Abstract
Background
Abnormal cardiac repolarization, indicated by a prolongation of the QT-interval, increases the risk for torsade de pointes, a potentially life-threatening arrhythmia. Many perioperatively administered drugs and conditions prolong the QT-interval. Despite several reports of perioperative torsade de pointes, systematic evidence regarding perioperative QT-interval prolongation is limited.
Methods
Serial postoperative 12-lead electrocardiograms were obtained from 469 adult patients undergoing major non-cardiac surgery under general anesthesia. Heart-rate corrected QT-interval duration (Fridericia’s formula) was the primary outcome. All perioperatively administered drugs were recorded. Emphasis was placed on absolute QTc prolongation >500ms and relative increases of 30 and 60ms.
Results
At the end of surgery, 80% of the patients (345/429) experienced a significant QTc interval prolongation (ΔQTc 23 ± 26ms (mean and SD), 95% CI 20 to 25ms, p<0.0001). Approximately 51% (219/429) had a QTc >440 ms, and 4% (16/429) a QTc >500ms. In 39% (166/429), the ΔQTc was >30ms, in 8% (34/429) >60ms, and in 0.5% (2/429) >100ms. No changes in ΔQTc occurred at subsequent time points. One patient developed torsade de pointes with a ΔQTc: 29ms (0.4% incidence rate). Several drugs had a large effect on ΔQTc: isoflurane; methadone; ketorolac; cefoxitin; zosyn; unasyn; epinephrine; ephedrine and calcium. Postoperative body temperature had a weak negative correlation with ΔQTc (r= −0.15, p=0.02); serum magnesium, potassium and calcium concentrations were not correlated.
Conclusions
Postoperative QT-interval prolongation is common. Several perioperatively administered drugs are associated with a substantial QT-interval prolongation. The exact cause and its clinical relevance are, however, unclear. Nevertheless, an association between postoperative QT-prolongation and risk for torsade de pointes is likely.
Introduction
In the last 30 years more than 40 cases of sometimes fatal perioperative torsade de pointes have been reported in the literature, six of them in the year 2011 alone.1–6 Abnormal cardiac repolarization is a well-known cause for malignant tachyarrhythmias, such as torsade de pointes, which can result in sudden cardiac death.7 Abnormal cardiac repolarization can be identified on the electrocardiogram as a prolonged QT interval (commonly, the heart rate-corrected QT interval [QTc] is reported).8 Typically, a QTc interval < 440ms is considered normal. QT prolongation can either be inherited, such as in the long QT syndrome, acquired, or a combination of the two. Acquired QT prolongation is often caused by drugs; well-known examples are antiarrhythmic drugs (sotalol, flecainide), cisapride and droperidol.9,10 Drug-induced QTc interval prolongation increases the risk for torsade de pointes and subsequent sudden cardiac death, and is often the result of drug-drug interaction or polypharmacy.11,12 Surgical patients under general anesthesia are simultaneously exposed to a multitude of mostly intravenously administered drugs, several of which are known to cause QTc prolongation. Typical drug classes include antibiotics, anti-nausea medications (odansetron or droperidol), inhalational anesthetics, and antihistamines.13–15 In addition, conditions conducive for QTc prolongation such as stress, hypothermia and electrolyte disturbances, particularly hypokalemia and hypomagnesemia, are common during major surgery.
We therefore hypothesized that patients undergoing major surgery under general anesthesia may be particularly vulnerable for acquired QTc prolongation due to the simultaneous exposure to the above mentioned risk factors. Previous research in the perioperative setting has exclusively focused on individual drugs and their effect on QTc prolongation. We aimed to investigate the cumulative effects of these drugs and conditions on acquired QTc prolongation in a cohort of patients undergoing major non-cardiac surgery under general anesthesia from the Vitamins in Nitrous Oxide (VINO) trial.
Materials and Methods
This study is an independent ancillary study to the VINO trial [NCT00655980]. All patients provided written informed consent and the study was approved by the Washington University School of Medicine Institutional Review Board (St. Louis, MO).
Design of the parent trial
The VINO trial has enrolled 625 patients to study the hypothesis that patients with a common gene variant in the folate cycle (MTHFR C677T) develop a higher risk for perioperative myocardial infarction after nitrous oxide anesthesia. The trial has three arms: in the first arm (n=250), patients receive 60% nitrous oxide during surgery and 1 mg vitamin B12 (cyanocobalamin) and 5 mg folic acid IV immediately before and after surgery; patients in arm 2 (n=250) receive 60% nitrous oxide but no B-vitamins, just a saline control, and patients in arm 3 (n=125) receive no nitrous oxide and no B-vitamins. The target patient population is adult patients with or at risk for coronary artery disease (defined as a combination of at least 3 out of 6 risk factors: history of stroke, diabetes, peripheral vascular disease, smoking, hypertension and hyperlipidemia) undergoing major non-cardiac surgery under general anesthesia. Exclusion criteria include contraindications against the use of nitrous oxide, folic acid and cyanocobalamin (vitamin B12). Other than for the interventions listed above, the perioperative and anesthetic regimen was at the discretion of the attending anesthesiologist.
Study Population
The eligible study population for this ancillary study included all patients from the VINO trial, who did not have atrial fibrillation and had analyzable baseline and follow-up electrocardiograms. Our goal was to have 500 evaluable patients for this ancillary study.
Measurements
Per study protocol, we obtained serial 12-lead electrocardiograms on all study patients at the following time points: (1) at baseline (immediately before surgery), (2) within 30 minutes of arrival in the post-anesthesia care unit, (3) on the morning of postoperative day 1 and 2 (although the parent study asked for an electrocardiogram on postoperative day 3, we excluded this time point for the ancillary study as less than 25% of the patients were still hospitalized and no electrocardiogram could be obtained).
