Abstract
To determine whether adiponectin may have synergistic effects in combination with the proinflammatory cytokine interleukin (IL)-1β regarding the production of proinflammatory mediators during arthritic joint inflammation, synovial cells from rheumatoid arthritis (RA) patients were treated with adiponectin, IL-1β, and their combination for 24 h. Culture supernatant was collected and analyzed by enzyme-linked immunosorbent assay for levels of IL-6, IL-8, prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs). Adiponectin-mediated intracellular signaling pathways were investigated to elucidate the molecular mechanisms underlying their synergy. The association of proinflammatory mediators with adiponectin was investigated in the synovial fluid of arthritis patients. Adiponectin functioned synergistically with IL-1β to activate IL-6, IL-8, and PGE2 expression in RA fibroblast-like synoviocytes; Levels of VEGF, MMP-1, and MMP-13 were not synergistically stimulated. Adiponectin and IL-1β each increased the expression of both adiponectin receptor 1 and IL-1 receptor 1. However, adiponectin and IL-1β did not synergistically support the degradation of IκB-α or the nuclear translocation of NF-κB. Synergistically increased gene expression was significantly inhibited by MG132, an NF-κB inhibitor. Supporting the in vitro results, IL-6 and IL-8 levels were positively associated with adiponectin in synovial joint fluid from patients with RA, but not osteoarthritis (OA). In conclusion, adiponectin and IL-1β may synergistically stimulate the production of proinflammatory mediators through unknown signaling pathways during arthritic joint inflammation. Adiponectin may be more important to the pathogenesis of RA than previously thought.
Keywords: adiponectin, interleukin-1β, obesity, osteoarthritis, rheumatoid arthritis, synovial fluid, synovial membrane
Introduction
Adipose tissue, which once was simply recognized as a lipid storage and release depot, is now considered an endocrine tissue (Ronti et al., 2006; Halberg et al., 2008). This tissue secretes various substances (known as adipokines), including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, leptin, adiponectin, resistin, visfatin, omenetin, and many others (Matsuzawa et al., 1999; Henry and Clarke, 2008). Much attention has been paid to the role of adiponectin in the past 10 yr because of its relationship with insulin sensitivity, glucose, and lipid metabolism. In addition, adiponectin, which circulates at relatively high levels in the bloodstream, exhibits potent anti-inflammatory (Wulster-Radcliffe et al., 2004), atheroprotective (Matsuda et al., 2002), and antidiabetic effects (Maeda et al., 2002). Adiponectin has also demonstrated beneficial effects against the development of obesity-related vascular diseases (Giannessi et al., 2007), and stimulates angiogenesis (Ribatti et al., 2007).
Recently, research interests have been devoted to elucidating the role of adiponectin in the pathophysiology of arthritis. Adiponectin may be involved in the pathogenesis of rheumatoid arthritis (RA) by stimulating the expression of IL-6, IL-8, matrix metalloproteinase (MMP)-1, and MMP-13 in RA fibroblast-like synoviocytes (FLSs) (Ehling et al., 2006; Choi et al., 2009; Kitahara et al., 2009). However, adiponectin has been suggested to have anti-inflammatory effects in the context of arthritis. It has been shown to significantly inhibit IL-1β-stimulated synovial cell proliferation in collagen-induced arthritic mice (Lee et al., 2008), and high-grade inflammation in RA patients has been negatively correlated with circulating adiponectin concentrations (Gonzalez-Gay et al., 2008). Furthermore, adiponectin has been negatively associated with leukocyte count in RA synovial fluid (Senolt et al., 2006). The controversy regarding adiponectin's biological role may be explained by adiponectin isoform-specific responses. Although there are three types of adiponectin (low molecular weight [LMW] trimeric form, middle molecular weight [MMW] exameric form, and high molecular weight [HMW] multimer), it circulates mainly as a LMW hexamer, which displays anti-inflammatory properties, and an HMW multimer, which may be responsible for proinflammatory effects (Neumeier et al., 2006).
IL-1β is known to strongly stimulate various proinflammatory mediators and MMPs, which bring about joint destruction, in various types of cells (Dayer et al., 1986; Kay and Calabrese, 2004). In particular, it greatly stimulates the production of cytokines that perpetuate inflammation and proteases that contribute to cartilage destruction in FLSs. Therefore, IL-1β is regarded as a key effector in rheumatoid arthritis (Bartok and Firestein, 2010). Meanwhile, both IL-1β and adiponectin levels have been shown to be elevated in the synovial fluid and sera of patients with RA (Symons et al., 1989; Schaffler et al., 2003; Otero et al., 2006).
In this study, to better understand the role of adiponectin in arthritis, we questioned whether adiponectin and IL-1β act synergistically in the production of proinflammatory mediators (IL-6, IL-8, and prostaglandin E2 [PGE2]), VEGF, and MMPs in RA FLSs.
