Abstract
Since its debut in the mid 70ties, electron crystallography has been a valuable alternative in the structure determination of biological macromolecules. Its reliance on single- or double-layered two-dimensionally ordered arrays and the ability to obtain structural information from small and disordered crystals make this approach particularly useful for the study of membrane proteins in a lipid bilayer environment. Despite its unique advantages, technological hurdles have kept electron crystallography from reaching its full potential. Addressing the issues, recent initiatives developed high-throughput pipelines for crystallization and screening. Adding progress in automating data collection, image analysis and phase extension methods, electron crystallography is poised to raise its profile and may lead the way in exploring the structural biology of macromolecular complexes.
Introduction
Publication in 1975 of the 7Å structure of the archebacterial proton pump bacteriorhodpsin by Unwin and Henderson marked the birth of electron crystallography and established that electron microscopy could be used to determine the structure of biological macromolecules at subnanometer resolutions [1]. However, another 15 years were needed to develop better imaging and computational tools that would allow near atomic resolution information to be retrieved from images of unstained, ordered biological samples. In 1990, these pioneering efforts culminated in the publication of the first near atomic resolution structure of bacteriorhodopsin and suggested that electron crystallography had the potential to become a competitive alternative to X-ray crystallography in the structure determination of integral membrane proteins [2]. Appearing only a few years later, the high-resolution structure of light harvesting complex II lent further momentum to a budding field that eventually would add successes such as the first high-resolution structures of glutathione transferase, and aquaporins [3–6]. Over time, however, X-ray crystallography gained the upper hand in the structure determination of membrane proteins (Figure 1). For the most part, this bias can be attributed to two causes (1) the size of the X-ray community is vast compared to that of 2D-crystallographers, and (2) the development of high-throughput pipelines which have addressed some of the issues that traditionally have hindered structural analyses of membrane proteins. Despite tremendous technological advances, however, X-ray crystallography of membrane proteins still lags far behind structure analysis of soluble proteins. Moreover, setting aside the accelerating progress in the structure determination of G protein-coupled receptors [7–11], the pace of structure determination of mammalian membrane proteins remains extremely slow. Adding to this, crystal structures of voltage-gated potassium channels and the prokaryotic drug transporter EmrE serve as a reminder that the evaluation of membrane protein structures outside of their natural context can lead to ambiguous or even erroneous results [12–15]. Mostly for the latter reason, electron crystallography remains a useful alternative approach because its idiosyncratic requirement for thin two-dimensional arrays creates a unique opportunity to observe membrane protein structures in a bilayer environment. Reinvigorated by Gonen’s 1.9Å structure of aquaporin 0 [16], the past 10 years have seen renewed interest in the further development of 2D-crystallography. Here we briefly discuss some of the exciting advances and provide an outlook how these developments may allow 2D-crystallography to gain more traction in the field of structural biology.
Figure 1. Summary of Unique Membrane Protein Structures.
Tracking progress back to the 1990ties, this figure shows the trends in structure determination of membrane proteins by different methods. Only fully determined 3D-structures were included in this count. Data for the structures solved by electron crystallography are further subdivided into structures that were defined at better than 5Å and those that were determined at lower resolutions. This comparison emphasizes that X-ray crystallography has a clear lead over electron crystallography. However, despite the tremendous efforts and resources that have been waged on the development of X-ray crystallographic pipelines, membrane proteins still remain a challenge.