To identify potential drug-induced QTc interval prolongation, we recorded all perioperatively administered drugs for all patients from the electronic patient record from preoperative holding to postoperative care unit, as well as all home medications. We did not retrieve medication data from nursing floors. In addition, serum electrolytes (K+, Ca2+, Mg2+) and temperature on admission to the postoperative care unit were recorded.
To assess the incidence of postoperative arrhythmias, and torsade de pointes in particular, we queried the electronic clinical patient database where all abnormal electrocardiogram rhythms that occurred in the postoperative anesthesia care unit were recorded. As standard of care in the postoperative anesthesia care unit, all patients are monitored with a continuous 3 lead electrocardiogram. In addition, we queried all reports from postoperative telemetry which consisted of a continuous 3-lead Holter electrocardiogram monitoring with audible alerts for patients deemed at high risk for cardiovascular complications by the care team. Approximately 52% (242/469) of our study population were on postoperative telemetry for >48 hours.
Statistical Analysis
The primary outcome variable was the change in QTc (corrected QT interval) between baseline and the other time points (ΔQTc). Electrocardiogram measurements were read and analyzed by hand by a single experienced anesthesiologist. QT-interval measurements are typically corrected for heart rate (our study used the Fridericia’s formula [; RR= interval between two QRS complexes]. The Fridericia’s correction is the recommended approach (of the classic formulae).10,16,17 Per Food and Drug Administration-endorsed International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guideline,17 we focused on identifying patients with an abnormal QTc >440ms and >500ms, and relative increases in ΔQTc of >30ms and >60ms. In general a QTc-interval >440ms is considered abnormal. The change in QTcF between baseline and end of surgery was determined by a two-sided paired t-test. The effects of drugs and other variables (e.g., gender) was determined by comparing ΔQTc between patients who received the drug vs. those who did not by unpaired t-test with unequal variances. The fraction of patients whose QTc was prolonged > 30 ms was tabulated for each drug. A one tail (for increasing the % > 30 ms) Fisher Exact test was then performed. A P-value corrected for multiple comparisons was then computed with the bootstrap method (500,000 samples). The effects of continuous variables such as age on ΔQTc was determined by linear correlation using the Pearson’s correlation coefficient. Except, when indicated all reported tests are two-sided and a p-value of <0.05 was considered statistically significant. IBM SPSS 20.0 (IBM, Armonk, NY) and SAS version 9.3 (SAS Institute Inc., Cary, NC) software packages were used for the statistical analysis.
Results
Postoperative QT-interval prolongation
This study was performed in a subset of patients participating in the VINO trial (n=469), and the main patient characteristics are described in table 1. At baseline, the mean QTc (Fridericia-corrected) was 418 ± 27ms; 17% (82/469) had a QTc > 440ms which is considered prolonged and two patients had a QTc >500 ms which indicates a high likelihood of long QT-syndrome. Because we were unable to obtain an electrocardiogram in 40/469 patients (8.5%) at the end of surgery, results are available only for 429 patients. The missing 40 patients had a virtually identical baseline QTc of 418 ± 25 ms.
Table 1.
Patient Characteristics
| Age (years) | 65 ± 10 |
| Male n (%) | 282 (60%) |
| Race | |
| White | 369 (78.7%) |
| Black | 94 (20.0%) |
| Asian | 2 (0.4%) |
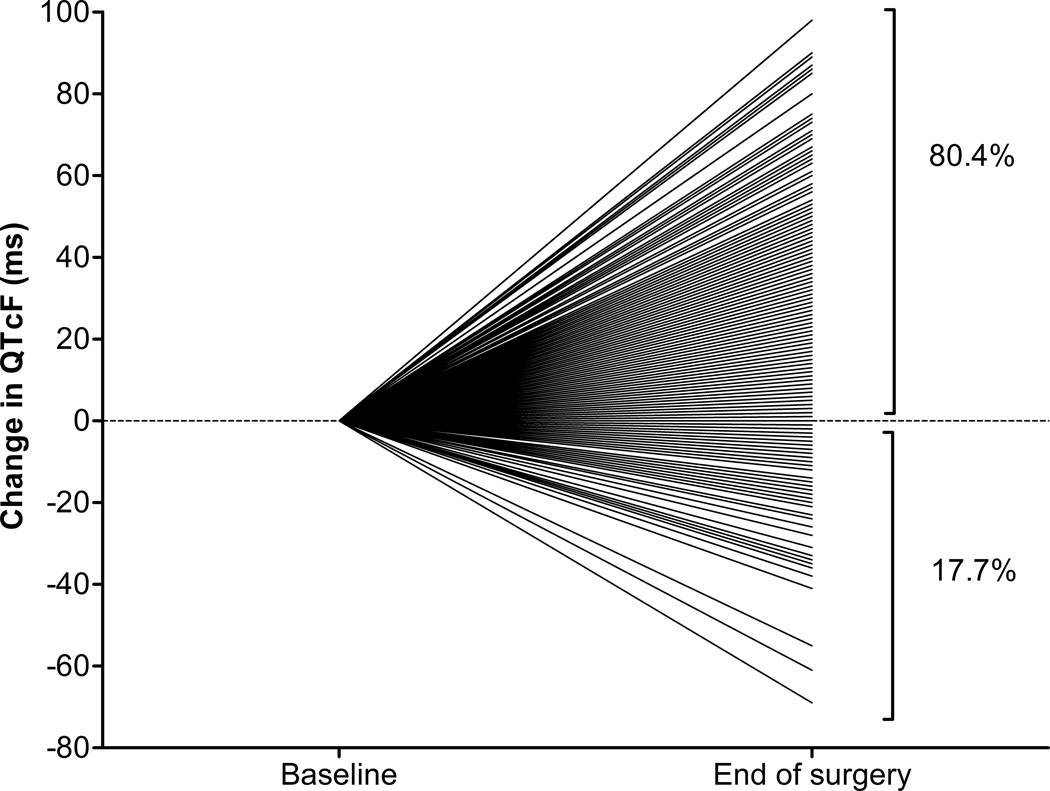
At the end of surgery, measured within 30 minutes of arrival in the postanesthesia care unit, 80% of the patients (345/429) experienced a significant prolongation of their respective QTc interval. The average increase was 23 ± 26ms (mean ± SD; 95% CI 20 to 25ms, p< 0.0001). Two percent (8/429) had no change in QTc and 18% (76/429) had a decrease in QTc-interval length (Figure 1). Approximately 51% (219/429) had a QTc >440 ms, and 4% (16/429) a QTc >500ms. In 39% of the patients (166/429), the QTc-prolongation (ΔQTcF) was >30ms, in 8% (34/429) >60ms, and in 0.5% (2/429) >100ms.