Results
Synergistic effect of adiponectin on the production of IL-6, IL-8, and PGE2 in IL-1β-stimulated RA FLSs
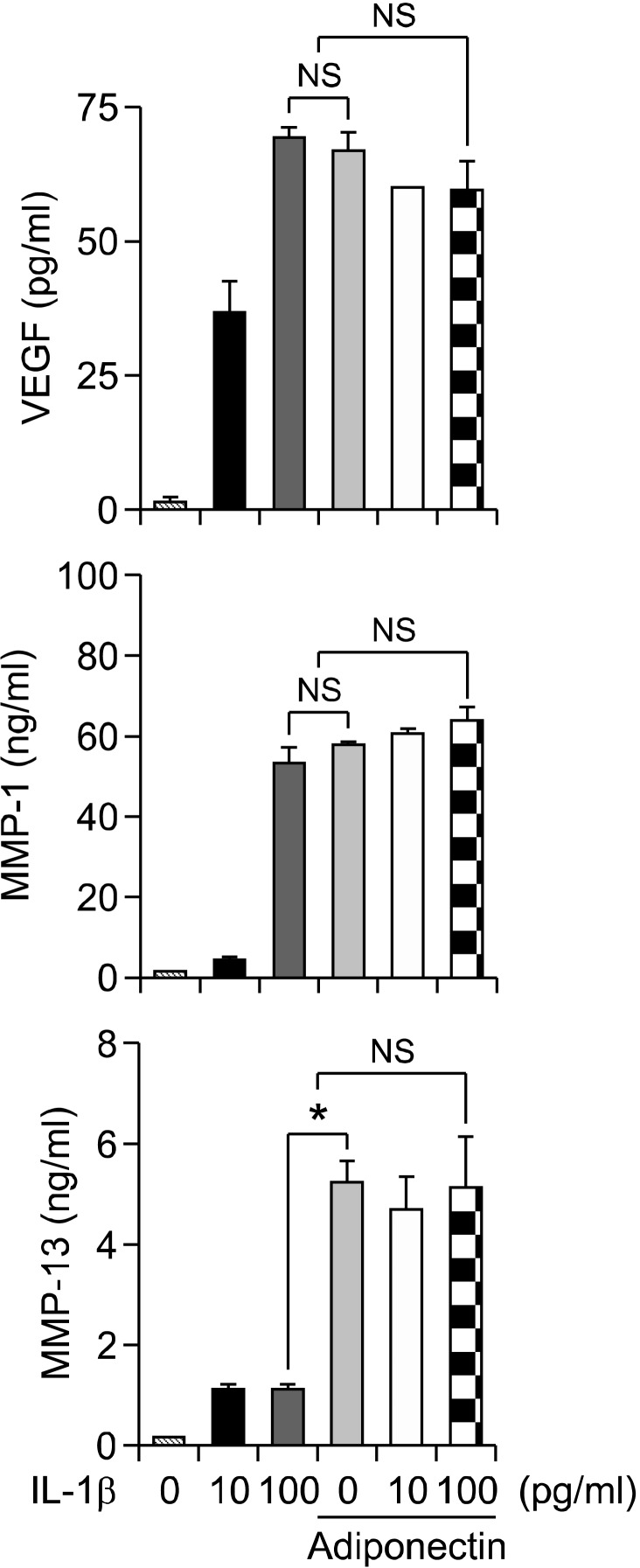
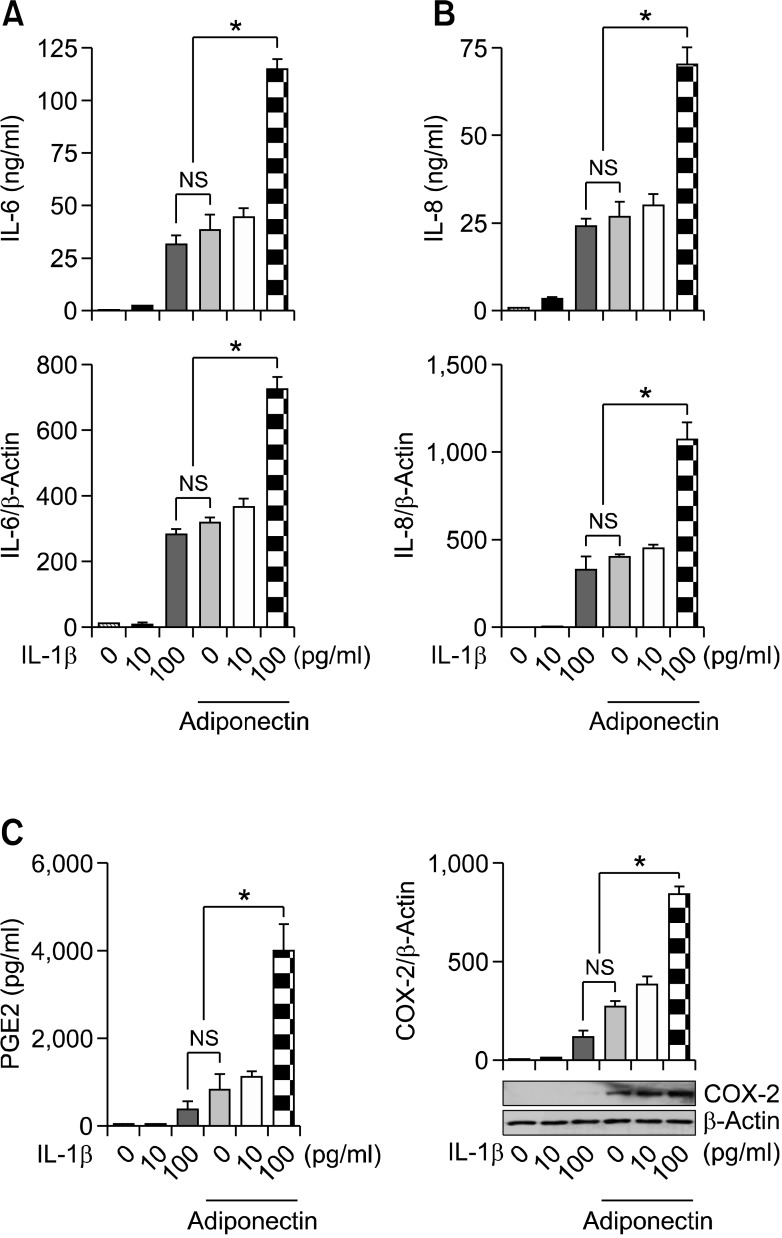
To evaluate the synergistic effects of adiponectin and IL-1β on inflammation and joint destruction in arthritic joints, cultured synovial cells were treated with IL-1β (10 or 100 pg/ml) and adiponectin (10 µg/ml) for 24 h. Supernatants were harvested and analyzed for levels of MMP-1, MMP-13, VEGF, IL-6, IL-8, and COX-2. As shown in Figure 1, adiponectin (10 µg/ml) stimulated the production of VEGF and MMP-1 similarly to IL-1β (100 pg/ml), while MMP-13 was stimulated more by adiponectin than by IL-1β. When synovial cells were stimulated with adiponectin and IL-1β at the same time, the protein expression of VEGF, MMP-1, and MMP-13 was not synergistically stimulated by the two cytokines in RA FLSs. In contrast, the levels of the proinflammatory mediators IL-6, IL-8, and PGE2 were further increased by concomitant stimulation with adiponectin and IL-1β compared with stimulation by either cytokine alone (Figure 2). In particular, PGE2 level was further increased approximately twofold by concomitant treatment with the two cytokines compared with accumulated treatment with individual cytokines. Consistent with the PGE2 level, the levels of mRNA and protein (COX-2), which were evaluated by real-time PCR and Western blot, respectively, were increased in a pattern identical to that of PGE2. More importantly, while the level of COX-2 measured in cells stimulated with 100 pg/ml of IL-1β was almost undetectable, the COX-2 level measured in cells stimulated with 10 µg/ml of adiponectin was detected weakly by Western blot. Combined treatment increased the COX-2 level approximately threefold compared with treatment with adiponectin alone.
Figure 1.
