Abstract
Fructose consumption predicts increased hepatic fibrosis in those with nonalcoholic fatty liver disease (NAFLD). Due to its ability to lower hepatic adenosine triphosphate (ATP) levels, habitual fructose consumption could result in more hepatic ATP depletion and impaired ATP recovery. The degree of ATP depletion following an intravenous fructose challenge test in low versus high fructose consumers was assessed. We evaluated diabetic adults enrolled in the Look AHEAD Fatty Liver Ancillary Study (n=244) for whom dietary fructose consumption estimated by a 130-item Food Frequency questionnaire, hepatic ATP measured by phosphorus MRS (31P MRS) and uric acid (UA) levels were performed (n=105). In a subset of participants (n=25), an intravenous fructose challenge was utilized to assess change in hepatic ATP content. The relationships between dietary fructose, UA and hepatic ATP depletion at baseline and following intravenous fructose challenge was evaluated in low (<15 g/d) vs. high (≥15 g/d) fructose consumers. High dietary fructose consumers had slightly lower baseline hepatic ATP levels and a greater absolute change in hepatic α-ATP/Pi ratio (0.08 vs. 0.03, p=0.05) and γ-ATP /Pi ratio following an intravenous fructose challenge (0.03 vs. 0.06, p=0.06). Patients with high UA (≥5.5 mg/dl) showed a lower minimum liver ATP/Pi ratio post-fructose challenge (4.5 vs. 7.0, p = 0.04).
Conclusions
High fructose consumption depletes hepatic ATP and impairs recovery from ATP depletion following an intravenous fructose challenge. Subjects with high UA show a greater nadir in hepatic ATP in response to fructose. Both high dietary fructose intake and elevated UA level may predict more severe hepatic ATP depletion in response to fructose and hence may be risk factors for the development and progression of NAFLD.
Key Words (MeSH): Nonalcoholic steatohepatitis, Diabetes mellitus, Obesity, Fructose metabolism, Uric acid, Fructose consumption
INTRODUCTION
The increasing prevalence of nonalcoholic fatty liver disease (NAFLD) parallels the rise in obesity and type 2 diabetes mellitus (T2DM). Patients with obesity and T2DM have not only a higher prevalence, but also more severe forms of NAFLD (i.e. steatohepatitis, hepatic fibrosis or cirrhosis) (1). The rapid rise in NAFLD supports the role for environmental factors, which in tandem with predisposing genetic factors, likely contribute to the pathogenesis and epidemic of NAFLD. In recent decades, there has not only been an increase in total energy consumption, but also a shift in the types of nutrients consumed.
Fructose is a simple monosaccharide found in plant sources. Most commercially available fructose is in the form of a disaccharide (mixture of glucose and fructose) in the form of high fructose corn syrup (HFCS). In the United States, fructose consumption has more than doubled in the past 30 years and has paralleled the rise in obesity and T2DM (2). Before 1900, Americans consumed approximately 15 grams of fructose/ day (4% of total calories), mainly through fruits and vegetables. However by 1994, Americans consumed approximately 55 grams of fructose/day (10% of total calories) (3) which is primarily accounted for by the marked increase in soft drink consumption (4, 5). Despite conservative estimates, patients with NAFLD consume 2–3 fold more fructose-containing beverages than matched controls (6). The parallel trend in rise of obesity, T2DM, NAFLD and fructose consumption makes fructose an attractive target for investigation.
Fructose induces both metabolic syndrome and NAFLD independent of energy intake (7–10). In fact, free fructose and glucose combinations (such as seen with HFCS) induce fatty liver more than sucrose despite the same fructose content (9). In overweight or obese adults, consumption of fructose- but not glucose-sweetened beverages increases de novo lipogenesis, promotes dyslipidemia, impairs insulin sensitivity, and increases visceral adiposity (11). Further, a longitudinal study of women (n=91,249) followed up over 8 years showed that those who consumed ≥ 1 serving of soft drinks per day were at twice the risk of developing T2DM as those who consumed < 1 serving per month (12). Thus, it is plausible that habitual and/or excessive fructose consumption may not only increase the risk for NAFLD (6, 13), but also exacerbate liver injury and promotes fibrosis progression in NAFLD (14).
Unlike glucose metabolism, there is no negative feedback mechanism regulating the phosphorylation of fructose to prevent hepatic ATP depletion (15). Upon entering the hepatocyte, fructose is rapidly phosphorylated by fructokinase to generate fructose-1-phosphate. Fructose induced hepatic ATP depletion has been demonstrated with low concentrations of fructose (1 mM) in a variety of cell types (16, 17) and in humans by both phosphorus magnetic resonance spectroscopy (31P MRS) (18, 19) and by liver biopsy (20). Cellular ATP depletion can cause an arrest in protein synthesis and induce inflammatory and prooxidative changes (16, 17, 20). Consistent with these findings, HFCS increases fatty acid synthesis (21), increases endoplasmic reticulum stress, promotes activation of the stress-related kinase, Jun N-terminal Kinase (JNK), induces mitochondrial dysfunction, and increases apoptotic activity (22, 23) in liver cells. Habitual fructose consumption may therefore lead to an unfavorable energy balance in the liver thus enhancing the susceptibility of hepatocytes to injury (24).
Fructose metabolism also causes rapid intracellular generation of UA. When fructose is rapidly phosphorylated, intracellular phosphate levels fall, resulting in the stimulation of AMP deaminase. Consequently, the increased stimulation of AMP deaminase shunts AMP towards the production of UA as opposed to the regeneration of ATP via AMP kinase (25). Following fructose ingestion, serum UA can increase by 1 to 4 mg/dl within 30 minutes (26). Further, in subjects who chronically consume a high fructose diet, fructose administration results in an enhanced rise in serum UA (26). Thus, increased UA may serve as a biomarker for increased fructose consumption and potentially as a marker of hepatic ATP depletion. Recent studies also suggest that UA may itself have proinflammatory and prooxidative effects (16, 17) that could be involved in the development and progression of NAFLD (27–29). Finally, both cell culture and experimental studies suggest that the continuous exposure to fructose results in the upregulation of both transporters (Glut 5) and enzymes (fructokinase) involved in fructose metabolism (30). Consistent with this data, subjects with NAFLD had higher hepatic fructokinase mRNA levels compared to subjects with other forms of chronic liver disease (6).