Technical Challenges - Crystallization
An undeniable advantage of 2D-crystallography is that the availability of correlation-based image processing tools [17] allows useful structural information to be generated even from small and moderately disordered 2D-arrays. In fact, reconstructions with resolutions of 5–10Å account for the overwhelming majority of structures that have been determined by this method [e.g. [18–20]]. While these structures make valuable contributions to their respective fields, they also emphasize that generating micron sized, well-ordered 2D-crystals of membrane embedded membrane proteins is as challenging as generating suitable 3D-crystals used for X-ray crystallography. Unlike the X-ray community, which received a large boost by the deployment of high-throughput pipelines for protein expression and crystallization, evaluation of parameter space in electron crystallography by and large remains a cumbersome manual process, taking about 20 minutes to evaluate each sample. Not only does this limit the number of conditions that can reasonably be tried, it also slowed systematic studies investigating what parameters are the most likely to exert a major influence on the 2D-crystallization behavior of membrane proteins [21,22]. Addressing this critical issue, and drawing on the expression pipelines already developed for the X-ray community, recent efforts in the 2D-community have been focused on moving into place largely automated processes for the high-throughput crystallization of membrane proteins by dialysis techniques or detergent removal by cyclodextrins [23•, 24•, 25, 26•, 27••]. Complementing these tools, automated microscopy protocols were developed over the past 10 years [25,28–30] and fortified by the development of “96-well format” methods that allow rapid preparation of negatively stained samples for screening [27]. The results of these efforts provide a much needed platform for accelerating the pace of electron crystallographic structure determination because screening a large number of conditions, and optimizing initial hits to generate micron sized, well-ordered samples seems now within reach.
Technical Challenges – Sample Preparation and Data Collection
Unlike X-ray crystallography, electron crystallographic data sets are stitched together from images and, where applicable, electron diffraction patterns collected from many crystals at various tilts ranging from 0–60°. While true projection data from untilted samples are readily obtained, collecting images from highly tilted crystals remains the most daunting challenge in electron crystallography because imperfections in sample embedding, lack of flatness in the supporting carbon film and instabilities such as sample drift during data collection greatly diminish the success rate for acquiring high-quality images. These issues are particularly acute in cases where the crystals are ordered to near atomic resolution because in these cases high-resolution images are required unless phase data can be obtained by phase extension of a low-resolution data set (see below). Collection of a sufficiently large number of high-resolution images typically takes many months if not years of effort, which starkly contrasts with X-ray crystallography, where data collection is fast and, in most cases, no longer requires the generation of heavy metal derivatives for phasing. This distinctive disadvantage of electron crystallography is of considerable concern and contributes very significantly to the fact that until recently, the structural biology community has been hesitant to invest into the development of electron crystallography. However, indications are that the current limitations can be overcome. For instance, sample instabilities during data collection can, in part, be negated by automated data acquisition because the latter allows many digital images to be taken, some of which will meet the stringent quality requirements. There also have been renewed efforts to better understand what limits image contrast at high resolutions, and how to overcome issues of specimen charging and movement [31]. Adding to this, improved sample preparation techniques may yield more uniformly preserved crystals, which in turn may increase success rates in both imaging and the collection of electron diffraction data. An approach in this direction is to embed crystalline samples between two evenly matched carbon films [32•,33]. This strategy has been key for obtaining atomic resolution data in the case of aquaporin 0 [16]. In contrast to single particle approaches, the increased background stemming from the second carbon layer is not of concern because the discreet data that emerge from averaging over thousands of molecules on the lattice have well defined signal-to-noise ratios and do not require individual molecules to be located for alignment. While the use of a carbon sandwich holds much promise, it remains unclear how generally applicable this technique will be. Specifically, crystals of the aquaporin 0 junction are several microns in size, double layered and contain little lipid. Because of this, the crystal sheets are more rigid than traditional, single-layered 2D-arrays, which in itself will improve properties such as sample flatness that is critical for obtaining high-resolution data. Moreover, it remains to be thoroughly tested whether the sandwich technique works well in cases where the protein has large soluble domains that may be susceptible to partial flattening or disordering upon contact with the second carbon film. A 6.5Å structure of the H+/K+-ATPase seems to suggest that the method may be suitable even in these cases [20]. However, the presence of the second carbon film may have been the reason for the limited resolution and the very large number of images that were required to obtain the reconstruction. It also remains to be seen whether this sample preservation technique generally results in an improved success rate with collecting high-resolution image data. Regardless of these open issues, use of a carbon sandwich will greatly facilitate data collection in many cases.
