Abstract
The late Na current is of pathophysiological importance for the heart. Ranolazine is an innovative anti-ischemic and antianginal agent that inhibits the late Na current, thereby reducing the Na-dependent Ca-overload, which improves diastolic tone and oxygen handling during myocardial ischemia. In addition, ranolazine seems to exert beneficial effects on diastolic cardiac function. Moreover, there are experimental and clinical data about its antiarrhythmic properties. A beneficial atrial selectivity of ranolazine has been suggested that may be helpful for the treatment of atrial fibrillation. The purpose of this review article is to discuss possible future clinical indications based on novel experimental and preclinical results and the significance of the available data.
Keywords: Late Na current, Intracellular Na overload, Late Na current inhibition, Ranolazine, Intracellular Ca overload, Diastolic dysfunction, Heart failure, Heart failure with preserved ejection fraction, Action potential duration, Arrhythmias, Atrial fibrillation, Ventricular arrhythmias, Treatment, Therapy
Introduction: The Late Na Current
Under physiological conditions, sarcolemmal Na channels open transiently and are quickly inactivated, thereby producing the peak Na current (INa) and, thus, the upstroke of the action potential. In addition, a late component of INa was described due to Na channels that remain active, inactivate with much slower kinetics, or reopen. The amplitude of this current is small with about 1 % of the amplitude of peak INa, but it may persist for hundreds of milliseconds. Because late INa can be elevated up to about 5 times under pathological conditions [1], one can imagine that the integral of this persistent current may exceed the one of peak INa leading to Na accumulation in the myocyte. It is known that intracellular Na concentration frequency dependently can increase by several mM [2]. In human heart failure, intracellular Na concentration can be up to 6–8 mM higher as compared to nonfailing myocardium [3].
Different Na channel isoforms are known (eg, Nav1.1–Nav1.8), with the pore-forming subunit Nav1.5 being the cardiac specific isoform. The contribution of Na channel isoforms for late INa and its modulation by different pathological conditions are not fully understood. Most of the studies identified Nav1.5 as the late INa producing channel [4]. Auxiliary ß subunits also exist [5]. There is one report that shows that neuronal isoforms Nav1.1 and Nav1.6 increase proportionally with increasing late INa in pressure-overloaded rat hearts [6]. Also, mutations in the Na channel gene SCN5A encoding for Nav1.5 that are associated with the long QT syndrome 3 produce slowed inactivation thereby increasing late INa [7].
Of broader clinical relevance are acquired disease states such as ischemia, myocardial infarction, and heart failure that are known to be associated with an elevated late INa [8–10]. We have shown that late INa is increased in myocytes from patients with atrial fibrillation (AF) [11••] and in isolated myocardium from patients with heart failure and diastolic dysfunction [12].
As mentioned above, an elevated late INa is considered to be a potent contributor of Na overload [12, 13]. Although Na overload itself cannot activate myofilaments directly, increasing Na levels can lead to Ca overload through the Na/Ca-exchanger (NCX). Normally, the NCX exchanges one Ca ion for three Na ions per cycle. However, the NCX can work in two different directions. In its forward mode, it eliminates Ca out of the cell to accomplish diastolic relaxation (in addition to sarcoplasmic reticulum Ca reuptake mainly through the sarcoplasmic reticulum Ca ATPase). In its reverse mode (usually during the action potential plateau), it transports Ca into the cell in exchange to transsarcolemmal elimination of Na. The activity and direction of the transport depends on the membrane potential and, thus, on the action potential as well as the intracellular Na and Ca concentration (ie, the electrochemical gradient). Na accumulation and prolonged action potential duration occur during myocardial hypoxia and promote reverse mode of the NCX [14]. The consequence is an impaired overall capacity of the cell to eliminate Ca from the cytosol leading to intracellular Ca overload. This leads to or aggravates diastolic dysfunction due to increased myofilaments activation [15]. Thus, diastolic activation of contractile proteins causing increased wall tension prolonging extravascular compression of intramural vessels reduces oxygen supply [16]. Additionally, elevated diastolic tone increases energy consumption and aggravates disturbed energy balance like a vicious cycle [17].