No synergistic effect of adiponectin plus IL-1β on the production of VEGF, MMP-1, or MMP-13 in RA FLSs. Synovial cells (2.5 × 105 cells/60 mm dish/2 ml serum-free media) were stimulated with IL-1β for 24 h at 10 or 100 pg/ml in the absence or presence of 10 µg/ml adiponectin, as described in the Materials and Methods section. The levels of VEGF, MMP-1, and MMP-13 were measured by ELISA from culture supernatants. Three independent experiments were performed in quadruplicate with FLSs from each patient. The data shown are representative of three independent experiments, and similar results were obtained from all three. Values are expressed as mean ± S.E.M.
Figure 2.
The synergistic effect of adiponectin plus IL-1β on the production of IL-6, IL-8, and PGE2 in RA FLSs. Culture supernatants were evaluated for (A) IL-6, (B) IL-8, and (C) PGE2 by ELISA, as in Figure 1. The cells were used for mRNA extraction and Western blot to detect COX-2 expression. The mRNA levels were measured by real-time RT-PCR. Three independent experiments were performed in quadruplicate with FLSs from each patient. The data shown are representative of three independent experiments, and similar results were obtained from all three. Values are expressed as mean ± S.E.M. *P < 0.05 versus treatment with IL-1β (100 pg/ml) or adiponectin (10 µg/ml) alone.
Molecular mechanisms underlying the synergistic effects of adiponectin and IL-1β
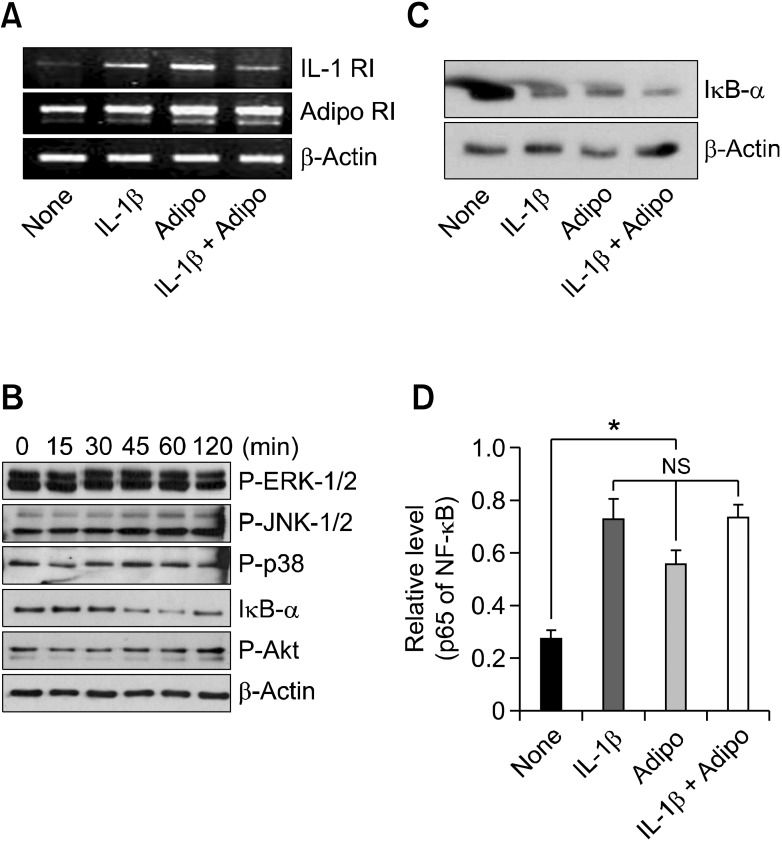
To study the molecular mechanisms by which adiponectin and IL-1β synergistically enhance the production of proinflammatory mediators, first we checked whether adiponectin and IL-1β increased the expression of IL-1 receptor (IL-1R1) and adiponectin receptor (AdipoR1), respectively. As shown in Figure 3A, adiponectin and IL-1β each increased the expression of both AdipoR1 and IL-1R1. This finding suggests that increased expression of their receptors may be a possible mechanism underlying the synergy between adiponectin and IL-1β regarding the expression of IL-6, IL-8 and COX-2.