Technical Challenges – Data Processing
Developed in the 70ties and 80ties, the MRC image processing programs are the most widely used for the processing of electron crystallographic data [17]. In the past, use of these programs required a significant amount of experience and was cumbersome since these programs were not designed with user comfort in mind. Over the past decade, this has been changed by the development of “2dx”. This user-friendly graphical interface makes usage of the MRC-software relatively straightforward and fast, shortening what used to take months of uninterrupted effort to as little as a few days [34,35]. While these advances eliminated one of the most important bottlenecks in electron crystallography, the hope is to completely automate data processing over the next few years. This is an exciting perspective because it is entirely doable. Moreover, coupling automated data collection with automated data processing opens the door to implement feedback loops where results from the data processing intelligently drive data collection without the need for operator input. While not emphasized here, recent years have seen the development of IPLT, a software suite that offers an alternative to the MRC programs for processing of images and electron diffraction patterns [36]. At present, there are too few comparative data to decide if one method has a clear advantage over the other. Regardless, automation of data processing pipelines has to be a priority in future efforts if electron crystallography is to develop its full potential.
Complementing the advances in data processing, Gonen’s laboratory recently implemented an approach that allows fragment based phase extension of electron diffraction data at high resolution. This potentially game changing approach is conceptually similar to phase extension protocols in X-ray crystallography [37••] and exploits that generic models, build by placing helical polyalanine fragments into ~7Å reconstructions, have sufficient phasing power to drive the interpretation of high-resolution electron diffraction data (Figure 2). This strategy is advantageous over the more traditional route of using phase information from images because factors such as specimen movement, effects of the contrast transfer function, or beam tilt do not adversely affect electron diffraction, making it far easier to record high-resolution electron diffraction data than collecting images of comparable quality. Fragment based phase extension has been validated for two types of membrane proteins – bacteriorhodopsin and aquaporins. An interesting future challenge will be to test whether the approach also works in cases where the membrane protein has large and crystallographically ordered extramembraneous domains. That notwithstanding and assuming that automated crystallization pipelines will make large and well-ordered 2D-arrays more readily available, fragment based phase extension is the most exciting progress that has recently been made in the field because it will greatly increase the speed and success rate in obtaining high-resolution structures by eliminating the need to record high-resolution images.
Figure 2. Fragment Based Phase Extension Method.
This figure is reproduced from Wisedchairsri et al [37••] and illustrates how a low-resolution reconstruction (Start) can serve as template for the phasing of high-resolution electron diffraction data. After placing generic alpha-helical segments into the experimental density map, phase probability distributions are calculated for the measured high-resolution amplitudes that are obtained from electron diffraction patterns. After combining the calculated high-resolution phases with the experimental phase data at lower resolution, an improved density map is calculated and established density modification is used to generate a more accurate model. Iteration of the process converges to a solution that is fully consistent with the previously determined high-resolution structures.
Conclusions
In light of recent technological advances, electron crystallography is finally poised to take off. Less clear though is its final destination. No doubt, analyzing the structures and dynamics of membrane proteins in bilayers is important, and will continue to make critical contributions to many fields. For instance, electron crystallography provides an avenue towards studying the detailed interactions between lipids and membrane proteins if the resolution is sufficiently high, and if the lipids are sufficiently close to those present in the natural membrane environment of any given membrane protein. Moreover, the existence of typical bilayer constraints such as lateral pressure may be important to accurately describe different, functionally relevant conformational states of a given membrane protein. However, circling back to the trends shown in Figure 1 and adding that the emergence of free electron lasers and nano-crystallography [38••,39] are likely to further accelerate the high-resolution exploration of membrane protein structure, it seems unlikely that electron crystallography will become competitive with X-ray methods in cases that concern the simple structure determination of individual membrane proteins. If not in this area, why else should one watch out for 2D-crystallography? To us, it seems that recent advances were essential for positioning electron crystallography to tackle the next, and far more complex challenge of conquering the structural biology of membrane associated macromolecular complexes and scaffolds. We believe that this area will be a promising hunting ground for electron crystallography because using the membrane to properly constrain the orientations and interactions between multiple partners will be a distinct advantage over trying to assemble such complexes in solution and in the presence of detergents.