Inhibition of Late INa
Ranolazine is an inhibitor of late INa and is available for clinical purposes since 2006 as an anti-ischemic agent (Ranexa® [Gilead Sciences, Inc., Foster City, CA]) [18, 19]. In cardiac myocytes from dogs and guinea pigs, ranolazine was shown to cause a concentration-, voltage-, and frequency-dependent inhibition of late INa [20]. Inhibitory effects of ranolazine on late INa were demonstrated in multicellular myocardium and in isolated cardiac myocytes [12, 21, 22]. Although ranolazine also has some peak INa inhibiting effects, it has an up to 38-fold higher potency for late INa as compared to peak INa with a half maximal inhibitory concentration (IC50) of 6.5 versus 244 μM [21] in ventricular myocytes with a therapeutic range of 2–8 μM. Regarding the binding site of ranolazine on the cardiac Na channel, two independent studies described mutation of a single amino acid residue, F1760A and F1759K, in the putative local anesthetic binding site of the cardiac Na channel Nav1.5 [7], and the skeletal muscle channel Nav1.4 [23], showing that this mutation reduced the late INa inhibiting effects of ranolazine. However, it remains unclear how ranolazine produces its selective effect on late INa.
Interestingly, in two very recent studies, additional modes of action of ranolazine were suggested. Beyder et al. [24•] could show a mechanosensitivity of Nav1.5 in isolated cardiac myocytes and that ranolazine was able to reduce this novel activation mechanism. However, this effect seemed to be independent of the suspected binding site F1760. Therefore, the exact molecular mode of action remains unexplained. Of not, a stretch-dependent effect on intracellular Na handling was previously proposed by us mainly through reverse mode NCX [25]. In addition, Lovelock et al. [26] could describe an effect of ranolazine on myofilament Ca sensitivity, thereby improving diastolic function.
Late INa Inhibition in Heart Failure: Effects on Cytosolic Na and Ca Handling
Diastolic heart failure is characterized by signs and symptoms of heart failure. These patients show a decreased compliance and relaxation of the ventricles and present with preserved ejection fraction (HFpEF). Unfortunately, there are no evidence-based agents for treating HFpEF. Because late INa is elevated in human heart failure [10], there is ongoing effort to investigate possible effects of ranolazine in conditions of heart failure.
Almost 20 years ago, there was an elegant in vivo study showing an improved diastolic function in noninfarcted ischemic hearts in a small number of patients before and in the presence of intravenous application of ranolazine [27]. Moreover, acute infusion of ranolazine in patients with long QT syndrome 3 with increased late INa caused a significant improvement of diastolic relaxation in parallel to an abbreviation of the QT time [28].
In addition, a recent echocardiographic study investigated the effects of ranolazine in patients with stable angina with preserved ejection fraction [29••]. After 2 months deceleration time E, isovolumic contraction time, and isovolumic relaxation time decreased, whereas ejection time increased. Global left ventricular function also improved, as indicated by a decrease in the myocardial performance index.
Interestingly, a subgroup analysis of the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary Syndromes (MERLIN TIMI-36) study revealed that patients with an acute coronary syndrome and elevated B-type natriuretic peptide levels (> 80 pg/mL), and thus increased wall stress, were at significantly higher risk of the primary trial end point of cardiovascular death and myocardial infarction at 1 year [30].
In contrast to the small number of clinical trials, there are a couple of experimental studies using ranolazine in vivo and in vitro in different heart failure models. First, in dogs with heart failure, acute infusion of ranolazine significantly reduced left ventricular end diastolic pressure, and increased ejection fraction as well as stroke volume [31]. Most importantly, these effects were observed in the absence of significant changes in heart rate or blood pressure. In another study, ranolazine was investigated as a chronic treatment option in a heart failure model again in dogs [32]. This study additionally examined the effects of ranolazine treatment in combination with the ß-blocker metoprolol or the angiotensin-converting enzyme (ACE) inhibitor enalapril. Ranolazine was capable to significantly prevent progressive left ventricular dysfunction as well as global and cellular myocardial remodeling.
