represent phospho β4 (S1424)/Total β4 and β4 (S1356)/Total β4. c [3H]palmitate-labeled proteins in PC3 whole cell lysates are visualized
An immunofluorescence staining experiment confirmed that DHHC3 ablation causes β4 disappearance, as seen either by staining of total β4 (in permeabilized cells, Supplemental Fig. S4c) or cell surface β4 (unpermeabilized cells, Fig. S4d). After Pepstatin A treatment of PC3 cells, total β4 no longer was lost due to DHHC3 ablation (Supplemental Fig. S4g), whereas cell-surface β4 expression still was substantially decreased (Supplemental Fig. S4h). These results again suggest that palmitoylation-deficient β4 can be rescued intracellularly (with Pepstatin A), but then does not reach the cell surface. To further support this notion, we analyzed phosphorylation of β4 integrin, which occurs proximal to the cell surface, at sites (S1424 and 1356) linked to hemidesmosome turnover [36, 37, 40]. As seen in Fig. 6c, there is a loss of S1424 and 1356 phosphorylation in DHHC3 ablated samples and this loss (measured as phosphorylation/protein) is even more obvious in the presence of Pepstatin A (when intracellular β4 integrin expression is partially rescued).
Upon DHHC3 ablation, the amount of intracellular α6 protein decreased by 59% in untreated cells (Fig. 6a, bottom panel, lanes 5, 6) and by 52% in Pepstatin A-treated cells (lanes 7, 8). Hence, Pepstatin A fails to rescue α6 from intracellular degradation. DHHC3 ablation caused cell surface α6 to decrease by 71–73% (Fig. 6a, bottom panel, lanes 1–4), comparable to the decrease seen for β4 protein (64–67%, lanes 1–4; and also see Supplemental Table 1). DHHC3 ablation, with or without Pepstatin A treatment, had little or no detectable effect on the palmitoylation of numerous unidentified proteins in PC3 whole cell lysates (Fig. 6c).
Discussion
DHHC3 palmitoylates α6β4 integrin
After narrowing the focus to 12 Golgi-resident DHHC enzymes, we utilized over-expression studies, shRNA knockdown, siRNA knockdown, and three different cellular environments to demonstrate that DHHC3 is the major PAT responsible for palmitoylation of α6β4 integrin. Although other DHHCs, such as DHHC2, partially stimulated β4 palmitoylation when overexpressed, knockdown of DHHC2 and other Golgi-resident DHHCs (other than DHHC3) did not diminish palmitoylation. We showed previously that DHHC2 palmitoylates tetraspanin proteins, but not α6β4 integrin [24]. Here we find that DHHC3 palmitoylates α6β4, but not tetraspanins (e.g., CD9, CD151). DHHC3 knockdown also failed to affect the palmitoylation of CD71 (transferrin receptor) and numerous unidentified proteins present in whole cell lysates in multiple experiments. These results are consistent with DHHC3 showing considerable substrate selectivity, while acting directly on α6β4.
DHHC3 may have several other substrates. Knockdown of DHHC3 decreased palmitoylation of multiple heterotrimeric G protein alpha subunits [34], and co-expression of DHHC3 promoted palmitoylation of regulator of G-protein signaling 4 (RGS4) protein [35], the γ2 subunit of GABA (A) receptors [33], cysteine-string protein (CSP)[41], and nearly a dozen other proteins [42]. However, in the majority of cases, co-expression results have not been confirmed by DHHC3 knockdown/ablation studies.
Although the amino acid sequence proximal to palmitoylated cysteines may play a critical role (e.g., [23]), other domains and amino acids, distant from palmitoylated cysteines, likely also contribute to DHHC3 substrate specificity [42]. In this regard, the seven membrane proximal cysteines in β4 (of which at least five may be palmitoylated [19]) show no discernible similarity to amino acid sequences in other reported DHHC3 substrates, such as G protein alpha subunits, or integrin α6 subunit. Furthermore, knockdown of DHHC3 diminished palmitoylation of the integrin α6 subunit, but not the α3 subunit, even though in both cases the target cysteine is flanked by the same amino acids (-LWKCGFFKR-) at the transmembrane–cytoplasmic interface.