We proposed the following hypotheses. First, subjects with higher habitual intake of fructose may be susceptible to lower ATP levels. Second, subjects with higher UA levels (either as a consequence of increased fructose consumption or as a surrogate marker of impaired hepatic energy homeostasis) may be at increased risk for hepatic ATP depletion from increased dietary consumption of fructose. To test these hypotheses, we evaluated the relationship of dietary fructose consumption and baseline UA levels to the baseline, nadir, and duration of hepatic ATP depletion by 31P MRS in a subset (n=25) of subjects enrolled in the Look AHEAD Fatty Liver Ancillary Study.
PATIENTS & METHODS
Human Subjects
The design and methods of the Look AHEAD study have been previously described (31). All participants were recruited by means of public advertisement and underwent complete medical history, examination, and laboratory tests to exclude viral hepatitis and other major diseases. Participants were eligible if they were between the ages of 45–76 years, had T2DM, a body mass index (BMI) ≥ 25 kg/m2, and were able to complete a maximal exercise test. Exclusion criteria included known chronic liver disease, cirrhosis, inflammatory bowel disease requiring treatment in the past year, consumption of > 14 alcoholic drinks/week, prior bariatric surgery or use of weight loss medications, uncontrolled medical conditions (e.g. HbA1c > 11%, blood pressure ≥160/100 mm/Hg), use of systemic corticosteroids, known conditions that would limit their lifespan (e.g. cancer) or their adherence to the study protocol (e.g. inability to engage in moderate exercise). Participants who weighed over 350 pounds or who had any contraindication to MR imaging were excluded from the MR portion of the study. Between January 2002 and April 2004, 244 Look AHEAD Study subjects enrolled at JHU also participated in the Look AHEAD Fatty Liver Ancillary Study. Informed consent was obtained from each participant included in the study which was approved by the Institutional Review Board.
Experimental Protocol
As a part of both the parent Look AHEAD trial, participants underwent extensive data collection at baseline and screening. Age, sex, race/ethnicity, and medication use were obtained by questionnaire. Lifetime alcohol use was estimated using the validated Skinner Lifetime Drinking History questionnaire (32). Weight, height and waist circumference were directly measured using standardized techniques. Blood samples were obtained in all patients after an overnight fast and included: UA, serum aminotransferases, glycosylated hemoglobin, creatinine, and lipid levels. Serum UA was quantified by autoanalyzer.
Usual food and nutrient intake in the preceding 6 months were obtained using a food frequency questionnaire (FFQ) modified slightly from the Diabetes Prevention Program FFQ. Estimates of the food and nutrient intake were conducted by the Look AHEAD Diet Assessment Center using the National Institute Health Habits and History Questionnaire (HHHQ)/DietSys program (version 3.0, 1993, National Cancer Institute, Rockville, MD) and the dataset was provided to the Look AHEAD Data Coordinating Center. The nutrient database was modified from the Diabetes Prevention Program database to incorporate new foods added for the Look AHEAD FFQ (33). Nutrient values for the added foods were obtained primarily from the Nutrition Data System for Research (version 4.01_30, 1999, Nutrition Coordination Center, Minneapolis, MN).
1H and 31P MRS were carried out on a 1.5 T whole body scanner (Philips Gyroscan ACS-NT, Philips Medical Systems, Best, The Netherlands) and hepatic fat and ATP was measured as previously reported (18, 34). 1H MR spectra were processed in the frequency domain using an in-house software ‘CSX’ (http://mri.kennedykrieger.org/). Areas under the water and fat signals were determined by integration after zero filling to 2048 data points and exponential broadening of 3 Hz. Percentage of hepatic fat was determined according to fat * 100 /(water + fat). 31P spectra data were processed using a circle-fitting (CFIT) program as previously described (35). 31P MRS allows for reproducible quantification and of phosphorus-containing metabolites (36). Hepatic 31P MRS detected in six resonances: phosphomonoesters; inorganic phosphate; phosphodiesters; and the nucleotide triphosphates, including γ, α, and β signals resolved sequentially. These latter three peaks are commonly referred to as ATP signals, although the γ and α signals may include adenosine diphosphate and uridine, guanosine, inosine and cytosine triphosphates contribute to these signals as well (37). ‘Hepatic ATP’ was expressed as β-ATP/total phosphorus.
A representative smaller sample (n=25) of participants underwent a fructose challenge test (34), performed in the morning, between 6:30 AM and 9:30 AM, after an overnight fast. Following intravenous catheter placement, a slow infusion of isotonic saline solution was started. After 2 baseline 31P MR spectra were obtained, fructose (250 mg/kg of body weight) dissolved in 100 mL of isotonic saline solution was rapidly infused over 30–60 seconds; further spectra were then collected every 5 minutes for 1 hour. The slow saline infusion was continued until the end of the study. Of the 25 subjects who underwent fructose challenge test, 16 subjects had FFQ and assessment of UA..