Our group investigated isolated trabeculae from human end-stage failing hearts that exhibited frequency-dependent diastolic dysfunction in vitro [12]. Addition of ranolazine did not cause negative inotropy but significantly ameliorated disturbed intracellular ion handling and reduced the increase in diastolic tension (ie, improved diastolic dysfunction [Fig. 1]). Interestingly, those trabeculae with severe diastolic dysfunction benefit from late INa inhibition even more. This hypothesis could be confirmed in experiments in isolated rabbit myocytes that were exposed to anemone toxin (ATX-II) to mimic increased late INa in heart failure. Using fluorescent dyes, the increase in cytosolic Na was paralleled by elevated intracellular diastolic Ca levels. Treatment with ranolazine significantly attenuated the effects of ATX-II leading to markedly reduced diastolic Ca levels in addition to lowering Na levels. Interestingly, ranolazine secondarily accelerated the NCX forward mode activity measured by analysis of Ca transients decay during caffeine applications [12]. This increase in NCX forward mode displays the link between ranolazine-modulated cytosolic Na and Ca in such a way that both stimuli of the reverse NCX mode in heart failure, elevated [Na]i and prolonged action potential duration, become attenuated by exposure to ranolazine, leading to improved Ca elimination during diastole. The results of this in vitro study led us to initiate the Ranolazine in Diastolic Heart Failure (RALI-DHF) trial (NCT01163734) to investigate intravenous ranolazine application in vivo followed by 2 weeks oral treatment in patients with diastolic dysfunction due to severe HFpEF in a small proof-of-concept placebo-controlled study (Fig. 2) [33].
Fig. 1.
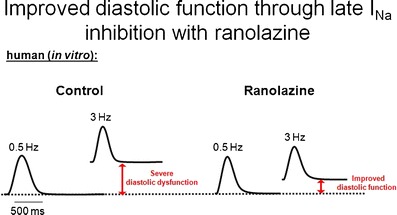
Original traces representing diastolic dysfunction in heart failure and improvement upon ranolazine (Adapted from Sossalla et al. [12].)
Fig. 2.
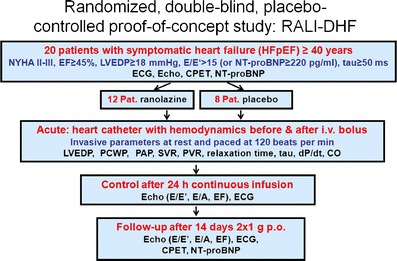
Schematic diagram showing the design of the RALI-DHF study. RALI-DHF Ranolazine in Diastolic Heart Failure; NYHA New York Heart Association class; EF ejection fraction; LVEDP left ventricular end diastolic pressure; NT-proBNP N-terminal pro–B-type natriuretic peptide; ECG electrocardiogram; Echo echocardiogram; CPET cardiopulmonary exercise testing; Pat patient; i.v. intravenous; PCWP pulmonary capillary wedge pressure; PAP pulmonary artery pressure; SVR systemic vascular resistance; PVR pulmonary vascular resistance; dP/dt; rate of pressure change in the ventricle
Interestingly, isolated papillary muscles from transgenic mice overexpressing Ca/calmodulin-dependent protein kinase II (CaMKII), which is known to be upregulated in heart failure [34] and to induce late INa, had frequency-dependent diastolic dysfunction [35]. Addition of ranolazine markedly improved diastolic tension under basal conditions but to a greater extent under frequency-induced stress, suggesting that CaMKII may be activated by increased Ca levels (and hence Ca overload) thereby activating late INa (Fig. 2). Indeed, direct effects of CaMKII on Na channels (and thus late INa) were described previously [36] and underlined by computational modeling [37] and even effects of reactive oxygen species on late INa were recently shown to be mediated by CaMKII [38].
Our findings are consistent with earlier studies in animals showing that ranolazine attenuates diastolic dysfunction in the hearts of rabbit and rat models during ischemia/reperfusion [39, 40], in the presence of ischemic metabolites [41] or reactive oxygen species [42], and in dogs with experimentally induced heart failure [22]. In summary, most of the experimental studies performing acute exposure to ranolazine in heart failure report on positive effects on diastolic performance. This is different to a long-term study in heart failure dogs where both improved diastolic parameters and ejection fraction were described [31].
Late INa Inhibition for the Treatment of Arrhythmias
Late INa is expected to influence electrophysiological cell properties in addition to cytosolic Na handling. Thus, it is not surprising that ranolazine has been shown to have beneficial effects on arrhythmias in vitro and in vivo. In general, ranolazine exerts antiarrhythmic capacities very likely via inhibition of late INa, but also peak INa and rapid delayed rectifier potassium current IKr under certain circumstances. While inhibition of late INa is the principal electrophysiological effect of ranolazine in ventricular myocardium, the inhibition of peak INa seems to be of great importance in atrial myocardium.