In the case of α6β4, the β4 protein might participate in recruitment of DHHC3 into proximity with both β4 and α6 in the Golgi. This would explain why α3β1 was unaffected by DHHC3. Consistent with a role for β4 in DHHC3 recruitment to α6, preliminary experiments indicate that DHHC3 knockdown did not affect palmitoylation of α6β1 in cells that lacked α6β4 (not shown).
Functional consequences of DHHC3-mediated α6β4 palmitoylation
Functional studies were chosen that specifically involve contributions from α6 and/or β4. Upon ablation of DHHC3, we observed a marked decrease in α6β4 palmitoylation, accompanied by impaired α6 integrin-dependent cell cable formation on 3D Matrigel. Matrigel cable formation results from tensional forces, acting through α6 integrin, which enables cell migration along “matrix guidance pathways” leading to formation of a network of cellular cables [43, 44]. DHHC3 ablation also diminished α6 and β4-dependent signaling, as evidenced by decreased antibody-triggered Src phosphorylation. Integrin α6β4 signaling through Src helps to regulate cancer cell motility and invasion [45]. We also noted that loss of β4 palmitoylation was accompanied by decreased phosphorylation of β4 at key sites (S1356 and 1424), which have been linked to hemidesmosome disassembly [36, 37, 40], a key step in EMT [46].
However, further studies showed that DHHC3 ablation caused a loss of endogenous α6β4 integrin expression on the cell surface, which likely explains reduced integrin-dependent functions. This result is in sharp contrast to results from β4 mutagenesis experiments [18, 19]. In those studies, β4 lacking palmitoylation was readily expressed on the surface of multiple cell types [18, 19]. With mutant β4 being expressed on cells, it was possible to show that β4 palmitoylation contributes to integrin-dependent cell signaling, spreading and proliferation [18, 19], as well as association with tetraspanin proteins such as CD9, CD81, and CD63 [18].
One major reason for the discrepancy between β4 mutagenesis results and DHHC3 ablation results may be that β4 mutants were analyzed when overexpressed [18, 19], whereas DHHC3 ablation was analyzed for effects on endogenous β4. Indeed in our own experiments, DHHC3 ablation caused loss of cell-surface expression for endogenous α6β4 (in PC3 and MDA-MB-231 cells), but not for α6β4 overexpressed in HEK293 cells. We suspect that for overexpressed α6β4, the normal cellular machinery for processing unpalmitoylated protein may be unavailable or insufficient. These findings emphasize that results based exclusively on overexpression of DHHC enzyme and/or substrate must be interpreted cautiously. Another reason that mutagenesis results could be erroneous is that replacement of cysteines by other amino acids may do more than simply prevent palmitoylation. Conceivably, removal of cysteines could additionally affect secondary structure, protein–protein interactions, protein stability, and/or sensitivity to proteolytic degradation. Further emphasizing the difficulty in interpreting cysteine-palmitoylation site mutagenesis results, replacement of cysteines can yield strikingly different results, depending on which replacement amino acids are used [47].
Loss of α6β4 stability and surface expression
In HEK293 cells, knockdown of DHHC3 did not decrease expression of α6β4. Consequently, a decrease in the amount of palmitoylation/protein could be readily observed. However, for endogenously expressed α6β4, DHHC3 ablation led to decreased α6β4 expression, as seen by immunoblotting, immunofluorescence microscopy, and cell-surface staining. Consequently, a decrease in palmitoylation/protein was not seen unless proteolytic degradation of β4 was blocked. This was best achieved using Pepstatin A, commonly used to inhibit cathepsin D, a lysosomal/endosomal aspartyl protease [48, 49]. Degradation of β4 was not appreciably rescued by bafilomycin-A1, a vacuolar-type H+-ATPase inhibitor that blocks lysosome acidification and protein degradation [50]. Integrin β4 was also not rescued by other inhibitors of lysosomes (chloroquine, NH4Cl; not shown) or inhibitors of lysosomal enzymes (leupeptin, E64). These results suggest that unpalmitoylated β4 is not degraded in lysosomes, but rather by cathepsin D in endosomes. By contrast, unpalmitoylated tetraspanin proteins resulting from DHHC2 ablation were degraded in lysosomes [24].