Figure 3.
Molecular mechanisms underlying the synergistic effects of adiponectin and IL-1β. (A) The expression of IL-1 receptor 1 (IL-1R1) and adiponectin receptor 1 (AdipoR1) were determined by semi-quantitative PCR. (B) Time course activation of various signaling pathways in adiponectin-stimulated RA FLSs. FLSs cultured (2.5 × 105 cells) in 60 mm dishes were serum-starved overnight and stimulated with either adiponectin or IL-1β for a given time. The cells were prepared for Western blot analysis. (C) IκB-α levels. (D) Nuclear levels of NF-κB in adiponectin and/or IL-1β-stimulated RA FLSs. IκB-α level was analyzed by Western blot after 60 min of stimulation. The nuclear level was evaluated after 90 min of stimulation, as described in the Materials and Methods section. For the Western analysis, three independent experiments were performed in one plate with FLSs from each patient. For the analysis of NF-κB level, three independent experiments were performed in quadruplicate with FLSs from each patient. The data shown are representative of three independent experiments, and similar results were obtained from all three. Values are expressed as mean ± S.E.M. *P < 0.05 versus treatment with IL-1β (100 pg/ml) or adiponectin (10 µg/ml) alone.
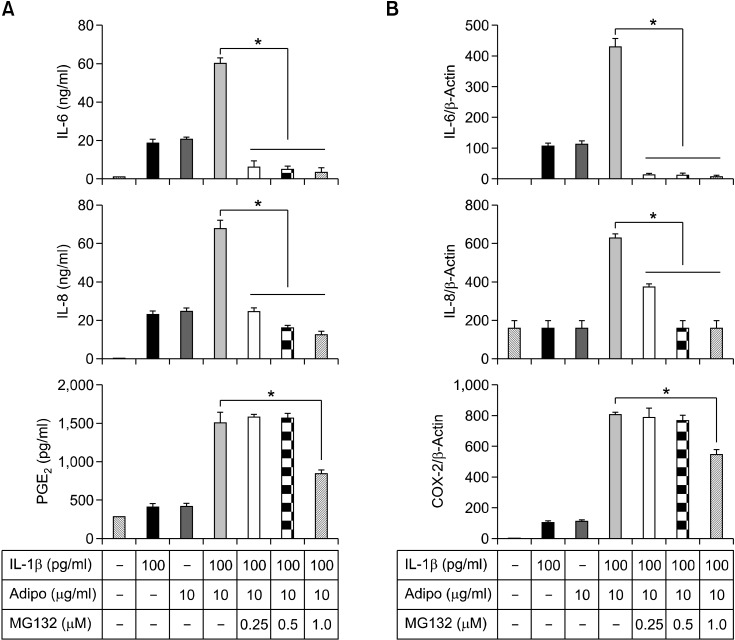
Next, we investigated adiponectin-mediated signaling pathways in RA FLSs. As shown in Figure 3B, adiponectin treatment (10 µg/ml) degraded IκB-α maximally at 60 min, while ERK1/2, P-38, and JNK-1/2 were not significantly phosphorylated in this system. Combined treatment with adiponectin and IL-1β could not significantly lead to the synergistic degradation of IκB-α (Figure 3C). In keeping with these results, the combined treatments did not act synergistically to increase the level of nuclear NF-κB (Figure 3D). Next, we determined whether the increase of IL-6, IL-8, and PGE2 levels by adiponectin plus IL-1β could be blocked by the NF-κB inhibitor MG132 (Figure 4). This inhibitor effectively inhibited the increase of IL-6, IL-8, and PGE2 levels produced by the combined stimulation of adiponectin and IL-1β at both protein and mRNA levels.
Figure 4.
The effects of the NF-κB inhibitor MG132 on the production of proinflammatory mediators such as IL-6, IL-8, and PGE2. Synovial cells (2.5 × 105 cells/60 mm dish/2 ml serum-free media) were stimulated with IL-1β (100 pg/ml) and/or adiponectin for 24 h in the presence of MG132 at concentrations of 0.25-1.0 µM. The supernatants and cells were used for ELISA (A) and real-time PCR (B), respectively. Three independent experiments were performed in quadruplicate with FLSs from each patient. The data shown are representative of three independent experiments, and similar results were obtained from all three. Values are expressed as mean ± S.E.M.