Figure 1. Relative Change in QTc-interval between baseline and end-of-surgery.
80% of the patients experience a prolongation of their respective QTc-interval. QTcF – corrected QTc interval (Fridericia’s formula)
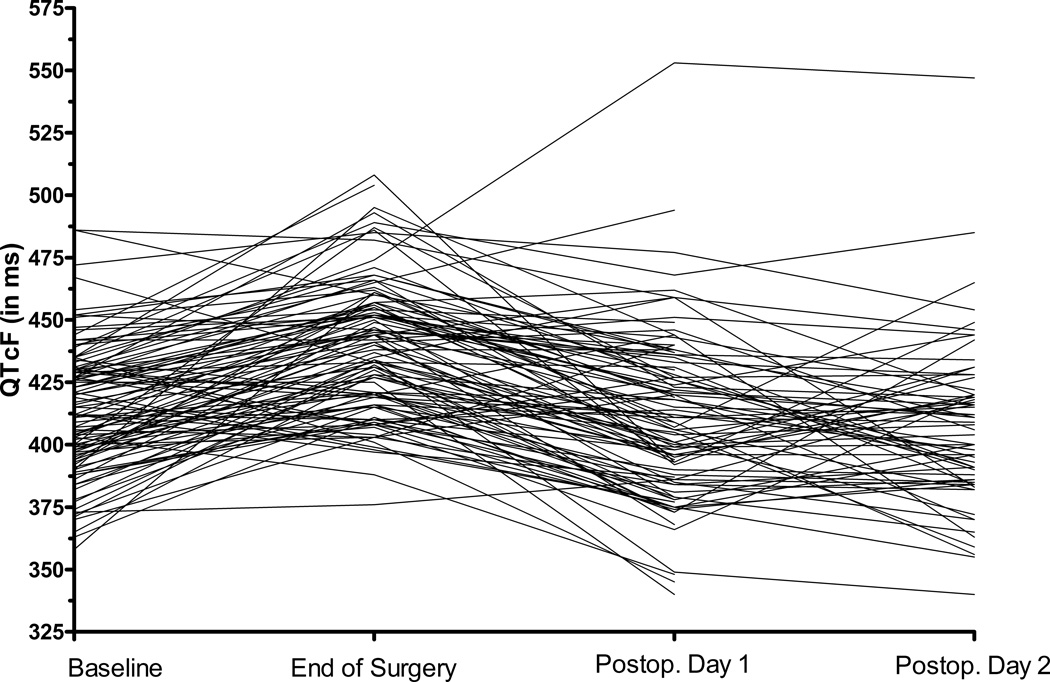
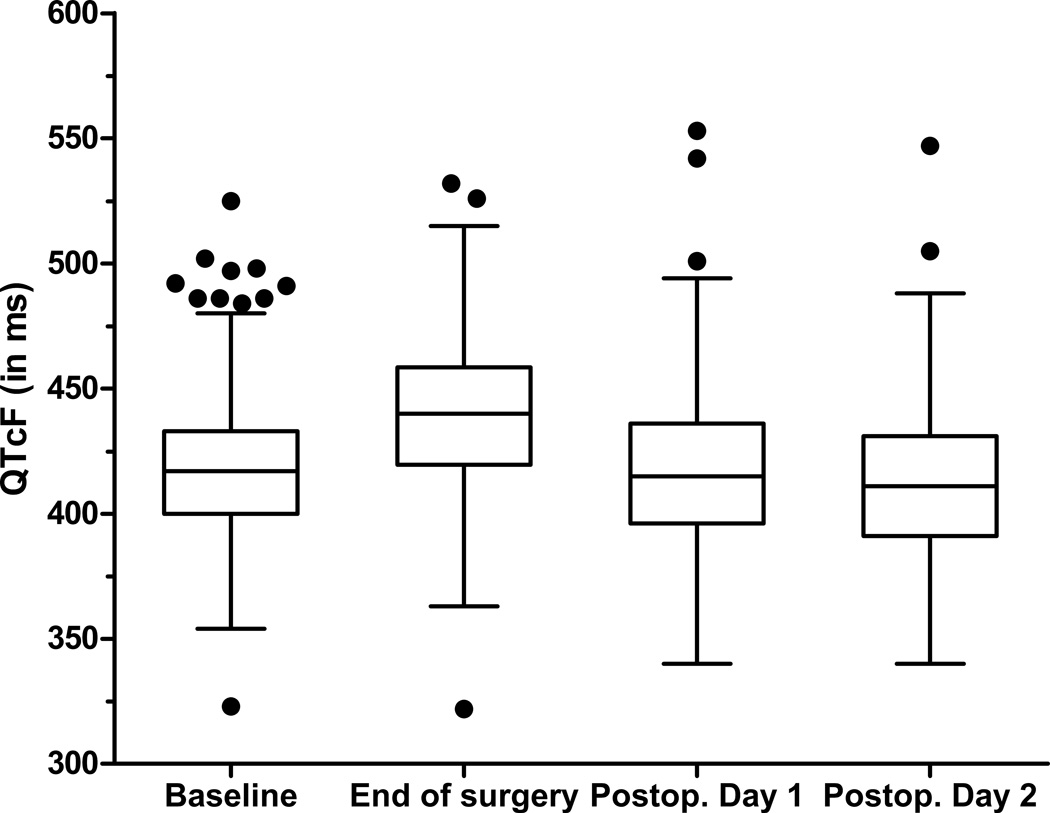
On the subsequent time points, no QTc-interval changes compared to baseline were detected: the mean QTc was 417 ± 30ms on postoperative day 1 and 412 ± 29ms on postoperative day 2 (Figure 2 and 3). To determine if the observed QTc-prolongation may have been influenced by a large change in heart rate, we compared the average heart rate at the four time points. The average heart rate increased steadily from baseline (70 ± 13/min), to end of surgery (76 ± 15/min) and postoperative day 1 (79 ± 13/min) and 2 (81 ± 13/min) while the only statistically significant QTc-prolongation was at the end of surgery.
Figure 2. Spaghetti plot of individual changes in QTc-interval.
QTcF – corrected QTc interval (Fridericia’s formula)
Figure 3. Box plots of postoperative changes in QTc-interval.
QTcF – corrected QTc interval (Fridericia’s formula)
Factors influencing the QTc-interval prolongation
To rule out that the trial intervention (B-vitamins) had an effect on postoperative QT-interval prolongation, we measured the change in QT/QTc in both study arms. B-vitamin treatment had a small, statistically non-significant effect on QTcF: 4 ms (95% CI −1.1 to 8.8ms, p=0.13). Because women are known to have a longer baseline QT-interval and a larger risk for developing drug-induced long QT-syndrome,18,19 we next investigated gender-specific effects on postoperative QTc-prolongation. In our study, women had a minimally larger, statistically non-significant, increase in postoperative QTc-prolongation (4ms, 95% CI −1to 9ms, p=0.11).
Several drugs had a pronounced effect on postoperative QTc-interval prolongation; table 2 lists the effects of each perioperatively administered medication on postoperative QTc-interval duration. Due to the substantial differences in the number of patients who received each drug, the fraction of patients who developed a QTc-prolongation >30ms is presented. Among home medications, 47% of patients who took angiotensin II receptor blockers had a QTc-interval increase of >30 ms. Among anesthesia drugs and analgesics, isoflurane (54%), methadone (53%) and ketorolac (58%) were associated with the most pronounced QTc-prolongation. Several antibiotics were associated with a marked postoperative QTc-interval prolongation: cefoxitin (65%), unasyn [ampicillin and sulbactam] (78%) and zosyn [piperacillin and tazobactam] (56%). Among the cardiovascular drugs, epinephrine had the strongest effect (80% of patients had a QTc-prolongation >30 ms); ephedrine (49%) and calcium (48%). Interestingly, hydralazine and metronidazole appear to be associated with a reduction in QTc-interval. Only 17% and 27%, respectively, of patients receiving these drugs had a QTc-prolongation >30ms.