Statistical analysis
This pilot study was conducted as exploratory hypothesis generating research to assess whether hepatic ATP depletion and/or UA levels may be associated with increased dietary fructose consumption and response to intravenous fructose challenge. Due to convenience sampling, this study could not be sufficiently powered to judge significance. We restricted our analyses to the 16 individuals with data on fructose intake, UA and dynamic 31P MRS. We defined “high” fructose consumption as fructose consumption >15 grams per day, a threshold in keeping with dietary fructose intake from vegetables and grains alone (3). For all the analyses we also used tertiles of fructose and fructose as continuous variables. Hyperuricemia was defined as UA > 5.5 mg/dl. Differences in the baseline hepatic ATP (β-ATP/total phosphorus), nadir value of ATP and recovery by fructose consumption (high vs. low) and UA level (hyperuricemia vs. normal) were assessed using nonparametric tests because of the non-normal distribution of 31P MRS data and the small sample size. Differences in other 31P MRS parameters (α-ATP/Pi, β-ATP/Pi, γ-ATP/Pi, PME/Pi, and PDE/Pi) were also evaluated and compared among the groups. All statistical analyses were conducted using STATA 9.2 (College Station, TX) and SAS 9.1 (SAS Institute, Cary, NC) and were not performed at the Look AHEAD data coordinating center. Differences were considered statistically significant when the p-values were less than 0.05. A “borderline” p-value of ≤ 0.06 was considered noteworthy of consideration as a trend toward significance.
RESULTS
Clinical characteristics of the study population
Of those enrolled in the Look AHEAD Fatty Liver Ancillary Study (n=244), 25 subjects had a successful MRS and completed an intravenous fructose challenge test. With the exception of lower BMI and total caloric intake, our study cohort was comparable in age, gender, serum UA, total fructose intake, % liver fat, liver biochemistries, or use of insulin sensitizing agents those Look AHEAD subjects who did not undergo intravenous fructose challenge test (Table 1).
Table 1.
Characteristics of Participants who Completed a Fructose Challenge Test
| INTRAVENOUS FRUCTOSE CHALLENGE TEST |
P-value | ||
|---|---|---|---|
| Not Completed N=219 |
Completed N=25 |
||
| Age (years) | 61.1 (6.1) | 60.3 (7.1) | 0.58 |
| Gender (Female, %) | 52% | 58% | 0.45 |
| Race, (White/Other, %) | 68% | 88% | 0.20 |
| BMI, kg/m2 | 36.3 (6.0) | 32.9 (3.0) | 0.001 |
| Serum uric acid, mg/dl | 5.2 (1.3) | 4.9 (1.2) | 0.76 |
| Total calorie intake, Cal/day | 1950 (1391, 2591) | 1502 (1182, 1771) | 0.01 |
| Fructose g/day | 16.8 (11.4, 24.3) | 17.1 (12.7-24) | 0.64 |
| Liver Outcomes | |||
| Steatosis, % Liver Fat | 5.2 (2.4, 11.6) | 5.8 (3.9, 16.1) | 0.14 |
| ALT | 21 (16, 28) | 23 (18,31) | 0.31 |
| AST | 18 (15, 23) | 22 (18,25) | 0.08 |
| Use of Metformin | 48% | 46% | 0.98 |
| Use of Thiazolidinediones | 31% | 50% | 0.07 |
| Use of Insulin | 16% | 0% | 0.02 |
Abbreviations: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartamine aminotransferase.
NAFLD (defined as >5% hepatic fat by MRS) was noted in 16 of 25 (64%) subjects. Among those with NAFLD the fat content ranged from 5% to 29%. Average fructose consumption in the high vs low fructose group was 22.3 ± 1.95 vs 11.13 ± 1.33 grams/day (p<0.001). The total energy intake in the high vs low fructose group was 1716 ± 242 vs 1497 ± 160 calories per day (p = 0.046). In the study cohort, the serum UA in subjects with high vs low UA was 6.39 ± 0.25 vs 4.35 ± 0.18 mg/dl (p<0.001).
Summary of the results of the ATP fructose challenge test