Expression levels and association of IL-6, IL-8, and PGE2 with adiponectin in the synovial fluid of arthritic patients
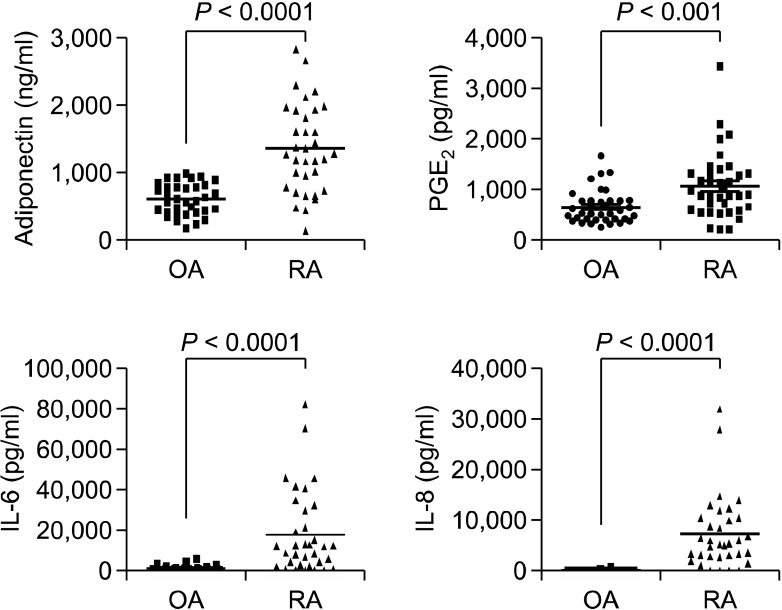
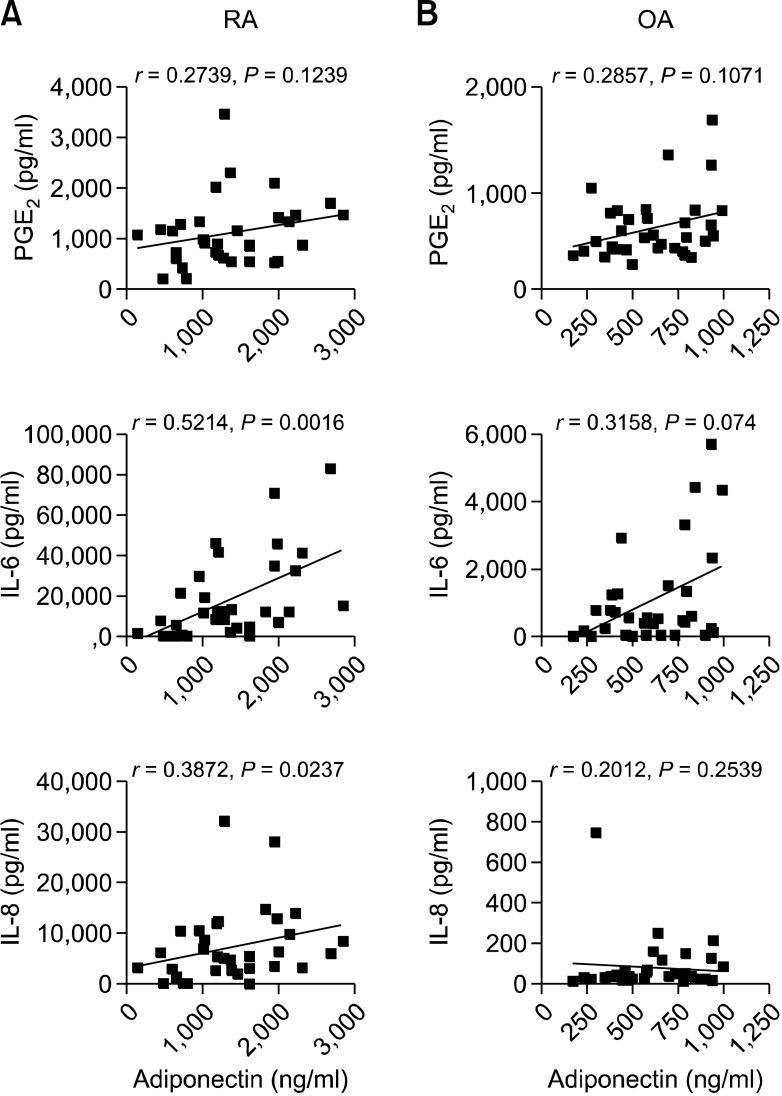
To evaluate whether the synergistic effect of adiponectin and IL-1β on the production of proinflammatory mediators is demonstrable in vivo, we investigated the expression levels and association of IL-6, IL-8, and PGE2 with adiponectin in the synovial fluid of patients with arthritis. As shown in Figure 5, the levels (mean ± S.D.) of adiponectin (1,358 ± 655.4 vs. 606.1 ± 231.1 ng/ml), IL-6 (17,868 ± 20,484 vs. 1,054 ± 1,435 pg/ml), IL-8 (7,238 ± 7,164 vs. 79.58 ± 129.6 pg/ml), and PGE2 (1,061 ± 642.8 vs. 650.6 ± 327.6 pg/ml) were significantly higher in RA patients than in OA patients. Next, we studied the association of IL-6, IL-8, and PGE2 levels with adiponectin level (Figure 6). In OA patients, the levels of none of these proinflammatory mediators were significantly associated with adiponectin level. However, in RA patients, IL-6 and IL-8 levels correlated positively with adiponectin level, while PGE2 level was not significantly correlated with adiponectin level.
Figure 5.
Expression levels of IL-6, IL-8, PGE2, and adiponectin in synovial fluid from patients with RA or OA. Collected joint fluid from 34 patients with RA or OA was dispensed into 1-ml aliquots and treated with hyaluronidase (50 µg/ml) for 1 h at room temperature. The joint fluid was diluted with diluent buffer to achieve the proper detection range for ELISA. The levels of proteins of interest in joint fluid were measured using a commercially available ELISA kit, according to the manufacturer's instructions.