Table 2.
Factors influencing postoperative QTc-interval prolongation
| Given/Used | % of those on drug with increase > 30 ms |
||||||
|---|---|---|---|---|---|---|---|
| Drug | Mean Change in QTc (ms) |
SD | n (%) | P value for t-test (2-tail) |
% with increase >30 ms |
P value Fisher Exact Test (1-tail) |
Bootstrap P value corrected for multiple comparison |
| Chronic Medications | |||||||
| Clopidogrel | 25.6 | 26.2 | 88 | 0.2301 | 43 | 0.2150 | 0.9999 |
| Heparin | 24.1 | 24.6 | 15 | 0.8366 | 40 | 0.5720 | 1.0000 |
| Warfarin | 18.4 | 23.9 | 36 | 0.3048 | 39 | 0.5658 | 1.0000 |
| Beta-Blocker | 21.9 | 27.0 | 221 | 0.5665 | 41 | 0.1918 | 0.9998 |
| ACE-Inhibitor | 23.9 | 28.8 | 158 | 0.4260 | 44 | 0.0731 | 0.9371 |
| Statin | 22.5 | 26.8 | 234 | 0.9203 | 39 | 0.4582 | 1.0000 |
| Angiotensin-R Blocker | 29.8 | 19.7 | 57 | 0.0196 | 47 | 0.0989 | 0.9823 |
| Nitrate | 24.3 | 26.8 | 36 | 0.6863 | 39 | 0.5772 | 1.0000 |
| Digoxin | 20.8 | 24.3 | 12 | 0.7932 | 42 | 0.5359 | 1.0000 |
| Calcium channel blocker | 21.5 | 26.0 | 125 | 0.5333 | 38 | 0.5877 | 1.0000 |
| Diuretic | 24.5 | 25.0 | 150 | 0.2580 | 41 | 0.3241 | 1.0000 |
| Perioperative Medications | |||||||
| Anesthetics and Anesthesia-related Drugs | |||||||
| Midazolam | 22.7 | 25.5 | 361 | 0.6941 | 39 | 0.4154 | 1.0000 |
| Nitrous Oxide | 23.3 | 25.4 | 351 | 0.2158 | 40 | 0.1722 | 0.9996 |
| Sevoflurane | 22.3 | 28.7 | 141 | 0.8883 | 39 | 0.5039 | 1.0000 |
| Desflurane | 22.2 | 23.9 | 268 | 0.7017 | 38 | 0.7442 | 1.0000 |
| Isoflurane | 29.7 | 26.5 | 26 | 0.1417 | 54 | 0.0779 | 0.9513 |
| Etomidate | 24.8 | 30.4 | 36 | 0.5893 | 44 | 0.2849 | 1.0000 |
| Propofol | 22.4 | 25.2 | 388 | 0.7582 | 39 | 0.5885 | 1.0000 |
| Dexmedetomidine | 25.3 | 22.5 | 3 | 0.8502 | 33 | 0.7706 | 1.0000 |
| Rocuronium | 21.5 | 23.1 | 201 | 0.4227 | 35 | 0.9260 | 1.0000 |
| Succinylcholine | 22.1 | 21.3 | 114 | 0.8388 | 40 | 0.3764 | 1.0000 |
| Vecuronium | 23.4 | 29.1 | 174 | 0.5695 | 43 | 0.2608 | 1.0000 |
| Neostigmine | 22.2 | 25.5 | 282 | 0.6861 | 37 | 0.8796 | 1.0000 |
| Antihistamines and H2-Blockers | |||||||
| Famotidine | 25.1 | 20.7 | 41 | 0.5066 | 34 | 0.7861 | 1.0000 |
| Cimetidine | 26.4 | 8.7 | 8 | 0.6704 | 38 | 0.6586 | 1.0000 |
| Dimenhydrinate | 31.8 | 26.9 | 8 | 0.3075 | 38 | 0.6586 | 1.0000 |