A pathophysiologically increased late INa by itself can alter cellular electrophysiology by two distinguished ways and thus increase the propensity for arrhythmias, i.e. i) elevation of late INa which prolongs cardiac action potentials, and ii) elevation of late INa which causes cellular Na-dependent Ca overload and thus the electrogenic transient inward current ITi.
Early afterdepolarizations are more likely to occur during a prolonged action potential duration, which can be induced by enhancing late INa. Moreover, transmural differences of late INa, and hence action potential duration, might increase transmural dispersion of repolarization and QT interval, which underlies the development of torsade de pointes arrhythmias [20].
In contrast, Ca overload, which occurs when late INa is elevated [12], and leakiness of the cardiac ryanodine receptor are believed to participate as crucial events in the initiation and propagation of spontaneous sarcoplasmic reticulum Ca release events and/or proarrhythmogenic Ca waves [43]. The consequence may be elimination of cytosolic Ca via the NCX, which generates ITi, which can give rise to delayed afterdepolarizations [44]. Song and coworkers [45] have shown the crucial importance of late INa for arrhythmias in guinea pig atrial myocytes. They observed early and delayed afterdepolarizations as well as triggered activity. Also, elevation of late INa induced a Ca-dependent ITi. All effects could be abolished by ranolazine or tetrodotoxin. Moreover, Ca chelating agents, Na/Ca-exchange blockers, and the sarcoplasmic reticulum Ca release inhibitor ryanodine could prevent delayed afterdepolarizations and triggered activity. Because early afterdepolarizations could not be prevented using these agents, it is suggested that action potential prolongation causes early afterdepolarizations in a Ca-independent manner.
First results of antiarrhythmic properties of ranolazine were reported in a guinea pig in vitro model of long-QT syndrome 3 [46] and in the presence of ATX-II [47], a selective inducer for late INa. In these studies, ranolazine reduced both afterdepolarizations as arrhythmic triggers and the transmural and temporal dispersion of repolarization as an arrhythmic substrate. Therefore, the efficacy of ranolazine was potentially ascribed to its late INa-blocking properties. Further studies underlined the antiarrhythmic effects of ranolazine; intact rat hearts subjected to ischemia/reperfusion showed a reduced incidence and duration of ventricular arrhythmias upon ranolazine treatment [48]. Recently, ranolazine had efficacy against both pacing-induced re-entrant and multifocal ventricular fibrillation in isolated-perfused rat hearts with H2O2-mediated early afterdepolarizations and triggered activity [49•].
Moreover, ranolazine reversed abnormalities of repolarization (prolonged action potential duration, beat-to-beat variability, and dispersion of action potential duration and early afterdepolarizations) of ventricular myocytes from failing canine hearts [22]. Also, it was shown recently that atrial myocytes from mice with long QT 3 mutation with increased late INa show greatly increased action potential duration and early afterdepolarizations, and ranolazine reduced action potential duration [50••].
However, ranolazine also inhibits IKr in cardiac myocytes [20]. Blocking IKr causes prolongation of the ventricular action potential. Therefore, the net effect of ranolazine on the action potential is mainly driven by the relative magnitude of reductions in late INa (inward) and IKr (outward) currents during the repolarization period.
It should be noted that there are also reports showing antiarrhythmic effects of ranolazine under conditions without elevated late INa. Antzelevitch et al. [20] revealed potent effects of ranolazine to suppress early afterdepolarizations in myocytes isolated from the middle of the left ventricular wall and Purkinje fiber preparations. Midmyocardial cells are known to have action potentials that prolong disproportionately relative compared to those of epi- or endocardial cell types in response to many QT-prolonging drugs [51, 52]. Moreover, these cells have the largest late INa while IKr is similar in all three cell types. Accordingly, ranolazine produces a preferential abbreviation of midmyocardial cell action potential duration, leading to a reduction in transmural dispersion of repolarization [20]. In contrast to other IKr blockers such as sotalol, extrasystolic activity and spontaneous torsade de pointes arrhythmias were never observed in this study. Their findings are in agreement with a report from an anesthetized dog model with chronic complete atrioventricular block in which ranolazine-attenuated torsade de pointes episodes induced by IKr blockers [53] and studies involving isolated guinea pig and rabbit hearts [46, 54].