As notable, the high-resolution structure of the tubulin α/β-dimer stands as a reminder that 2D-crystallography is not limited to the exploration of membrane proteins. However, the 2D-crystallization of soluble proteins on substrates such as lipid monolayers, spread at water-air interfaces, never gained much traction, mostly because these 2D-arrays tend to be fragile and often do not survive the transfer to the carbon support films for data recording [40,41]. Ultimately, this is a material sciences problem, and there no longer seems to be a reason why substrates more stable than lipid monolayers could not be developed. Why would it be advantageous to revisit this area and to push for further developments of such approaches? A simple answer emerges from proteomics studies showing that >50% of all macromolecular complexes are composed from >5–6 different protein subunits [42]. Even if one ignores that current strategies to express and purify such assemblies are entirely inadequate, the limited stability, compositional and conformational heterogeneity of these complexes pose additional challenges that will make growing even nanometer-sized 3D-crystals of these assemblies very difficult if not impossible. In contrast, very small 2D-arrays can yield useful data. To illustrate this point, Figure 3 shows examples of projection structures that were obtained from 20×20 unit cells (<0.2×0.2µm), cut from single images of two oligomeric membrane proteins, a truncated gap-junction [43] and a human copper transporter [19]. Statistically significant phase data were obtained to at least 9Å resolution in both cases. While still challenging and potentially affected by molecular heterogeneity, the odds of obtaining such limited arrays of more complex macromolecular assemblies seem better than the odds for growing nanometer sized 3D-crystals. Clearly, resolution will likely be limited and significantly lower than needed to resolve near-atomic detail. However, routine electron crystallographic reconstruction of macromolecular complexes at subnanometer resolutions would have a tremendous impact because fitting of available subunit crystal structures into the subnanometer molecular envelopes would allow the construction of pseudo atomic models [44], which in turn would enable informed biochemical and cell biological studies. That prospect alone appears sufficient to advocate for further development of electron crystallography. In the case of macromolecular complexes, the lower dimensionality of the parameter space governing the formation of 2D-arrays, and the reduced need for purified material give electron crystallography a unique edge over other structural methods and make it appear likely that the importance of electron crystallography will grow over the years to come.
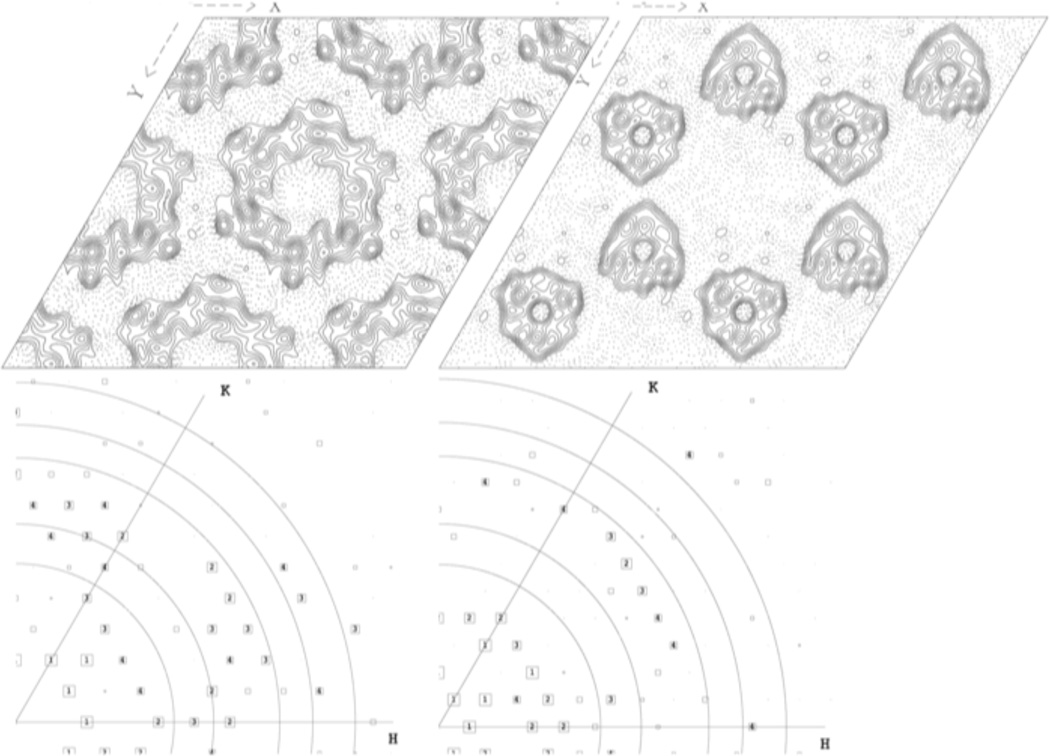
Figure 3. Reaching Subnanometer Resolutions From Small Arrays.
Determining the structures of macromolecular complexes represents one of the largest challenges in structural biology. The figure illustrates how even small 2D-arrays can yield subnanometer resolutions. Shown are 9Å projection structures of a dodecameric gap junction channel (~350kDa, top left, [43]) and dimers of the trimeric human copper transporter hCTR1 (~120kDa, top right, [19,45]). Data were obtained from a single image and no symmetry was imposed for the purpose of this illustration. Solid lines represent protein, contoured at 0.3α. Dotted lines represent non-protein densities (eg lipid, water). Bottom panels show the data readout from the transform of the corrected images. Significance of the data is indicated by the size of the boxes where numbers 1 to 4 mark the most reliable data in decreasing order. Circles were added to indicate resolution at 15, 12, 9, 8 and 7Å respectively. If, for simplicity, dodecameric gap junction channels and dimers of copper transporter trimers were considered to represent the equivalents of one copy of a macromolecular complex, as few as 400 (gap junction) or 800 (copper transporter) copies, are fully sufficient to retrieve reliable information at better than 10Å resolution, even if no symmetry is present.