| Diphenhydramine | 24.4 | 17.2 | 8 | 0.8381 | 38 | 0.6586 | 1.0000 |
| Analgesics | |||||||
| Fentanyl | 22.9 | 25.5 | 390 | 0.3956 | 40 | 0.1065 | 0.9866 |
| Morphine | 24.2 | 24.2 | 109 | 0.4278 | 40 | 0.3802 | 1.0000 |
| Hydromorphone | 18.8 | 23.3 | 170 | 0.0161 | 35 | 0.9303 | 1.0000 |
| Methadone | 30.7 | 28.8 | 15 | 0.2098 | 53 | 0.1794 | 0.9997 |
| Meperidine | 24.4 | 22.7 | 13 | 0.7922 | 31 | 0.8101 | 1.0000 |
| Ketorolac | 28.0 | 28.0 | 26 | 0.2645 | 58 | 0.0340 | 0.7045 |
| Acetaminophen | 20.2 | 27.3 | 11 | 0.7603 | 36 | 0.6746 | 1.0000 |
| Antiemetics | |||||||
| Ondansetron | 23.0 | 25.5 | 299 | 0.5786 | 38 | 0.6030 | 1.0000 |
| Droperdol | 23.2 | 26.5 | 13 | 0.9205 | 38 | 0.6123 | 1.0000 |
| Antibiotics | |||||||
| Cefazolin | 21.5 | 25.4 | 306 | 0.2111 | 36 | 0.9579 | 1.0000 |
| Cefoxitin | 33.3 | 21.2 | 17 | 0.0788 | 65 | 0.0245 | 0.6110 |
| Ciprofloxacin | 28.8 | 27.3 | 12 | 0.3906 | 42 | 0.5253 | 1.0000 |
| Metronidazole | 17.3 | 22.4 | 15 | 0.4276 | 27 | 0.8959 | 1.0000 |
| Vancomycin | 19.9 | 28.8 | 83 | 0.2936 | 33 | 0.9217 | 1.0000 |
| Zosyn | 30.8 | 24.6 | 9 | 0.3324 | 56 | 0.2378 | 1.0000 |
| Clindamycin | 21.4 | 17.9 | 13 | 0.8713 | 46 | 0.3861 | 1.0000 |
| Unasyn | 36.0 | 13.7 | 9 | 0.1131 | 78 | 0.0195 | 0.4950 |
| Cardiovascular Drugs | |||||||
| Calcium | 26.8 | 25.8 | 58 | 0.1738 | 48 | 0.0723 | 0.9358 |
| Potassium | 22.8 | 27.1 | 16 | 0.9640 | 38 | 0.6343 | 1.0000 |
| Metoprolol | 21.6 | 24.2 | 38 | 0.8075 | 42 | 0.3869 | 1.0000 |
| Hydralazine | 12.1 | 22.4 | 12 | 0.1548 | 17 | 0.9773 | 1.0000 |
| Labetalol | 24.5 | 24.9 | 36 | 0.5572 | 47 | 0.1787 | 0.9997 |
| Glycopyrrolate | 23.1 | 25.4 | 323 | 0.3957 | 38 | 0.6348 | 1.0000 |
| Epinephrine | 55.2 | 32.7 | 5 | 0.0042 | 80 | 0.0762 | 0.9468 |
| Ephedrine | 28.0 | 27.3 | 138 | 0.0022 | 49 | 0.0015 | 0.0468 |
| Phenylephrine | 22.6 | 25.0 | 274 | 0.9576 | 39 | 0.3811 | 1.0000 |
| Other | |||||||
| Metoclopramide | 29.7 | 17.8 | 28 | 0.1290 | 43 | 0.3902 | 1.0000 |
| Albuterol | 23.9 | 20.3 | 27 | 0.7712 | 37 | 0.6458 | 1.0000 |
| Lidocaine | 21.9 | 26.0 | 355 | 0.2404 | 37 | 0.8986 | 1.0000 |
| Protamine | 22.5 | 24.5 | 42 | 0.9943 | 31 | 0.8959 | 1.0000 |
| Dexamethasone | 26.3 | 25.6 | 78 | 0.1551 | 43 | 0.1965 | 0.9999 |
| Non Pharmaceuticals | |||||||
| Gender - female | 25.0 | 25.5 | 164 | 0.1172 | 43 | ||
| B-Vitamins | 24.7 | 26.6 | 189 | 0.1225 | 46 | ||
ACE – angiotensin converting enzyme
Postoperative body temperature had a weak negative correlation with postoperative QTc-interval prolongation (Pearson’s r= −0.15, p=0.02); serum magnesium, potassium and calcium concentrations were not correlated.