Late INa Inhibition for the Treatment of Atrial Fibrillation
Rhythm control remains important in the treatment of AF, but cannot be effectively achieved without the risk of potential side effects such as proarrhythmia, hypotension, or sometimes organ toxicity with current drugs (eg, dronedarone or amiodarone), except for ß-blockers. Thus, there is a demand for novel pharmacological strategies to treat AF. Ranolazine has potent effects on atrial arrhythmias (eg, AF) that are worth mentioning. In the MERLIN TIMI-36 trial a significant reduction of supraventricular tachycardias was observed in patients that were treated with ranolazine. Although a low incidence of AF was found, patients treated with ranolazine were less likely to have a new onset of AF. While 75 patients developed new AF in the placebo group, only 55 individuals had new-onset AF during treatment with ranolazine. However, this trial was not designed and statistically powered to investigate new onset of AF. Nevertheless, in addition to this remarkable finding during assessment of ranolazine’s safety, further studies are warranted.
Murdock et al. [55] investigated high-dose ranolazine as a pill in the pocket approach. They found that 72 % of the patients with paroxysmal AF converted to sinus rhythm after application of 2,000 mg of ranolazine (single dose). Although very promising, the limitation of this study is that no placebo collective was included. In another pilot project, ranolazine was helpful in maintaining sinus rhythm in patients with resistant AF in whom more established measures had failed [56].
Finally, there is preliminary evidence from a recent abstract that investigated the effects of ranolazine compared to amiodarone to prevent AF following bypass surgery [57]. In this retrospective trial, baseline characteristics such as age, important other diseases, drug pretreatment, and others were not statistical different between both groups. Ranolazine (generally 1,500 mg preoperatively followed by 1,000 mg twice daily for 10 days) was given to 111 patients and 145 patients were treated with amiodarone (generally 400 mg preoperatively followed by 200 mg twice daily for 10 days). Patients treated with ranolazine were significantly less likely to experience AF, with an incidence of 15 % compared to 26 % in patients treated with amiodarone. Although the result of this retrospective trial is promising, selection bias cannot be ruled out as a cause for the reduced incidence of AF with ranolazine. So far, clinical data seems to be promising but it is limited due to trial design and number of patients.
One major problem concerning Na channel blockers in the past is the fact that they can cause ventricular proarrhythmia as it was demonstrated in the Cardiac Arrhythmia Suppression Trial (CAST) trial [58]. Hence, atrial selective peak INa inhibition would be an attractive approach for the treatment of atrial rhythm disorders due to the lack of ventricular proarrhythmia. It has been reported that ranolazine acts as an atrial selective peak INa inhibitor [59] but it selectively inhibits late INa in ventricular myocytes [20]. While it remains unclear how ranolazine selectively inhibits late INa in ventricular myocytes, its capacity to inhibit peak INa in atrial myocytes was largely attributed to electrophysiological differences between atrial and ventricular myocardium [59]. In the latter report the authors have properly announced some atrial capacities that may account for the atrial selective profile (inhibition of peak INa) of ranolazine which was formerly described as an inactivated state blocker [22]: i) the half inactivation voltage is 16 mV more negative in atrial compared to ventricular myocytes; and ii) a more depolarized resting membrane potential in atrial cells that is generally accepted.
Although ranolazine was described to act as an inactivated state blocker, recent studies suggest that it preferentially binds rather to open versus inactivated Na channels, staying trapped in the channel during inactivation and unbinding during resting state [23, 60]. During rapid recovery from inhibition at the resting state, inhibition of peak INa might be atrial selective independent of open or inactivated blocking properties of the agent. This might be explained by a smaller fraction of rested Na channels at resting membrane potential in atrial compared to ventricular myocytes.
Accordingly, we also have shown that ranolazine inhibits peak INa in human atrial myocytes [11••]. We further found that inhibition of Na channels by ranolazine was frequency dependent, such as the higher the frequency the higher the inhibition rate. Faster activation rates are associated with abolished diastolic intervals, and the slow repolarization of the action potential phase 3 causes a slower unbinding of ranolazine from the channel. This phenomenon causes accumulation of block at fast, but not at slow frequencies. This might be explained by the capacity of ranolazine to dissociate rapidly from the resting state of the Na channel and may be of therapeutic importance during high atrial frequencies as it is known for AF.