Figure 6.
Association of adiponectin with IL-6, IL-8, or PGE2 in synovial fluid from patients with RA or OA. To determine the degree of linearity between 2 variables, data were compared using Spearman's correlation test (2-tailed). Differences were considered significant at P < 0.05.
Discussion
Adiponectin has been known to play various roles in the pathogenesis of various diseases. In our previous report, we suggested that adiponectin is a stimulant of MMP-1 and MMP-13 expression as strong as IL-1β, even though adiponectin is also an inducer of proinflammatory mediators (Choi et al., 2009). Regarding adiponectin's synergy with IL-1β, Kusunoki et al. reported that adiponectin stimulates PGE2 production in RA synovial fibroblasts (Kusunoki et al., 2010). Consistent with that report, our data also found that adiponectin stimulated the PGE2 production and COX-2 expression, and greatly increased the COX-2 expression in the presence of IL-1β. The production of IL-6 and IL-8 by adiponectin in RA FLSs has already been reported (Ehling et al., 2006; Kitahara et al., 2009); however, the synergy of adiponectin and IL-1β in the production of proinflammatory mediator is, to our knowledge, reported here for the first time. Contradictory to adiponectin's synergy with IL-1β, adiponectin treatment mitigates the severity of arthritis in mice with collagen-induced arthritis; additionally, in vitro experiments with RA FLSs indicated that adiponectin significantly inhibits IL-1β-induced RA FLS proliferation, while increasing IL-6 expression in IL-1β-stimulated RA FLSs (Lee et al., 2008). All of these findings suggest that adiponectin's synergy with IL-1β may not necessarily lead to more severe inflammation in arthritic joints, but may sometimes be overwhelmed by other factors at the local inflammatory site. In addition, the above controversial reports of adiponectin may be due to a potential dual effect of adiponectin, having an anti-inflammatory and pro-inflammatory action according to its exposure time and concentration (Brochu-Gaudreau et al., 2010).
Adiponectin activates intracellular signaling pathways through the adiponectin receptors AdipoR1 and AdipoR2. COX-2 expression is significantly inhibited when these receptors are expressed in RA FLSs and reduced by RNA interference (RNAi) technology, suggesting that adiponectin activates intracellular signaling pathways through adiponectin receptors (Kusunoki et al., 2010). However, adiponectin-mediated intracellular signaling pathways are not much studied by Western blot in RA FLSs. Therefore, various inhibitors of MAPK signaling pathways have been tested to indirectly evaluate adiponectin-induced intracellular signaling pathways. In particular, p38 MAPK inhibitor SB203580 treatment resulted in a significant reduction of adiponectin-dependent IL-6 synthesis in RA FLSs (Ehling et al., 2006). Furthermore, adiponectin-induced activation of p38 and NF-κB pathways has been demonstrated by Western blot in human chondrosarcoma cells (Chiu et al., 2009). Recently, adiponectin-induced MCP-1 secretion was downregulated by p38 MAPK and protein kinase C inhibitors (Frommer et al., 2010). All of these findings emphasized that adiponectin stimulates proinflammatory mediators through the p38 signaling pathway. However, in the present study, Western blot data revealed that NF-κB signaling pathways are likely to be more important in adiponectin-induced gene expression of RA FLSs than other pathways. In contrast, 10 ng/ml IL-1β strongly stimulated MAPK and IκB pathways in our previous report (Bang et al., 2009). In this study, the NF-κB inhibitor MG132 greatly inhibited IL-6, IL-8 and PGE2 production in adiponectin- and IL-1β-stimulated RA FLSs. However, the specific synergistic mechanisms are not explained here. Unknown signaling pathways other than IκB or MAPK pathways seem to be involved in the synergistic action in this system. The MMP-1, MMP-13, and VEGF genes, which are not synergistically stimulated, may be expressed by a set of transcription factors through the common signaling pathways activated by adiponectin or IL-1β. Expression of IL-6, IL-8, and COX-2 may be synergistically increased through transcription factors of different signaling pathways activated by adiponectin and IL-1β. In addition, 10 ng/ml IL-1β is not a physiological concentration; rather, 10-100 pg/ml IL-1β is a more physiological concentration in the synovial fluid of RA patients (Kahle et al., 1992). Therefore, the combined stimulation of adiponectin (10 µg/ml) and IL-1β (100 pg/ml) is feasible in physiological conditions. Their synergistic effect on the production of proinflammatory mediators may shed some light on the question of how to properly control their level during the pathogenesis of inflammatory arthritis.
To provide greater insight into their synergistic effects, we investigated the levels of proinflammatory mediators in synovial fluids and their association with adiponectin. The level of IL-1β in synovial fluid was below the detection limit for ELISA; therefore, we could not measure the level of IL-1β. However, the positive association of IL-6 and IL-8 with adiponectin in RA synovial fluids can, at least in part, tell us that adiponectin plays an important role in the production of these proinflammatory mediators during joint inflammation. In support of this hypothesis, IL-6 levels are strongly associated with IL-8 levels in RA synovial fluid (Spearman r = 0.6999, P < 0.0001), but not in OA synovial fluid, suggesting that the expression levels of adiponectin, IL-6, and IL-8 are associated with each other in inflammatory arthritic joints. Meanwhile, various cell types in arthritic joints contribute to the production of proinflammatory mediators such as IL-6, IL-8, and PGE2. Therefore, the synergistic effect of adiponectin and IL-1β on the production of such proinflammatory mediators should be tested in other cell types, such as endothelial cells and immune cells. Because other various factors such as hypoxia are involved in the production of proinflammatory mediators, we are unable to declare their synergistic effect on the basis of this correlation study. In conclusion, we demonstrate for the first time that adiponectin and IL-1β may act synergistically on the production of proinflammatory mediators such as IL-6, IL-8, and PGE2 in inflammatory arthritic joints. Considering that there are still some patients who are refractory to the biologics, such as blockers of TNF-α, IL-1β, and IL-6, new biologics are necessary to improve arthritis treatment. Therefore, adiponectin can be considered an additional potential therapeutic target to control inflammation in arthritic joints. A blocker against adiponectin may be useful to supplement the effective biologics that are currently available.