Highlights.
Automation of 2D-crystallization and sample screening for high-throughput analysis
Graphical interfaces facilitate data analysis
Fragment based phase extension – a novel alternative to imaging at high resolution
Small arrays - a way to tackle the structural biology of macromolecular complexes
Acknowledgements
Work in the Unger Laboratory is supported by PHS grants F31 NS076213 (C.R.P.), P01 GM067166 and R01 GM094479 (V.M.U).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975;93:123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- 2.Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, Downing KH. Model for the structure of bacteriorhodopsin based on high-resolution electron cryomicroscopy. J Mol Biol. 1990;213:899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlbrandt W, Wang DN, Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 4.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 5.Ren G, Reddy VS, Cheng A, Melnyk P, Mitra AK. Visualization of a water-selective pore by electron crystallography in vitreous ice. Proc Natl Acad Sci U S A. 2001;98:1398–1403. doi: 10.1073/pnas.041489198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm PJ, Bhakat P, Jegerschold C, Gyobu N, Mitsuoka K, Fujiyoshi Y, Morgenstern R, Hebert H. Structural basis for detoxification and oxidative stress protection in membranes. J Mol Biol. 2006;360:934–945. doi: 10.1016/j.jmb.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)�adrenergic receptor. Nature. 2011;469:241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, et al. Structures of the CXCR4 chemokine GPCR with smallmolecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuldiner S. When biochemistry meets structural biology: the cautionary tale of EmrE. Trends Biochem Sci. 2007;32:252–258. doi: 10.1016/j.tibs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Tate CG. Comparison of three structures of the multidrug transporter EmrE. Curr Opin Struct Biol. 2006;16:457–464. doi: 10.1016/j.sbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Bezanilla F. The voltage-sensor structure in a voltage-gated channel. Trends Biochem Sci. 2005;30:166–168. doi: 10.1016/j.tibs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Cohen BE, Grabe M, Jan LY. Answers and questions from the KvAP structures. Neuron. 2003;39:395–400. doi: 10.1016/s0896-6273(03)00472-0. [DOI] [PubMed] [Google Scholar]
- 16.Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;429:193–197. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- 17.Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9–16. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Ikeda Y, Abe Y, Kuma H, Kang D, Hamasaki N, Hirai T. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. J Mol Biol. 2010;397:179–189. doi: 10.1016/j.jmb.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 19.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci U S A. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe K, Tani K, Nishizawa T, Fujiyoshi Y. Inter-subunit interaction of gastric H+,K+�ATPase prevents reverse reaction of the transport cycle. EMBO J. 2009;28:1637–1643. doi: 10.1038/emboj.