Because Burashnikov et al. investigated myocytes without electrical remodeling (but this usually occurs during AF), these data are limited to conditions where electrical remodeling has not taken place yet [59]. Therefore, we additionally investigated atrial myocytes from patients with chronic AF and showed significantly reduced peak INa density (~16 %) in AF versus sinus rhythm, which was accompanied by a 26 % lower expression of Nav1.5 while neuronal Na channel isoforms were upregulated [11••]. In contrast, late INa was significantly increased in myocytes from AF atria by about 26 %. In a second step we exposed myocytes to ranolazine and found a marked reduction of late INa by about 60 % in myocytes from patients with AF but only by about 18 % in myocytes from patients with sinus rhythm. Although late INa integral per beat decreases with increasing frequencies, the high frequency (and thus an increasing late INa integral per minute) during AF might largely counteract this effect, leading to Na-dependent Ca overload Thus, it is likely that these effects of ranolazine independent of the action potential duration are important in human AF where Ca overload is also present [61].
But what may be the net effect of ranolazine on action potential duration in patients with AF with shortened action potential duration (as usually shown in AF)? Although there is competition between effects of ranolazine on late INa and IKr, which also determines action potential duration, we have observed that ranolazine rather prolongs action potential in human atrial myocytes (Maier L, unpublished). Furthermore, it was shown that ranolazine does not shorten but rather prolongs action potential duration in dog atrial myocytes [59]. This may have been due to the fact that ranolazine also inhibits IKr. Inhibition of IKr would counteract this phenomenon leading to prolonged atrial refractoriness. Indeed, it was previously shown that ranolazine prolongs atrial action potential duration, which leads to elimination of diastolic intervals and a more depolarized takeoff potential at rapid rates [59]. This effect may further potentiate the atrial selectivity for peak INa inhibition and possibly the clinical effectiveness of ranolazine. Taken together, inhibition of late INa via ranolazine may possess beneficial effects on cellular Na-dependent Ca overload similar to its property to block peak INa and IKr, which are accepted strategies for the treatment of AF.
Moreover, Kumar et al. [62] investigated atrial electrical properties of ranolazine in an intact porcine heart. They found that ranolazine increased atrial effective refractory period and prolonged conduction time in a frequency-dependent manner. These effects were more pronounced in the atria than in ventricle, as was also observed previously [59]. Intravenous application of ranolazine also decreased acetylcholine-induced AF duration, the dominant frequency of the arrhythmia, and tended to suppress reinduction of AF in a porcine model [63]. The same group further provided evidence that intrapericardial ranolazine exhibits striking atrial antiarrhythmic actions in the intact porcine heart [64]. This was evidenced by increases in refractoriness and here in AF inducibility.
Recently, Sicouri et al. [65] investigated possible synergistic effects of ranolazine in combination with chronic amiodarone on AF. Their data indicate that the combination of both agents produced an atrial-selective inhibition of Na channel parameters and prevented induction of acetylcholine-induced AF, that was much greater than treatment alone, and greater than the algebraic sum of the individual treatments. Therefore, this study points to a synergism of the effects of the two therapies. Regarding severe organ toxicity, which is regular produced by amiodarone, it is of special interest that dronedarone in combination with low doses of ranolazine similarly resulted in atrial-selective depression of sodium channel–dependent parameters and effective suppression of AF [66••].
It also has been shown that ranolazine exerts antiarrhythmic action in canine pulmonary vein sleeve preparations, where this agent caused a frequency-dependent inhibition of Na channel activity leading to post-repolarization refractoriness, conduction slowing, and suppression of triggered activity [67].
Finally, we do not know which AF patient might benefit most from ranolazine, and thus, there is a huge need for clinical studies to investigate the effects of ranolazine on persistent and paroxysmal AF. Therefore, two placebo-controlled studies were recently initiated, including the Ranolazine in Atrial Fibrillation Following An Electrical Cardioversion (RAFFAELLO) trial investigating ranolazine in patients with persistent AF after electric cardioversion and how efficient sinus rhythm can be maintained over a period of 4 months (www.ClinicalTrials.gov; NCT01534962). The HARMONY trial investigates a combination of ranolazine and low-dose dronedarone in patients with paroxysmal AF assessing AF burden (www.ClinicalTrials.gov: NCT01522651) [68].