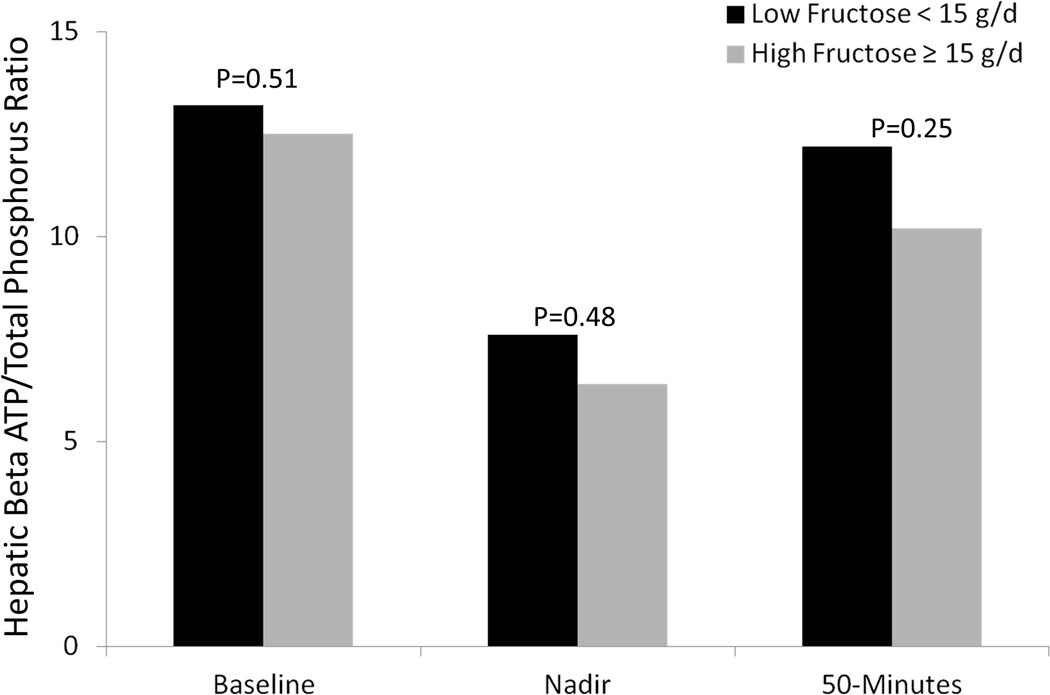
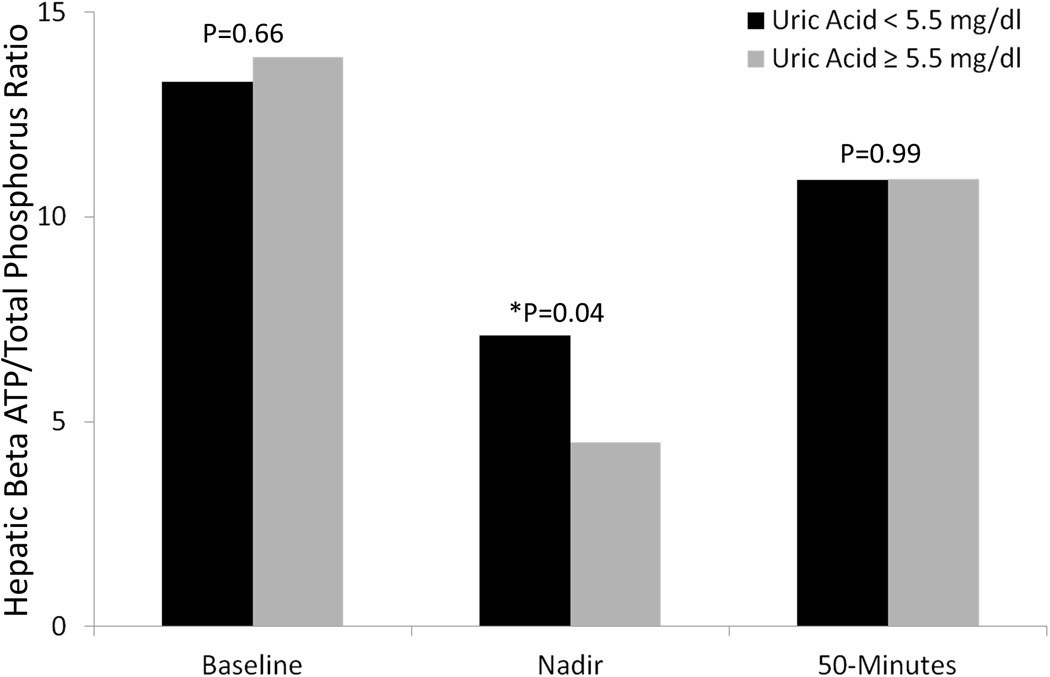
Of the 25 patients who completed the intravenous fructose challenge test, patients with high dietary fructose consumption had lower mean hepatic ATP and ATP/total phosphate ratio at baseline as compared to those who consumed lower amounts of fructose (Figure 1). Patients who consumed higher amounts of fructose also had lower β-ATP/Pi and γ-ATP/Pi at nadir and 50 minutes following the intravenous fructose challenge. Similarly, at baseline, patients with hyperuricemia had lower mean baseline hepatic ATP levels as well as lower ATP levels at the nadir and 50-minutes following intravenous fructose challenge. The mean hepatic ATP/total phosphate ratio in patients with hyperuricemia dropped further than in patients without hyperuricemia (p=0.04) suggesting less hepatic “reserve” (Figure 2), although levels at baseline and at recovery were comparable.
Figure 1.
Response to Intravenous Fructose Challenge, by Fructose Intake
Figure 2.
Response to Intravenous Fructose Challenge, by Uric Acid Level
Changes in 31P-MRS metabolites at baseline and 50 minutes post intravenous fructose challenge as a function of the level of fructose consumption are depicted in Table 2. When compared to baseline ATP levels, patients with high fructose consumption had significant declines in α-ATP and a trend toward a decline in β-ATP post intravenous fructose challenge (p= 0.002 and 0.06 respectively). In contrast, α-ATP and β-ATP did not decline significantly from baseline in low fructose consumers after the acute fructose challenge (p=0.56 and 0.1 respectively). There was a significant difference between high and low fructose consumers following the acute fructose challenge occurred in α-ATP (p=0.05).
Table 2.
P-MRS Metabolites at Baseline and 50 Minutes Post Intravenous Fructose, by Fructose Intake
|
High Fructose (≥15 g/day) |
P value Δ Baseline- 50 minutes In High Fructose Consumers |
Low fructose (<15 g/day) |
P value Δ Baseline- 50 minutes in Low Fructose Consumers |
P value Δ Baseline- 50 minutes High vs Low Fructose Consumers |
|
|---|---|---|---|---|---|
| N=9 | N=7 | ||||
| α-ATP/ total Pi | |||||
| Baseline | 0.30 (0.02) | 0.24 (0.02) | |||
| Nadir 50 min |
0.17 (0.03) 0.22 (0.01) |
0.17 (0.02) 0.21 (0.02) |
|||
| Absolute Change | 0.08 (0.02) | 0.002 | 0.03 (0.02) | 0.1 | 0.05 |
| Percent change | −24.52 (5.3) | −11.87(5.8) | 0.13 | ||
| β-ATP/ total Pi | |||||
| Baseline | 12.5 (0.7) | 13.2 (0.7) | |||
| Nadir 50 min |
6.4 (3.5) 10.2 (1.0) |
7.6 (2.9) 12.2 (1.3) |
|||
| Absolute Change | 2.3 (1.1) | 0.06 | 1.0 (1.6) | 0.56 | 0.48 |
| Percent change | −17.3 (8.5) | −5.3 (13.1) | 0.44 | ||
| γ-ATP/ total Pi | |||||
| Baseline | 0.13( 0.01) | 0.15 (0.004) | |||
| Nadir 50 min |
0.05 (0.02) 0.09 (0.01) |
0.06 (0.2) 0.09 (0.01) |
|||
| Absolute Change | 0.03 (0.01) | 0.05 | 0.06 (0.007) | <0.001 | 0.06 |
| Percent change | −22.61 (10.24) | −40.03 (3.8) | 0.14 | ||
| PME/ total Pi | |||||
| Baseline | 0.11 ( 0.01) | 0.12 (0.01) | |||
| Nadir 50 min |
0.11 (0.01) 0.18 (0.01) |
0.09 (0.04) 0.16 (0.02) |
|||
| Absolute Change | −0.06 (0.02) | 0.01 | −0.04 (0.01) | 0.03 | 0.37 |
| Percent change | 80.86 ( 29.32) | 40.70 (19.39) | 0.30 | ||
| PDE/ total P | |||||
| Baseline | 0.18 (0.01) | 0.15 (0.01) | |||
| Nadir 50 min |
0.12 (0.03) 0.14 (0.02) |
0.11 (0.04) 0.13 (0.02) |
|||
| Absolute Change | 0.04 (0.02) | 0.08 | 0.02 (0.02) | 0.32 | 0.57 |
| Percent change | −20.13 (8.81) | −11.75 (11.01) | 0.56 | ||
Abbreviations: ATP, adenosine triphosphate; Pi, inorganic phosphate; PME, phosphomonoesters; PDE, phosphodiesters.