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt-Krey I. Electron crystallography of membrane proteins: two-dimensional crystallization and screening by electron microscopy. Methods. 2007;41:417–426. doi: 10.1016/j.ymeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Krey I, Lundqvist G, Morgenstern R, Hebert H. Parameters for the two-dimensional crystallization of the membrane protein microsomal glutathione transferase. J Struct Biol. 1998;123:87–96. doi: 10.1006/jsbi.1998.4018. [DOI] [PubMed] [Google Scholar]
- 23.Signorell GA, Kaufmann TC, Kukulski W, Engel A, Remigy HW. Controlled 2D crystallization of membrane proteins using methyl-beta-cyclodextrin. J Struct Biol. 2007;157:321–328. doi: 10.1016/j.jsb.2006.07.011. [DOI] [PubMed] [Google Scholar]; • This article describes an alternative to the dialysis approach towards membrane protein 2D-crystallization. This method is notable because it allows detergent removal, and thereby reconstitution, to be precisely controlled.
- 24.Vink M, Derr K, Love J, Stokes DL, Ubarretxena-Belandia I. A high-throughput strategy to screen 2D crystallization trials of membrane proteins. J Struct Biol. 2007;160:295–304. doi: 10.1016/j.jsb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This contribution describes the development of a 96-well block for high-throughput 2D-crystallization of membrane proteins.
- 25.Hu M, Vink M, Kim C, Derr K, Koss J, D'Amico K, Cheng A, Pulokas J, Ubarretxena-Belandia I, Stokes D. Automated electron microscopy for evaluating two-dimensional crystallization of membrane proteins. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacovache I, Biasini M, Kowal J, Kukulski W, Chami M, van der Goot FG, Engel A, Remigy HW. The 2DX robot: a membrane protein 2D crystallization Swiss Army knife. J Struct Biol. 2010;169:370–378. doi: 10.1016/j.jsb.2009.12.001. [DOI] [PubMed] [Google Scholar]; • The article desdribes the fully automated 2D-crystallization of membrane proteins by the cyclodextrin method. The development of this tool has the potential to greatly accelearte the success rates for obtaining large, well-ordered 2D-crystals
- 27.Kim C, Vink M, Hu M, Love J, Stokes DL, Ubarretxena-Belandia I. An automated pipeline to screen membrane protein 2D crystallization. J Struct Funct Genomics. 2010;11:155–166. doi: 10.1007/s10969-010-9088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article describes a fully automated pipeline for screening of 2D-crystallization experiments. The work described here is transformative in that it vastly extends the reach of a single operator, allowing a much larger number of crystallization conditions to be evaluated
- 28.Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Carragher B, Kisseberth N, Kriegman D, Milligan RA, Potter CS, Pulokas J, Reilein A. Leginon: an automated system for acquisition of images from vitreous ice specimens. J Struct Biol. 2000;132:33–45. doi: 10.1006/jsbi.2000.4314. [DOI] [PubMed] [Google Scholar]
- 30.Potter CS, Chu H, Frey B, Green C, Kisseberth N, Madden TJ, Miller KL, Nahrstedt K, Pulokas J, Reilein A, et al. Leginon: a system for fully automated acquisition of 1000 electron micrographs a day. Ultramicroscopy. 1999;77:153–161. doi: 10.1016/s0304-3991(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 31.Glaeser RM, McMullan G, Faruqi AR, Henderson R. Images of paraffin monolayer crystals with perfect contrast: minimization of beam-induced specimen motion. Ultramicroscopy. 2010;111:90–100. doi: 10.1016/j.ultramic.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyobu N, Tani K, Hiroaki Y, Kamegawa A, Mitsuoka K, Fujiyoshi Y. Improved specimen preparation for cryo-electron microscopy using a symmetric carbon sandwich technique. J Struct Biol. 2004;146:325–333. doi: 10.1016/j.jsb.2004.01.012. [DOI] [PubMed] [Google Scholar]; • The technique introduces the carbon sandwich technique. This approach holds promise to improve success rates of data collection at high=resolution