Conclusions
In summary, there are increasingly experimental and preclinical data for a beneficial role of ranolazine in diastolic dysfunction and cardiac arrhythmias in addition to its current antianginal role. We believe that further experimental studies and future clinical trials will shed light onto the potential impact and future indications for ranolazine to treat patients possibly with certain arrhythmias, forms of heart failure most likely with diastolic dysfunction and HFpEF.
Acknowledgments
Dr. Lars Maier is funded by the Deutsche Forschungsgemeinschaft (MA 1982/2-2, MA 1982/4-1, TPA03 SFB 1002), as well as by the Leducq Transatlantic Networks of Excellence “Alliance for CaMK Signaling in Heart Disease” and “Redox and Nitrosative Regulation of Cardiac Remodeling: Novel Therapeutic Approaches for Heart Failure.”
Disclosures
Dr. Lars Maier acknowledges research grants and funding (including travel expense compensation and consulting fees) from CVT, Gilead, and MENARINI/Berlin-Chemie.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71:1231–1241. doi: 10.1161/01.RES.71.5.1231. [DOI] [PubMed] [Google Scholar]
- 2.Maier LS, Pieske B, Allen D Influence of stimulation frequency on [Na+]i and contractile function in Langendorff-perfused rat heart. Am J Physiol 1997;H1246–54 [DOI] [PubMed]
- 3.Pieske B, Maier LS, Piacentino V, 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.CIR.0000023042.50192.F4. [DOI] [PubMed] [Google Scholar]
- 4.Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog Biophys Mol Biol. 2008;96:421–451. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner S, Maier LS. Modulation of cardiac Na and Ca currents by CaM and CaMKII. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S26–S33. doi: 10.1111/j.1540-8167.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Xi Y, Wu G, Yang L, Han K, Du Y, Wang T, Lei X, Bai X, Ma A. Increased late sodium currents are related to transcription of neuronal isoforms in a pressure-overload model. Eur J Heart Fail. 2009;11:749–757. doi: 10.1093/eurjhf/hfp092. [DOI] [PubMed] [Google Scholar]
- 7.Fredj S, Lindegger N, Sampson KJ, Carmeliet P, Kass RS. Altered Na+ channels promote pause-induced spontaneous diastolic activity in long QT syndrome type 3 myocytes. Circ Res. 2006;99:1225–1232. doi: 10.1161/01.RES.0000251305.25604.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, El-Sherif T, Gidh-Jain M, Qin D, El-Sherif N. Alterations of sodium channel kinetics and gene expression in the postinfarction remodeled myocardium. J Cardiovasc Electrophysiol. 2001;12:218–225. doi: 10.1046/j.1540-8167.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- 9.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts–role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Makielski JC, Farley AL. Na(+) current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kogler H. Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther. 2007;321:213–220. doi: 10.1124/jpet.106.115519. [DOI] [PubMed] [Google Scholar]
- 17.Brandes R, Maier LS, Bers DM. Regulation of mitochondrial [NADH] by cytosolic [Ca2+] and work in trabeculae from hypertrophic and normal rat hearts. Circ Res. 1998;82:1189–1198. doi: 10.1161/01.RES.82.11.1189. [DOI] [PubMed] [Google Scholar]
- 18.Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 19.Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, Pepine CJ, Wang W, Nelson JJ, Hebert DA, Wolff AA. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 22.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.• Beyder A, Strege PR, Reyes S, Bernard CE, Terzic A, Makielski J, Ackerman MJ, Farrugia G Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel NaV1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–2706. This is the first report showing a novel mechanism of how Na channel scan be activated (ie, by mechanosensitivity) and how ranolazine may decrease this mechanism. [DOI] [PMC free article] [PubMed]
- 25.von Lewinski D, Stumme B, Maier LS, Luers C, Bers DM, Pieske B. Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc Res. 2003;57:1052–1061. doi: 10.1016/S0008-6363(02)00830-1. [DOI] [PubMed] [Google Scholar]
- 26.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110(6):841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- 28.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueredo VM, Pressman GS, Romero-Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther. 2011;16:168–172. doi: 10.1177/1074248410382105. [DOI] [PubMed] [Google Scholar]
- 30.Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, Theroux P, Bode C, Braunwald E. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol. 2010;55:1189–1196. doi: 10.1016/j.jacc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 31.Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, Biesiadecki BJ, Blackburn B, Wolff A, Stanley WC. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail. 2002;8:416–422. doi: 10.1054/jcaf.2002.129232. [DOI] [PubMed] [Google Scholar]