Postoperative torsade de pointes and ventricular arrhythmias
For 243 out 469 patients (52%) postoperative telemetry data were available. One patient developed torsade de pointes on postoperative day 1; his QTcF was prolonged by 29ms (from 439ms to 468ms). The observed incidence rate for postoperative torsade de pointes was 0.4% (1/242). Non-sustained monomorphic ventricular tachycardia (non-torsade; < 30 seconds duration) occurred in 11/242 patients (incidence rate: 5%); all were self-terminated. Ventricular tachycardia was not associated with QTcF-prolongation: the mean change in QTcF compared to baseline at the time of the event was −12ms. Premature ventricular contractions occurred in 27/242 patients (incidence rate 11%). The mean change in QTcF at the time of the event was 15ms, suggesting a moderate association with QTc-interval prolongation.
Discussion
The goal of the study was to investigate postoperative QTc-interval prolongation in a large cohort of adult patients undergoing non-cardiac surgery. Our study confirmed the hypothesis that the majority of patients experience a marked QTc-interval prolongation postoperatively. The average increase in QTc was 23ms, but a large number of patients experienced a much longer QTc-prolongation with some patients exceeding an increase of 60ms or an absolute QTc >500ms. Interestingly, the observed QTc-prolongation was only present during the stay in the postoperative anesthesia care unit but not on the following postoperative days.
What is the likely cause for the observed QTc-prolongation? We would like to point out that the granularity of our study design does not allow us to draw definitive conclusions. Despite having all administered drug data, patients in our study had serial electrocardiograms, but not a continuous Holter-electrocardiogram monitoring, which would have allowed for a much more detailed investigation of QTc-interval dispersion. Given these constraints, we nevertheless believe the cause for the observed QTc-interval prolongation is a combination of several influencing factors. Cardiac repolarization, as indicated by the duration of the QT/QTc-interval, can be prolonged due to inherited or acquired factors or a combination thereof. In fact, between 5–20% of all patients who develop drug-induced torsade de pointes have subclinical (inherited) long-QT syndrome.20,21 Since the vast majority of patients in our study experienced a QT-interval prolongation and the prevalence of inherited long QT-syndrome is low, it is very likely that the cause for the observed QTc-interval prolongation was acquired. Given the clear evidence that several drugs were associated with a statistically significant QTc-prolongation in our study, drug-induced QT-prolongation appears to be a major contributor to the observed QTc-prolongation. Because individual drugs mostly showed a median QTc-prolonging effect of <10ms and the average observed postoperative QTc-interval prolongation was 23ms, drug-drug interactions and cumulative effect of several drugswere likely contributing to the postoperative QTc-interval prolongation.
However, surgical stress – a concept difficult to quantify and measure – may also have been an important contributor. The observed large effects of epinephrine on QTc-interval duration would be consistent with this notion; however the median heart rate was only slightly elevated in the postoperative period and showed no correlation with the QTc-interval prolongation. Moreover, it cannot be ruled out that the stress during anesthesia emergence resulting from extubation, neuromuscular reversal, and pain had a marked effect on postoperative QTc-interval.
Our study identified several drugs that had a pronounced effect on the QTc-interval. Many of them, such as several antibiotics, and methadone have long been known to affect QTc-duration. What was surprising was that neither ondansetron nor droperidol were associated with postoperative QTc-interval prolongation. Both drugs have been shown to cause QTc-interval prolongation in the perioperative setting,13,22 and droperidol even received a black box warning from the Food and Drug Administration. However, other studies have found no or little effect of droperidol on QTc-interval duration,23,24 so the overall strength of evidence is unclear. It should also be pointed out that when corrected for multiple comparisons, nearly all p-values became non-significant. This part of the analysis should be interpreted with caution and as exploratory.
It is important to emphasize that QTc-interval prolongation is only an intermediate outcome measure that is associated with, but does not cause, torsade de pointes. Torsade de pointes is a unique, potentially catastrophic tachyarrhythmia that is caused by an abnormal cardiac repolarization and thought to be triggered by a premature ventricular contraction.9,11 The occurrence of torsade de pointes is probabilistic and correlated with the duration of the QT/QTc-interval.25 Each 10ms increase in QTc-interval duration exponentially increases the risk for developing torsade de pointes by 5–7%.26,27 Data from congenital long QT-syndrome show that a QTc-interval duration of >500ms increases the risk for torsade de pointes by 2–3-fold.28 Perioperative torsade de pointes is a rare event and as of 2011, 37 cases have been reported in the literature. The fact that one of our study patients, who had a QTc-interval prolongation from 439ms to 468ms (29ms), developed torsade de pointes may be a mere coincidence or an indicator that perioperative torsade de pointes are more common than previously assumed and substantially underreported. The fact that in contrast to other tachyarrhythmias such as ventricular tachycardia or ventricular fibrillation most instances of torsade de pointes are self-limited may contribute to underreporting.