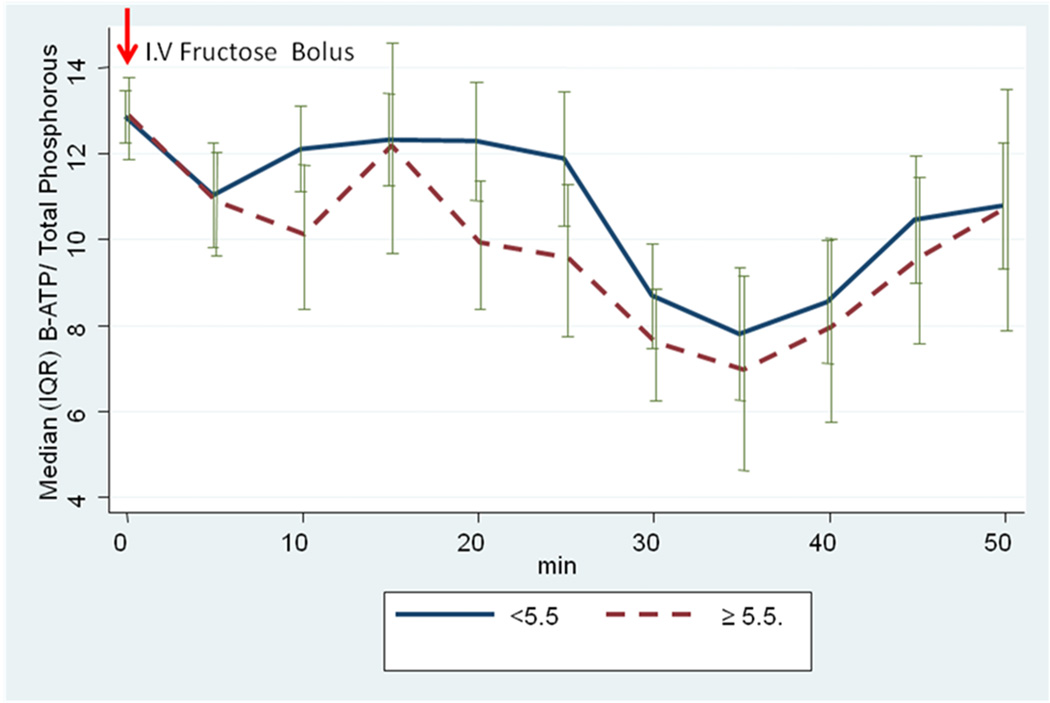
The relationships among changes in 31P-MRS metabolites at baseline and 50 minutes following intravenous fructose challenge and the presence or absence of hyperuricemia are depicted in Table 3. Patients with hyperuricemia had a trend toward a decline in α-ATP compared to patients without baseline hyperuricemia (p=0.06). The median decline in β-ATP/ Pi ratio was lower at nearly all time-points (5–50 minutes) following intravenous fructose challenge in patients with compared to those without baseline hyperuricemia (Figure 3). A statistically significant greater nadir was noted in patients with NAFLD versus without NAFLD (7.15 vs 4.58, p=0.03). No association between hepatic ATP levels and ALT, BMI, or alcohol consumption was observed.
Table 3.