Methods
Reagents
Recombinant adiponectin and IL-1β expressed in E. coli was purchased from ProSpec (Rehovot, Israel). MG132 was obtained from Sigma-Aldrich Korea (Young-In, Korea).
Synovial cell culture and collection of synovial fluids from patients
All in vitro experiments were carried out with fibroblast-like synoviocytes derived from patients with rheumatoid arthritis. After obtaining informed consent, synovial tissues were collected from RA patients. They met the 1987 American College of Rheumatology criteria for the diagnosis of RA and had been treated with nonbiological disease-modifying antirheumatic drugs (DMARDs) and were underwent therapeutic joint surgery. FLSs were isolated and grown in Dulbecco's Modified Essential Medium (DMEM, low glucose) (Gibco-BRL, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco-BRL) and 1× Antibiotic-Antimycotic (Gibco-BRL) as described previously (Kim et al., 2007). After the cells had grown to confluence, they were split at a 1:4 ratio. FLS passages 3-6 from three patients were used for all experiments. The synovial joint fluids were collected from 34 RA and OA patients. This study was approved by the Institutional Review Board of Kyung Hee University Hosipital at KANGDONG. The joint fluid was collected at Kyung Hee Hospital, Seoul, Korea.
ELISA
Synovial cells (2.5 × 105 cells/60 mm dish/2 ml serum-free media) were treated with recombinant adiponectin (10 µg/ml), IL-1β (10 or 100 pg/ml) or their combination (ProSpec, Rehovot, Israel). Conditioned media was collected 24 h later as described previously (Choi et al., 2009). Three independent experiments were performed in quadruplicate. Each experiment was performed using synovial cells from different patients. For the assessment of MMP-1, MMP-13, VEGF, IL-6, IL-8, PGE2, adiponectin levels in joint fluid, the collected joint fluid from 34 patients with RA or OA were dispensed into 1 ml aliquots and treated with hyaluronidase at 50 µg/ml for 1 h at room temperature. The joint fluid was diluted with diluent's buffer for the proper detection range with ELISA. The levels of proteins of interest in joint fluid were measured using a commercial ELISA kit from R&D Systems. For the measurement of transcription factor, NF-κB, in the nucleus, FLSs were seeded (5 × 106 cells) into 100 mm dishes and grown to 80% confluence. The cells were serum-starved overnight and stimulated by adiponectin, IL-1β or their combination for 90 min at the concentration as described above. Subsequently, the cells were washed twice in PBS and treated with lysis buffer and the extraction of transcription factors from the nucleus was performed according to the manufacturer's protocol (Active Motif, Seoul, Korea).
RT-PCR
FLSs (2.5 × 105 cells) were cultured overnight in 60 mm dishes containing 2 ml of media. Cells were incubated with serum-free media for 2 h and new serum-free media was replaced just prior to the addition of adiponectin or IL-1β and cultured for 24 h. Supernatants were collected for ELISA and the cells were used for RT-PCR. Trizol was used to extract total RNA from the cells. Complementary DNA was synthesized from 1 µg of total RNA in a 20 µl reverse transcription reaction mixture. For semi-quantitative PCR, aliquots of cDNA were amplified in a 25 µl PCR mixture according to the protocol provided by the manufacturer (TaKaRa Bio, Kyoto, Japan). The PCR conditions for the IL-1 receptor 1 (IL-1R1) and adiponectin receptor (AdipoR1) were as follows: 35 cycles of 95℃ for 45 s, 55℃ for 45 s, and 72℃ for 45 s. PCR products were subjected to electrophoresis on 1.0% agarose gels containing ethidium bromide, and the bands were visualized under UV light.
For real-time quantitative PCR, the reaction was carried out using the LightCycler PCR system (Roche Diagnostics, Meylan, France) using the DNA binding SYBR Green I dye and primers to detect the PCR products as described previously (Choi et al., 2009, 2010). The results are calculated as ratios of gene transcripts to β-actin transcripts, with the quantity of transcripts in each sample expressed as a copy number. The ratio of IL-6, IL-8 and COX-2/β-actin mRNA was assigned a value of 100%, with the corresponding results calculated as relative percentages. The primer were synthesized by Bioneer Co. Ltd. (Seoul, Republic of Korea), and their sequences are listed in Supplemental Data Table S1.