In a recent scientific statement from the American Heart Association and the American College of Cardiology titled “Prevention of Torsade de Pointes in Hospital Settings” the authors raise several important points.25 First, they convincingly point out that hospitalized patients are at higher risk for torsade de pointes. One of the major causes for the increased risk is polypharmacy or drug-drug interactions which was also shown in a recent report from an intensive care unit setting.12 Consistent with this observation is recent evidence from a well conducted prospective study in the intensive care unit that found a high incidence of QT-interval prolongation and torsade de pointes.29 Second, the scientific statement points out that increased vigilance, particularly for high-risk patients, can potentially identify patients at increased risk for QT-prolongation and torsade de pointes as classic premonitory signs often precede the initiation of torsade de pointes (e.g., short-long-short sequence of R-R intervals).
The magnitude of the observed postoperative QTc-prolongation in our study was substantial (23ms). In comparison, during new drug development the current international guidelines target drug-induced QTc-interval prolongations of 5ms in so called thorough QT studies. Drugs that prolong the QTc-interval by more than 5ms are often removed from further development.16,17,30
This study had several potential limitations. First, the study was an ancillary study to a clinical trial and we cannot rule out that the study intervention (B-vitamins and nitrous oxide) had measurable effects on the findings, despite being statistically non-significant in the statistical analysis. Second, in our study patients had serial 12-lead electrocardiograms but not a continuous Holter electrocardiogram monitoring which would have allowed us to determine the full range of QTc-interval dispersion, particularly during surgery. Using a spot electrocardiogram on arrival in the postanesthesia care unit is somewhat random and drugs that were given shortly before the electrocardiogram measurement or clinical events may have had a larger effect on the observed change in QTc-interval. Furthermore, this study setup limited our ability to measure the effects of short-acting drugs including anesthetic agents whose short half-life would have eliminated most drug effects on QTc by the time the patient arrived in the postanesthesia care unit. It is therefore impossible to draw any conclusions regarding the effects of short-acting intraoperatively administered drugs on QTc-interval prolongation. Third, our sample size was robust enough to determine the overall effects on QTc-interval duration, but too small to allow for a robust multivariate analysis of all administered drugs. With more than 60 drugs and several additional covariates, a multivariate analysis would have probably resulted in many false negatives and become inconclusive.
In summary, our study shows that postoperative QTc-interval prolongation is common. Several perioperatively administered drugs are associated with a substantial QT-interval prolongation. Drug-drug interactions appear to be a major contributing factor to postoperative QTc-prolongation. The exact cause of postoperative QTc-prolongation and its clinical relevance, however, are unclear. Nevertheless, an association between postoperative QTc-prolongation and risk for torsade de pointes is likely. It therefore seems prudent to increase the vigilance for perioperative QTc-prolongation. Inexpensive measures may include the assessment of the preoperative baseline QTc-interval duration, the display of the QTc-interval duration on vital sign monitors and the avoidance of potentially dangerous drug-drug interactions.
Final Box Summary.
What we already know about this topic
Many commonly used drugs in perioperative medicine can prolong QT interval, although a large study examining incidence of QT interval prolongation postoperatively has not been performed
What this article tells us that is new
In a prospective study of nearly 500 non-cardiac surgery patients, minor prolongation of QT interval was common, with a 4% incidence of marked prolongation (QTc > 500 msec), and there was one case of torsades de pointes with modest QT prolongation.
QT prolongation was associated with multiple drugs, including opioids, general anesthetics, antibiotics, and cardio-active drugs.
Acknowledgments
The study was supported, in parts, by grants from the National Institutes of Health, Bethesda, MD (NIHK23 GM087534 to PN and UL1RR024992 to Washington University Institute of Clinical and Translational Sciences), the Foundation for Anesthesia Education and Research (FAER), and the Division of Clinical and Translational Research, Department of Anesthesiology, Washington University. Dr. Nagele reports receiving research support from Roche Diagnostics (Indianapolis, IN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Heide K, de Haes A, Wietasch GJ, Wiesfeld AC, Hendriks HG. Torsades de pointes during laparoscopic adrenalectomy of a pheochromocytoma: A case report. J Med Case Rep. 2011;5:368. doi: 10.1186/1752-1947-5-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tajiri O, Ito H, Yago Y, Masumori Y. [Torsade de pointes (TdP) observed during general anesthesia for cerebral aneurysm clipping in a patient with QT prolongation] Masui. 2011;60:1090–1093. [PubMed] [Google Scholar]
- 3.Tacken MC, Bracke FA, Van Zundert AA. Torsade de pointes during sevoflurane anesthesia and fluconazole infusion in a patient with long QT syndrome. A case report. Acta Anaesthesiol Belg. 2011;62:105–108. [PubMed] [Google Scholar]