P-MRS Metabolites at Baseline and 50 Minutes Post Intravenous Fructose. by Uric Acid Levels
|
Increased Uric Acid (≥5.5 mg/dl) |
P value Δ Baseline- 50 minutes increased uric acid |
Normal Uric Acid (<5.5 mg/dl) |
P value Δ Baseline- 50 minutes normal uric acid |
P value Mean Δ Baseline- 50 minutes high vs normal |
|
|---|---|---|---|---|---|
| N=8 | N=17 | ||||
| α-ATP/ total Pi | |||||
| Baseline | 0.3 (0.02) | 0.3 (0.01) | |||
| Nadir 50 min |
0.15 (0.03) 0.2 (0.03) |
0.17 (0.03) 0.2 (0.01) |
|||
| Absolute Change | 0.1 (0.02) | 0.004 | 0.04 (0.01) | 0.003 | 0.06 |
| Percent change | −28.9 (6.2) | −14.3 (4.2) | 0.06 | ||
| β-ATP/ total Pi | |||||
| Baseline | 13.9 (1.5) | 13.3 (0.5) | |||
| Nadir 50 min |
4.5 (2.3) 10.92 (1.4) |
7.1 (2.8) 10.9 (0.7) |
|||
| Absolute Change | − 2.9 (1.4) | 0.07 | − 2.4 (0.9) | 0.02 | 0.76 |
| Percent change | −18.5 (9.4) | −16.2 (7.2) | 0.85 | ||
| γ-ATP/ total Pi | |||||
| Baseline | 0.14 (0.01) | 0.14 (0.01) | |||
| Nadir 50 min |
0.04 (0.02) 0.08 (0.01) |
0.06 (0.02) 0.09 (0.01) |
|||
| Absolute Change | 0.06 (0.01) | <0.001 | 0.05 (0.01) | <0.001 | 0.47 |
| Percent change | −43.4 (5.8) | −32.9 (6.4) | 0.31 | ||
| PME/ total Pi | |||||
| Baseline | 0.15 (0.01) | 0.11 (0.01) | |||
| Nadir 50 min |
0.09 (0.03) 0.15 (0.01) |
0.11 (0.02) 0.19 (0.01) |
|||
| Absolute Change | −0.003 (0.02) | 0.86 | −0.07 (0.01) | <0.001 | 0.005 |
| Percent change | 9.91 (15.8) | 81.8 (20.3) | 0.03 | ||
| PDE/ total Pi | |||||
| Baseline | 0.15 (0.01) | 0.18 (0.01) | |||
| Nadir 50 min |
0.13 (0.03) 0.15 (0.01) |
0.11 (0.04) 0.14 (0.01) |
|||
| Absolute Change | 0.0003 (0.01) | 0.97 | 0.04 (0.01) | 0.0094 | 0.09 |
| Percent change | 3.7 (9.1) | −19.5 ( 6.1) | 0.04 | ||
Abbreviations: ATP, adenosine triphosphate; Pi, inorganic phosphate; PME, phosphomonoesters; PDE, phosphodiesters.
Figure 3.
Median (IQR) Changes After I.V. Fructose, by Uric Acid Status
DISCUSSION
Our study shows that fructose intake triggers transient declines in hepatic Pi, α-ATP, β-ATP and γ-ATP, findings consistent with hepatic ATP utilization during the initial phases of fructose metabolism. Interestingly, individuals with obesity and T2DM who habitually consumed increased dietary fructose were more susceptible to hepatic α-ATP and γ-ATP depletion following an acute intravenous fructose bolus than similar patients who consumed less dietary fructose. Thus, fructose provides a metabolic perturbation to the liver which can be utilized to characterize inter-individual differences in hepatic energy homeostasis, variability in disease severity and progression among patients with NAFLD.
We speculate that the association between habitual consumption of high fructose diets and susceptibility to hepatic ATP depletion after an acute fructose challenge may reflect, at least in part, compensatory up-regulation of fructose metabolizing enzymes in high fructose consumers. Hydrolysis of ATP during fructose metabolism generates ADP and AMP. The latter is either rephosphorylated by AMP kinase to regenerate ATP, or further degraded to adenosine and ultimately, UA. UA tends to accumulate when the rate of ATP hydrolysis outstrips its regeneration. Thus, it is particularly interesting that hyperuricemic subjects had lower baseline α-ATP/Pi as well as a greater absolute and percent change from baseline in α-ATP, β-ATP, and γ-ATP following intravenous fructose challenge than those with normal serum UA levels. Together, this data suggest that habitual consumption of high fructose-containing diets provides a metabolic challenge that may impair hepatic energy homeostasis in patients with underlying insulin resistance (IR). Further, increased serum UA may serve as a surrogate serologic marker identifying individuals who are unable to replenish liver ATP stores effectively.
The decrease in absolute levels of hepatic ATP in viral and alcoholic hepatitis (38, 39) and obesity (18, 19) has been interpreted as “energy deficit” or impaired “ATP homeostasis”. Humans with IR and hepatic steatosis also have decreased hepatocellular ATP (40). Even in metabolically well controlled T2DM, hepatic energy metabolism could be impaired when compared to age- and BMI-matched and young lean controls (40). Individuals with T2DM had 26% and 23% lower γ-ATP (1.68 ± 0.11; 2.26 ± 0.20; 2.20 ± 0.09 mmol/L; P < 0.05) than age- and BMI-matched controls and young healthy individuals, respectively. Further, they had 28% and 31% lower Pi than did individuals from the matched control and young healthy control groups (0.96 ± 0.06; 1.33 ± 0.13; 1.41 ± 0.07 mmol/L; P < 0.05). Even after adjustment for hepatic lipid volume fraction, hepatic ATP and Pi related negatively to hepatic insulin sensitivity (r =−0.665, P = 0.010, r =−0.680, P = 0.007) but not to whole-body insulin sensitivity. These data suggest that impaired hepatic energy metabolism and IR could precede the development of steatosis in individuals with T2DM (40).
Likewise, it is conceivable that mitochondrial ATP synthesis might also be decreased in pre-diabetic patients with NAFLD. In support of this contention, patients with NASH exhibit alternations and/or abnormalities of their mitochrondria (41). Impaired energy homeostasis could also result from hepatocellular γ-ATP depletion due to increased ATP utilization by energy-demanding processes such as Na+/K+ adenosine triphosphatases, lipogenesis, or gluconeogenesis. Although loss of functional hepatocytes due to necrosis and replacement with fat and collagen may serve as yet another explanation for hepatic γ-ATP depletion, our study group of subjects with obesity and T2DM lacked overt clinical or laboratory evidence of liver damage. In such subjects, a dietary history of increased fructose consumption correlated with reduced hepatic content of ATP, suggesting that metabolism of fructose may provide a previously unsuspected threat to hepatic energy homeostasis.