Upon addition of Pepstatin A, intracellular β4 was rescued from proteolysis to a markedly greater extent than cell surface β4. These results indicate that unpalmitoylated β4, despite rescue from proteolysis, is still unable to reach the cell surface. We speculate that inability to associate with tetraspanin proteins such as CD9, CD81, CD63 (as seen previously for palmitoylation-deficient mutant β4 [18]) may play a role in preventing cell-surface expression. In this regard, loss of association with tetraspanins CD9 and CD81 prevented MT1-MMP from reaching the cell surface [51]. DHHC3 ablation did not diminish the cell-surface expression of ten other proteins, consistent with DHHC3 directly and selectively affecting α6β4.
Because DHHC3 has an unknown number of other substrates, besides α6β4, indirect effects of DHHC3 ablation on α6β4 plasma membrane targeting are possible. However, indirect effects are unlikely to be a major issue for the several reasons. First, indirect effects, caused by DHHC3 ablation, would be expected to affect plasma membrane targeting of both palmitoylated and unpalmitoylated α6β4 to a similar extent. However, that is not the case. In the presence of Pepstatin A, DHHC3 ablation caused only a 12.9% decrease in palmitoylation/protein for cell surface β4, but a 41.4% loss for intracellular β4 (Supplemental Table 1). Hence, the subset of β4, which remains palmitoylated, is preferentially getting to the cell surface, whereas the unpalmitoylated population is more enriched intracellularly. Second, if there were indirect effects, they would need to be very specific. Whereas cell-surface expression of α6 and β4 decreased, there was no decrease in molecules related to α6β4 (other integrins α3β1 and α2β1), molecules associated with α6β4 (tetraspanins CD81, CD9, and CD82), and assorted other molecules (EWI-F, CD98, MHC1, CD147) in response to DHHC3 ablation (Supplemental Fig. S3). Third, there is ample precedent for DHHC-type enzymes directly affecting membrane targeting of proteins [52–54]. However, we could not find any published examples of DHHC-type enzymes having indirect effects on membrane targeting events.
Summary and implications
Despite the functional importance of α6β4 palmitoylation [18, 19], the responsible PAT had not been identified. Now we show that DHHC3 is largely responsible for α6β4 palmitoylation in 3 different cell lines. Given the wide expression of DHHC3 (see Gene Expression Atlas), it should be available to palmitoylate widely expressed α6β4 in many, if not all cell types. Here we have uncovered a new approach, through DHHC3, by which to modulate the expression and function of α6β4. Because α6β4 plays key roles on nearly all epithelial cells and on many other normal and cancer cell types, we predict that DHHC3 may also be needed to support α6β4 expression and function in those same cells. On cancer cells α6 integrins contribute to tumor initiation, growth, invasion, metastasis, EMT, and drug resistance [9–14]. Consequently, these integrins have emerged as potential cancer drug targets. Gene expression for ZDHHC3 is elevated in several malignant cell lines from prostate, breast and colorectal cancers, and also from patient prostate and breast carcinoma samples [55]. Hence, it may be reasonable to affect α6 integrin expression and function in cancer cells by targeting DHHC3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Hong-Xing Wang for assistance with confocal microscopy and for providing a control vector. This work was supported by National Institutes of Health Grant GM38903 (to MEH).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Rabinovitz I, Gipson IK, Mercurio AM. Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell. 2001;12:4030–4043. doi: 10.1091/mbc.12.12.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Pereda JM, Ortega E, Alonso-Garcia N, Gomez-Hernandez M, Sonnenberg A. Advances and perspectives of the architecture of hemidesmosomes: lessons from structural biology. Cell Adh Migr. 2009;3:361–364. doi: 10.4161/cam.3.4.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georges-Labouesse EN, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of the alpha-6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 4.Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18:29–42. doi: 10.1016/S0945-053X(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 5.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 6.Borland G, Cushley W. Positioning the immune system: unexpected roles for alpha6-integrins. Immunology. 2004;111:381–383. doi: 10.1111/j.0019-2805.2004.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haworth O, Hardie DL, Burman A, Rainger GE, Eksteen B, Adams DH, Salmon M, Nash GB, Buckley CD. A role for the integrin alpha6beta1 in the differential distribution of CD4 and CD8 T-cell subsets within the rheumatoid synovium. Rheumatology (Oxford) 2008;47:1329–1334. doi: 10.1093/rheumatology/ken263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMillan NA, Payne E, Frazer IH, Evander M. Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology. 1999;261:271–279. doi: 10.1006/viro.1999.9825. [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb EA, Mercurio AM. Mobilization and activation of a signaling competent alpha6beta4integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 2005;24:413–423. doi: 10.1007/s10555-005-5133-4. [DOI] [PubMed] [Google Scholar]
- 10.Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12:e3. doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Yang XH, Flores LM, Li Q, Zhou P, Xu F, Krop IE, Hemler ME. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 2010;70:2256–2263. doi: 10.1158/0008-5472.CAN-09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M. Colombel, C. L. Eaton, F. Hamdy, E. Ricci, G. van der Pluijm, M. Cecchini, F. Mege-Lechevallier, P. Clezardin, G. Thalmann (2011) Increased expression of putative cancer stem cell markers in primary prostate cancer is associated with progression of bone metastases. Prostate [DOI] [PubMed]
- 14.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 16.Sterk LM, Geuijen CA, van Den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- 17.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion and fusion events, and define a novel type of membrane microdomain. Ann Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Kovalenko OV, Tang W, Claas C, Stipp CS, Hemler ME. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J Cell Biol. 2004;167:1231–1240. doi: 10.1083/jcb.200404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnoux-Palacios L, Dans M, Van’t Hof W, Mariotti A, Pepe A, Meneguzzi G, Resh MD, Giancotti FG. Compartmentalization of integrin {alpha}6{beta}4 signaling in lipid rafts. J Cell Biol. 2003;162:1189–1196. doi: 10.1083/jcb.200305006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsumi R, Fukata Y, Fukata M. Discovery of protein-palmitoylating enzymes. Pflugers Arch. 2008;456:1199–1206. doi: 10.1007/s00424-008-0465-x. [DOI] [PubMed] [Google Scholar]
- 22.Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma C, Yang XH, Hemler ME. DHHC2 affects palmitoylation and stability of tetraspanins CD9 and CD151. Mol Biol Cell. 2008;19:3415–3425. doi: 10.1091/mbc.E07-11-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2008;105:5384–5389. doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez E, Girones N, Davis RJ. Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J Biol Chem. 1990;265:16644–16655. [PubMed] [Google Scholar]
- 27.Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J Biol Chem. 2001;276:31936–31944. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 28.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Seehafer JG, Slupsky JR, Tang SC, Masellis-Smith A, Shaw AR. Myristic acid is incorporated into the two acylatable domains of the functional glycoprotein CD9 in ester, but not in amide bonds. Biochim Biophys Acta. 1990;1039:218–226. doi: 10.1016/0167-4838(90)90189-M. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Claas C, Kraeft SK, Chen LB, Wang Z, Kreidberg JA, Hemler ME. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell. 2002;13:767–781. doi: 10.1091/mbc.01-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berditchevski F, Odintsova E, Sawada S, Gilbert E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signalling. J Biol Chem. 2002;277:36991–37000. doi: 10.1074/jbc.M205265200. [DOI] [PubMed] [Google Scholar]