- 32.Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, Stanley WC, Sabbah HN. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–H2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: Background, aims, and design of the RALI-DHF study. Clin Cardiol. 2011;34:426–432. doi: 10.1002/clc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 35.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann W, Jacobshagen C, Wagner S, Maier LS (2011) Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res Cardiol 263–272 [DOI] [PMC free article] [PubMed]
- 36.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/Calmodulin Kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res. 1994;28:1231–1237. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Song Y, Belardinelli L, Shryock JC. The late Na+ current (INa) inhibitor ranolazine attenuates effects of palmitoyl-L-carnitine to increase late INa and cause ventricular diastolic dysfunction. J Pharmacol Exp Ther. 2009;330:550–557. doi: 10.1124/jpet.109.151936. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama K, Hara A, Hashizume H, Ushikubi F, Abiko Y. Ranolazine attenuates palmitoyl-L-carnitine-induced mechanical and metabolic derangement in the isolated, perfused rat heart. J Pharm Pharmacol. 2000;52:709–715. doi: 10.1211/0022357001774381. [DOI] [PubMed] [Google Scholar]
- 43.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardi-ac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 45.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 47.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Dhalla AK, Wang WQ, Dow J, Shryock JC, Belardinelli L, Bhandari A, Kloner RA. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1923–H1929. doi: 10.1152/ajpheart.00173.2009. [DOI] [PubMed] [Google Scholar]
- 49.Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, Karagueuzian HS. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol. 2011;57:366–375. doi: 10.1016/j.jacc.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res. 2011;92:67–72. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 51.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.RES.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 52.Zygmunt A, Goodrow R, Antzelevitch C (2000) I NaCa contributes to electrical heterogeneity within the canine ventricle. Am J Physiol Heart Circ Physiol H1671–8 [DOI] [PubMed]
- 53.Antoons G, Oros A, Beekman JD, Engelen MA, Houtman MJ, Belardinelli L, Stengl M, Vos MA. Late Na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55:801–809. doi: 10.1016/j.jacc.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Shryock J, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- 55.Murdock DK, Kersten M, Kaliebe J, Larrain G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–267. [PMC free article] [PubMed] [Google Scholar]
- 56.Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8:175–181. [PMC free article] [PubMed] [Google Scholar]
- 57.Miles RH, Murdock DK. Ranolazine verses amiodarone for prophylaxis against atrial fibrillation following coronary artery bypass surgery. Heart Rhythm. 2010;7:258. doi: 10.1016/j.hrthm.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 58.Hohnloser SH, Singh BN. Proarrhythmia with class III antiarrhythmic drugs: definition, electrophysiologic mechanisms, incidence, predisposing factors, and clinical implications. J Cardiovasc Electrophysiol. 1995;6:920–936. doi: 10.1111/j.1540-8167.1995.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 59.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajamani S, Shryock JC, Belardinelli L. Block of tetrodotoxin-sensitive, Na(V)1.7 and tetrodotoxin-resistant, Na(V)1.8, Na+ channels by ranolazine. Channels (Austin) 2008;2:449–460. doi: 10.4161/chan.2.6.7362. [DOI] [PubMed] [Google Scholar]
- 61.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 62.Kumar K, Nearing BD, Bartoli CR, Kwaku KF, Belardinelli L, Verrier RL. Effect of ranolazine on ventricular vulnerability and defibrillation threshold in the intact porcine heart. J Cardiovasc Electrophysiol. 2008;19:1073–1079. doi: 10.1111/j.1540-8167.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 63.Kumar K, Nearing BD, Carvas M, Nascimento BC, Acar M, Belardinelli L, Verrier RL. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. doi: 10.1111/j.1540-8167.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 64.Carvas M, Nascimento BC, Acar M, Nearing BD, Belardinelli L, Verrier RL. Intrapericardial ranolazine prolongs atrial refractory period and markedly reduces atrial fibrillation inducibility in the intact porcine heart. J Cardiovasc Pharmacol. 2010;55:286–291. doi: 10.1097/FJC.0b013e3181d26416. [DOI] [PubMed] [Google Scholar]
- 65.Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of ranolazine and dronedarone to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mullard A. Second chance for dronedarone after recent setback? Lancet. 2012;379:601. doi: 10.1016/S0140-6736(12)60251-7. [DOI] [PubMed] [Google Scholar]