An alternative explanation may reflect the impaired ATP generation in response to fructose ingestion that is unique to fructose metabolism. As discussed in the Introduction, fructose is known to induce transient ATP depletion due to its rapid phosphorylation (42). The scavenger enzyme AMP deaminase 2 reclaims additional phosphates from ADP, and in the process generates the waste product UA. Of note, AMP kinase is the master regulator of cellular energy flux in the liver. Under normal physiologic conditions, increased cellular content of AMP activates AMP kinase and result in prompt regeneration of ATP. However, under conditions where AMP kinase activity is low (as may occur in the setting of IR), AMP is deaminated and increased production of UA (as opposed to ATP) is favored. Fructose is also known to upregulate both its main transporter (Glut 5) and its major enzyme (fructokinase) (30). Both fructokinase protein and activity in murine hepatocytes increase following incubation with fructose (6). Furthermore, laboratory rats fed diets high in fructose show an increase in Glut5 in the intestinal epithelium and an increase in fructokinase in their liver compared to controls (30). Likewise, subjects with NAFLD and higher intake of fructose have higher levels of fructokinase mRNA in their liver biopsies compared to control subjects with liver disease (6). Humans given a high fructose diet show a more marked increase in UA in response to fructose (26). These studies suggest that the effects of fructose to upregulate its enzymes could lead to a greater ATP depletion and hyperuricemia in response to fructose. In turn, a more severe ATP depletion could be a mechanism for potentiating cell injury in subjects with NAFLD.
Elevated UA predicted both the baseline and nadir of ATP depletion. This finding, which may be explained by a tight link between generation of UA and ATP depletion in response to fructose administration, suggests that UA may be a biomarker of fructokinase activity levels. The higher UA increase induced by fructose in cirrhotic patients therefore appears to be a good marker the diseased liver’s inability to efficiently resynthesize ATP from its breakdown products (43). Alternatively, the habitual consumption of fructose may not allow for efficient resynthesis of ATP. Regardless, these data could provide an explanation for why an elevated UA may be a predictor for NAFLD. However, we did not find UA as a predictor for more advanced liver disease in our recent study, whereas the amount of fructose intake did correlate with hepatic fibrosis (14). Clearly further studies are needed to better understand the role of UA in NAFLD and the progression of liver disease.
Our study has some limitations. First, our study is an observational, cross sectional study without a true control population and no randomized intervention designed to affect the end points. Second, histology was unavailable for analysis as liver biopsies are not considered ethical in subjects without any evidence of liver disease. Third, this study was not powered to assess clinically significant differences between groups. Despite this limitation, interesting insights regarding the potential mechanism(s) which may underlie fructose-related liver injury were gained. Forth, our study population consisted of patients with known T2DM whom has already received nutritional counseling. Thus, the total fructose and caloric intake were lower in our study population than might have been observed in a general population. Despite this, the striking finding was that we were still able to show a difference in hepatic ATP and baseline and following intravenous fructose challenge in subjects who consumed more fructose. Further, despite this low threshold for defining fructose consumption, differences in UA levels also correlated with the severity of ATP depletion observed in response to fructose.
In conclusion, patients with obesity and T2DM with increased habitual dietary fructose consumption show reduced hepatic ATP concentrations compared to those with minimal dietary fructose intake. These data support our hypothesis that increased dietary fructose consumption may impair hepatocellular energy homeostasis and thus could be a risk factor for progressive liver injury. Further, hyperuricemia may serve as a surrogate marker of hepatic ATP depletion following exposure to fructose in patients with IR, and potentially NAFLD. The presence of hyperuricemia in patients with IR may help clinicians to identify patients “at risk” for cellular injury from fructose and hence may highlight subjects at risk for progression of NAFLD. Impaired hepatic energy homeostasis attributable to increased dietary fructose consumption underscores the urgent and dire need for increased public awareness of the risks associated with high fructose consumption.
Acknowledgments
Funding: The study was supported by NIH/NIDDK grant RO1-DK060427 and UO1-DK57149 and the John Hopkins University School of Medicine General Clinical Research Center M01-RR00052. Dr. Richard Johnson is supported by grant HL-68607. Dr. Manal Abdelmalek is supported by a NIH/NIDDK K23 Career Development Award (K23-DK062116).
Abbreviations
- AMP
adenosine monophosphate
- AMPK
adenosine monophosphate kinase
- ATP
adenosine triphosphate
- BMI
body mass index
- CI
confidence interval
- HDL
high-density lipoprotein
- HFCS
high fructose corn syrup
- HOMA-IR
homeostasis model assessment of insulin resistance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MRS
magnetic resonance spectroscopy
- T2DM
type 2 diabetes mellitus
- UA
uric acid
Footnotes
Potential conflict of interest: R.J.J.: Published a lay book “The Sugar Fix” that discusses the potential role of fructose in obesity and fatty liver and has a patent application on lowering uric acid to reduce fatty liver disease. All other authors have no conflict of interest.
The Fatty Liver Subgroup of the Look AHEAD Research Group includes: Jeanne M. Clark, MD, MPH (PI), Charalett Diggs, RN (PC), Anna Mae Diehl, MD (former PI; now at Duke University), Frederick L. Brancati, MD, MHS, Stephen Crawford, PhD, Susanne Bonekamp, PhD, DVM, Alena Horska, PhD, Mariana Lazo, MD, PhD, ScM, and Steven Solga, MD from The Johns Hopkins University.
Presented at the American Association for the Study of Liver Disease (AASLD) 2009.
Author Conduct
Manal F. Abdelmalek: Generation of research idea, data interpretation, and drafting, review, revision and approval of final manuscript.
Mariana Lazo: Data analysis, data interpretation, drafting and revision of manuscript.
Alena Horska: Generation of data, interpretation of data, revision of manuscript.