- 4.Mandal B, Kaur G, Batra YK, Mahajan S. Manifestation of Long QT syndrome with normal QTc interval under anesthesia: A case report. Paediatr Anaesth. 2011;21:1265–1267. doi: 10.1111/j.1460-9592.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Lee JH, An EH, Song JG, Park PH. Postanesthetic torsade de pointes in a patient with unrecognized long QT syndrome: A case report. Korean J Anesthesiol. 2011;60:294–297. doi: 10.4097/kjae.2011.60.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaguchi E, Kawano H, Kawahito S, Kitahata H, Oshita S. [Torsade de pointes associated with severe bradycardia after induction of general anesthesia] Masui. 2011;60:1097–1100. [PubMed] [Google Scholar]
- 7.Roden DM. Long-QT Syndrome. N Engl J Med. 2008;358:169–176. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- 8.Pollard CE, Abi Gerges N, Bridgland-Taylor MH, Easter A, Hammond TG, Valentin JP. An introduction to QT interval prolongation and non-clinical approaches to assessing and reducing risk. Br J Pharmacol. 2010;159:12–21. doi: 10.1111/j.1476-5381.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden DM. Drug-Induced Prolongation of the QT Interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 10.Kannankeril P, Roden DM, Darbar D. Drug-Induced Long QT Syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heist EK, Ruskin JN. Drug-Induced Arrhythmia. Circulation. 2010;122:1426–1435. doi: 10.1161/CIRCULATIONAHA.109.894725. [DOI] [PubMed] [Google Scholar]
- 12.Smithburger PL, Seybert AL, Armahizer MJ, Kane-Gill SL. QT prolongation in the intensive care unit: Commonly used medications and the impact of drug–drug interactions. Expert Opin Drug Safety. 2010;9:699–712. doi: 10.1517/14740331003739188. [DOI] [PubMed] [Google Scholar]
- 13.Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–1100. doi: 10.1097/00000542-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Han DW, Park K, Jang SB, Kern SE. Modeling the effect of sevoflurane on corrected QT prolongation: A pharmacodynamic analysis. Anesthesiology. 2010;113:806–811. doi: 10.1097/ALN.0b013e3181f26d34. [DOI] [PubMed] [Google Scholar]
- 15.Curry TB, Gaver R, White RD. Acquired long QT syndrome and elective anesthesia in children. Paediatr Anaesth. 2006;16:471–478. doi: 10.1111/j.1460-9592.2005.01746.x. [DOI] [PubMed] [Google Scholar]
- 16.Darpo B, Nebout T, Sager PT. Clinical Evaluation of QT/QTc Prolongation and Proarrhythmic Potential for Nonantiarrhythmic Drugs: The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 Guideline. J Clin Pharmacol. 2006;46:498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, Food and Drug Administration: E14 Clinical Evaluation of QT/QTc. Interval Prolongation and. Proarrhythmic Potential for. Non-Antiarrhythmic Drugs. 2005 [Google Scholar]
- 18.Drici M-D, Clément N. Is Gender a Risk Factor for Adverse Drug Reactions?: The Example of Drug-Induced Long QT Syndrome. Drug Safety. 2001;24:575–585. doi: 10.2165/00002018-200124080-00002. [DOI] [PubMed] [Google Scholar]
- 19.Abi-Gerges N, Philp K, Pollard C, Wakefield I, Hammond TG, Valentin J-P. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol. 2004;18:139–151. doi: 10.1111/j.1472-8206.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- 20.Lehtonen A, Fodstad H, Laitinen-Forsblom P, Toivonen L, Kontula K, Swan H. Further evidence of inherited long QT syndrome gene mutations in antiarrhythmic drug–associated torsades de pointes. Heart Rhythm. 2007;4:603–607. doi: 10.1016/j.hrthm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, Hohnloser SH, Shimizu W, Schwartz PJ, Stanton M, Murray KT, Norris K, George AL, Roden DM. Allelic Variants in Long-QT Disease Genes in Patients With Drug-Associated Torsades de Pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 22.Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation: A clinical drug interaction study. Anesthesiology. 2008;109:206–212. doi: 10.1097/ALN.0b013e31817fd8c8. [DOI] [PubMed] [Google Scholar]
- 23.Nuttall GA, Eckerman KM, Jacob KA, Pawlaski EM, Wigersma SK, Marienau ME, Oliver WC, Narr BJ, Ackerman MJ. Does low-dose droperidol administration increase the risk of drug-induced QT prolongation and torsade de pointes in the general surgical population? Anesthesiology. 2007;107:531–536. doi: 10.1097/01.anes.0000281893.39781.64. [DOI] [PubMed] [Google Scholar]
- 24.White PF, Song D, Abrao J, Klein KW, Navarette B. Effect of low-dose droperidol on the QT interval during and after general anesthesia: A placebo-controlled study. Anesthesiology. 2005;102:1101–1105. doi: 10.1097/00000542-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W. Prevention of Torsade de Pointes in Hospital Settings: A Scientific Statement From the American Heart Association and the American College of Cardiology Foundation Endorsed by the American Association of Critical-Care Nurses and the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A., Jr The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 27.Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, Benhorin J, Locati EH, Towbin JA, Keating MT, Lehmann MH, Hall WJ. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 28.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 29.Pickham DP, Helfenbein EMS, Shinn JAMA, Chan GP, Funk MP, Weinacker AMD, Liu J-NMS, Drew BJP. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: Results of the QT in Practice (QTIP) Study. Crit Care Med. 2011;40:394–399. doi: 10.1097/CCM.0b013e318232db4a. [DOI] [PubMed] [Google Scholar]
- 30.Chapel S, Hutmacher MM, Bockbrader H, de Greef R, Lalonde RL. Comparison of QTc Data Analysis Methods Recommended by the ICH E14 Guidance and Exposure-Response Analysis: Case Study of a Thorough QT Study of Asenapine. Clin Pharmacol Ther. 2011;89:75–80. doi: 10.1038/clpt.2010.220. [DOI] [PubMed] [Google Scholar]