- 32.Charrin S, Manie S, Oualid M, Billard M, Boucheix C, Rubinstein E. Differential stability of tetraspanin/tetraspanin interactions: role of palmitoylation. FEBS Lett. 2002;516:139–144. doi: 10.1016/S0014-5793(02)02522-X. [DOI] [PubMed] [Google Scholar]
- 33.Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29:435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Xie Y, Wolff DW, Abel PW, Tu Y. DHHC protein-dependent palmitoylation protects regulator of G-protein signaling 4 from proteasome degradation. FEBS Lett. 2010;584:4570–4574. doi: 10.1016/j.febslet.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germain EC, Santos TM, Rabinovitz I. Phosphorylation of a novel site on the {beta}4 integrin at the trailing edge of migrating cells promotes hemidesmosome disassembly. Mol Biol Cell. 2009;20:56–67. doi: 10.1091/mbc.E08-06-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashyap T, Germain E, Roche M, Lyle S, Rabinovitz I. Role of β4 integrin phosphorylation in human invasive squamous cell carcinoma: regulation of hemidesmosome stability modulates cell migration. Lab Invest. 2011;91:1414–1426. doi: 10.1038/labinvest.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelmsen K, Litjens SH, Kuikman I, Margadant C, van Rheenen J, Sonnenberg A. Serine phosphorylation of the integrin beta4 subunit is necessary for epidermal growth factor receptor induced hemidesmosome disruption. Mol Biol Cell. 2007;18:3512–3522. doi: 10.1091/mbc.E07-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greaves J, Salaun C, Fukata Y, Fukata M, Chamberlain LH. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem. 2008;283:25014–25026. doi: 10.1074/jbc.M802140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol. 1995;147:873–883. [PMC free article] [PubMed] [Google Scholar]
- 44.Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113–123. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- 45.Kim TH, Kim HI, Soung YH, Shaw LA, Chung J. Integrin (alpha6beta4) signals through Src to increase expression of S100A4, a metastasis-promoting factor: implications for cancer cell invasion. Mol Cancer Res. 2009;7:1605–1612. doi: 10.1158/1541-7786.MCR-09-0102. [DOI] [PubMed] [Google Scholar]
- 46.Takkunen M, Grenman R, Hukkanen M, Korhonen M, de Garcia HA, Virtanen I. Snail-dependent and -independent epithelial–mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem. 2006;54:1263–1275. doi: 10.1369/jhc.6A6958.2006. [DOI] [PubMed] [Google Scholar]
- 47.Greaves J, Prescott GR, Fukata Y, Fukata M, Salaun C, Chamberlain LH. The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediate membrane interactions and recognition by DHHC palmitoyl transferases. Mol Biol Cell. 2009;20:1845–1854. doi: 10.1091/mbc.E08-09-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett AJ (1979) Cathepsin D: the lysosomal aspartic proteinase. Ciba Found Symp, pp 37–50 [DOI] [PubMed]
- 49.Diment S, Leech MS, Stahl PD. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988;263:6901–6907. [PubMed] [Google Scholar]
- 50.van Weert AW, Dunn KW, Gueze HJ, Maxfield FR, Stoorvogel W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafleur MA, Xu D, Hemler ME. Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell. 2009;20:2030–2040. doi: 10.1091/mbc.E08-11-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Natl Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 53.J. Korycka, A. Lach, E. Heger, D. M. Boguslawska, M. Wolny, M. Toporkiewicz, K. Augoff, J. Korzeniewski, A. F. Sikorski (2011) Human DHHC proteins: a spotlight on the hidden player of palmitoylation. Eur J Cell Biol [DOI] [PubMed]
- 54.Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. 2011;1808:2981–2994. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Lukk M, Kapushesky M, Nikkila J, Parkinson H, Goncalves A, Huber W, Ukkonen E, Brazma A. A global map of human gene expression. Nat Biotechnol. 2010;28:322–324. doi: 10.1038/nbt0410-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.