- 33.Abeyrathne PD, Chami M, Pantelic RS, Goldie KN, Stahlberg H. Preparation of 2D crystals of membrane proteins for high-resolution electron crystallography data collection. Methods Enzymol. 2010;481:25–43. doi: 10.1016/S0076-6879(10)81001-8. [DOI] [PubMed] [Google Scholar]
- 34.Gipson B, Zeng X, Stahlberg H. 2dx_merge: data management and merging for 2D crystal images. J Struct Biol. 2007;160:375–384. doi: 10.1016/j.jsb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gipson B, Zeng X, Zhang ZY, Stahlberg H. 2dx--user-friendly image processing for 2D crystals. J Struct Biol. 2007;157:64–72. doi: 10.1016/j.jsb.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Philippsen A, Schenk AD, Signorell GA, Mariani V, Berneche S, Engel A. Collaborative EM image processing with the IPLT image processing library and toolbox. J Struct Biol. 2007;157:28–37. doi: 10.1016/j.jsb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Wisedchaisri G, Gonen T. Fragment-based phase extension for three-dimensional structure determination of membrane proteins by electron crystallography. Structure. 2011;19:976–987. doi: 10.1016/j.str.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study represents the potentially most significant advance towards increasing the success rate for high-resolution struture determination by electron crystallography. The transformative idea driving this study was to use low-resolution density maps as a template to calculate phases for high-resolution electron diffraction amplitudes. The key advance is that this approach eliminates the need to collect high-resolution images from highly tilted crystals, which respents by far the largest bottleneck at the level of data collection
- 38.Fromme P, Spence JC. Femtosecond nanocrystallography using X-ray lasers for membrane protein structure determination. Curr Opin Struct Biol. 2011;21:509–516. doi: 10.1016/j.sbi.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study presents proof of principle that high-resolution X-ray structures can be obtained from nano-sized 3D-crystals. This transformative approach will change how X-ray crystallographic data will be collected and analyzed in the future.
- 39.Chapman HN, Fromme P, Barty A, White TA, Kirian RA, Aquila A, Hunter MS, Schulz J, DePonte DP, Weierstall U, et al. Femtosecond X-ray protein nanocrystallography. Nature. 2011;470:73–77. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darst SA, Ahlers M, Meller PH, Kubalek EW, Blankenburg R, Ribi HO, Ringsdorf H, Kornberg RD. Two-dimensional crystals of streptavidin on biotinylated lipid layers and their interactions with biotinylated macromolecules. Biophys J. 1991;59:387–396. doi: 10.1016/S0006-3495(91)82232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornberg RD, Darst SA. Two-dimensional crystals of proteins on lipid bilayers. Curr Opin Struct Biol. 1991;1:642–646. [Google Scholar]
- 42.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 43.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 44.Volkmann N. Putting structure into context: fitting of atomic models into electron microscopic and electron tomographic reconstructions. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A. 2006;103:3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]