Susanna Bonekamp: Generation of research data, interpretation of data, review and revision of manuscript
Edward Lipkin: Generation of data, interpretation of results, critical review and revision of manuscript.
Ashok Balasubramanyam: Generation of data, interpretation of results, critical review and revision of manuscript.
John Bantle: Generation of data, interpretation of results, critical review and revision of manuscript.
Richard Johnson: Data interpretation, provided substantial contribution to scientific interpretation of data, drafting and critical review of the manuscript for important intellectual content.
Anna Mae Diehl: Obtained grant funding for Hopkins Look Ahead Liver Ancillary Study, provided substantial contributions to conception and design, interpretation of data, critical revision of manuscript for important intellectual content, final approval for the version to be published.
Jeanne M. Clark: Generation of data, interpretation of data, drafting, review, revision, critical revision of manuscript for important intellectual content, and final approval for the version to be published.
REFERENCES
- 1.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Vos MBKJ, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 4.Putnam J, Alshouse JE. Food Consumption, Prices and Expenditures. 1970–1997. Washington, DC: Food and Rural Economics Division, Economics Research Service, US Dept of Agriculture; 1999. Statistical Bulletin No. 965. [Google Scholar]
- 5.French SA, Lin BH, Guthrie JF. National trends in soft drink consumption among children and adolescents age 6 to 17 years: prevalence, amounts, and sources, 1977/1978 to 1994/1998. J Am Diet Assoc. 2003;103:1326–1331. doi: 10.1016/s0002-8223(03)01076-9. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Lozada LG, Mu W, Roncal C, Sautin YY, Abdelmalek M, Reungjui S, Le M, et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr. 2010;49:1–9. doi: 10.1007/s00394-009-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 11.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 13.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977;162:601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, Sanchez-Lozada LG, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. Jama. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 19.Nair S, V PC, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003;98:466–470. doi: 10.1111/j.1572-0241.2003.07221.x. [DOI] [PubMed] [Google Scholar]
- 20.Bode JC, Zelder O, Rumpelt HJ, Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973;3:436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 21.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, Lee FY, et al. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131–1138. doi: 10.1002/hep.510290428. [DOI] [PubMed] [Google Scholar]
- 23.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 24.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1311. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol. 2009;50:1029–1034. doi: 10.1016/j.jhep.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Petta S, Camma C, Cabibi D, Di Marco V, Craxi A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 34:757–766. doi: 10.1111/j.1365-2036.2011.04788.x. [DOI] [PubMed] [Google Scholar]
- 29.Afzali A, Weiss NS, Boyko EJ, Ioannou GN. Association between serum uric acid level and chronic liver disease in the United States. Hepatology. 52:578–589. doi: 10.1002/hep.23717. [DOI] [PubMed] [Google Scholar]
- 30.Korieh A, Crouzoulon G. Dietary regulation of fructose metabolism in the intestine and in the liver of the rat. Duration of the effects of a high fructose diet after the return to the standard diet. Arch Int Physiol Biochim Biophys. 1991;99:455–460. [PubMed] [Google Scholar]
- 31.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 32.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 33.Vitolins MZ, Anderson AM, Delahanty L, Raynor H, Miller GD, Mobley C, Reeves R, et al. Action for Health in Diabetes (Look AHEAD) trial: baseline evaluation of selected nutrients and food group intake. J Am Diet Assoc. 2009;109:1367–1375. doi: 10.1016/j.jada.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solga SF, Horska A, Hemker S, Crawford S, Diggs C, Diehl AM, Brancati FL, et al. Hepatic fat and adenosine triphosphate measurement in overweight and obese adults using 1H and 31P magnetic resonance spectroscopy. Liver Int. 2008;28:675–681. doi: 10.1111/j.1478-3231.2008.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabr RE, Ouwerkerk R, Bottomley PA. Quantifying in vivo MR spectra with circles. J Magn Reson. 2006;179:152–163. doi: 10.1016/j.jmr.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chmelik M, Schmid AI, Gruber S, Szendroedi J, Krssak M, Trattnig S, Moser E, et al. Three-dimensional high-resolution magnetic resonance spectroscopic imaging for absolute quantification of 31P metabolites in human liver. Magn Reson Med. 2008;60:796–802. doi: 10.1002/mrm.21762. [DOI] [PubMed] [Google Scholar]
- 37.Gallis JL, Delmas-Beauvieux MC, Biran M, Rousse N, Durand T, Canioni P. Is cellular integrity responsible for the partial NMR invisibility of ATP in isolated ischemic rat liver? NMR Biomed. 1991;4:279–285. doi: 10.1002/nbm.1940040606. [DOI] [PubMed] [Google Scholar]
- 38.Meyerhoff DJ, Boska MD, Thomas AM, Weiner MW. Alcoholic liver disease: quantitative image-guided P-31 MR spectroscopy. Radiology. 1989;173:393–400. doi: 10.1148/radiology.173.2.2798871. [DOI] [PubMed] [Google Scholar]
- 39.Rajanayagam V, Lee RR, Ackerman Z, Bradley WG, Ross BD. Quantitative P-31 MR spectroscopy of the liver in alcoholic cirrhosis. J Magn Reson Imaging. 1992;2:183–190. doi: 10.1002/jmri.1880020211. [DOI] [PubMed] [Google Scholar]
- 40.Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, Moser E, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50:1079–1086. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell SH, de Freitas LA, Park SH, Moreno ML, Redick JA, Davis CA, Sisson BJ, et al. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888–1895. doi: 10.1002/hep.22851. [DOI] [PubMed] [Google Scholar]
- 42.Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]
- 43.Budillon G, Citarella C, Loguercio C, Nardone G, Sicolo P, Del Vecchio Blanco C. Hyperuricemia induced by fructose load in liver cirrhosis. Ital J Gastroenterol. 1992;24:373–377. [PubMed] [Google Scholar]