Western blot analysis
FLSs cultured (2.5 × 105 cells) in 60 mm dishes were serum-starved overnight and stimulated by adiponectin or IL-1β for a given time. As described previously (Choi et al., 2009), the cells are prepared for Western blot analysis and the samples were separated using 12% SDS-PAGE, and were then transferred to Hybond-ECL membranes (Amersham, Arlington Heights, IL). The membranes were first blocked with 6% nonfat milk dissolved in TBST buffer (10 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.05% Tween 20). The blots were then probed with various rabbit polyclonal antibodies for IκB-α, p-ERK1/2, p-P38, p-JNK-1/2, COX-2 and β-actin (Cell Signaling Technology, Beverly, MA) diluted 1:1000 in TBS at 4℃ for overnight, and incubated with 1:1000 dilutions of goat anti-rabbit IgG secondary antibody coupled with horseradish peroxidase. The blots were developed using the ECL method (Amersham). For re-probing, the blots were incubated in the stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) at 50℃ for 30 min with occasional agitation.
Statistical analysis
The in vitro experimental data are expressed as the mean ± standard error of the mean (SEM) of quadruplicate samples. Differences between groups were compared with the Mann-Whitney test. The level of IL-6, IL-8, PGE2, and adiponectin in joint fluid of RA and OA patients was compared between groups with the Mann-Whitney test. To determine the degree of linearity between 2 variables, data were compared using Spearman's correlation test (2-tailed). Prism software 4 (Graphpad Software, San Diego, CA) was used for statistical analysis and graphing. Differences were considered significant at P < 0.05.
Acknowledgements
This study was supported by the program of Kyung Hee University for the young researcher in medial science (KHU-2009-1442).
Abbreviations
- FLS
fibroblast-like synoviocytes
- OA
osteoarthritis
- RA
rheumatoid arthritis
Footnotes
The authors declare that they have no competing interests.
Supplemental data
Supplemental data include a table and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-44-7-04.pdf.
References
- 1.Bang JS, Oh da H, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 4.Chiu YC, Shieh DC, Tong KM, Chen CP, Huang KC, Chen PC, Fong YC, Hsu HC, Tang CH. Involvement of AdipoR receptor in adiponectin-induced motility and alpha2beta1 integrin upregulation in human chondrosarcoma cells. Carcinogenesis. 2009;30:1651–1659. doi: 10.1093/carcin/bgp156. [DOI] [PubMed] [Google Scholar]
- 5.Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH, Choi SY, Yang HI, Yoo MC, Kim KS. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res Ther. 2009;11:R161. doi: 10.1186/ar2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HM, Oh da H, Bang JS, Yang HI, Yoo MC, Kim KS. Differential effect of IL-1beta and TNFalpha on the production of IL-6, IL-8 and PGE2 in fibroblast-like synoviocytes and THP-1 macrophages. Rheumatol Int. 2010;30:1025–1033. doi: 10.1007/s00296-009-1089-y. [DOI] [PubMed] [Google Scholar]
- 7.Dayer JM, de Rochemonteix B, Burrus B, Demczuk S, Dinarello CA. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986;77:645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 9.Frommer KW, Zimmermann B, Meier FM, Schroder D, Heil M, Schaffler A, Buchler C, Steinmeyer J, Brentano F, Gay S, Muller-Ladner U, Neumann E. Adiponectin-mediated changes in effector cells involved in the pathophysiology of rheumatoid arthritis. Arthritis Rheum. 2010;62:2886–2899. doi: 10.1002/art.27616. [DOI] [PubMed] [Google Scholar]
- 10.Giannessi D, Maltinti M, Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol Res. 2007;56:459–467. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Gay MA, Llorca J, Garcia-Unzueta MT, Gonzalez-Juanatey C, De Matias JM, Martin J, Redelinghuys M, Woodiwiss AJ, Norton GR, Dessein PH. High-grade inflammation, circulating adiponectin concentrations and cardiovascular risk factors in severe rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:596–603. [PubMed] [Google Scholar]
- 12.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry BA, Clarke IJ. Adipose tissue hormones and the regulation of food intake. J Neuroendocrinol. 2008;20:842–849. doi: 10.1111/j.1365-2826.2008.1730.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahle P, Saal JG, Schaudt K, Zacher J, Fritz P, Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Ann Rheum Dis. 1992;51:731–734. doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- 16.Kim KS, Park EK, Ju SM, Jung HS, Bang JS, Kim C, Lee YA, Hong SJ, Lee SH, Yang HI, Yoo MC. Taurine chloramine differentially inhibits matrix metalloproteinase 1 and 13 synthesis in interleukin-1beta stimulated fibroblast-like synoviocytes. Arthritis Res Ther. 2007;9:R80. doi: 10.1186/ar2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2009;378:218–223. doi: 10.1016/j.bbrc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki N, Kitahara K, Kojima F, Tanaka N, Kaneko K, Endo H, Suguro T, Kawai S. Adiponectin stimulates prostaglandin E(2) production in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62:1641–1649. doi: 10.1002/art.27450. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW, Kim JH, Park MC, Park YB, Lee SK. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand J Rheumatol. 2008;37:260–268. doi: 10.1080/03009740801910346. [DOI] [PubMed] [Google Scholar]
- 20.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 23.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, Wrede C, Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 24.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. doi: 10.1124/pr.59.2.3. [DOI] [PubMed] [Google Scholar]
- 26.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 27.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 28.Senolt L, Pavelka K, Housa D, Haluzik M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247–252. doi: 10.1016/j.cyto.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Symons JA, McDowell TL, di Giovine FS, Wood NC, Capper SJ, Duff GW. Interleukin 1 in rheumatoid arthritis: potentiation of immune responses within the joint. Lymphokine Res. 1989;8:365–372. [PubMed] [Google Scholar]
- 30.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data include a table and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-44-7-04.pdf.