Abstract
Summary
In exploring relationships between vitamin D status in childhood and cortical bone, little relationship was observed with plasma concentrations of 25-hydroxyvitamin-D2 [25(OH)D2], whereas 25-hydroxyvitamin-D3 [25(OH)D3] was positively related to cortical bone mineral content (BMCC) and cortical thickness, suggesting D3 exerts a beneficial effect on cortical bone development in contrast to D2.
Introduction
The study is aimed to determine whether vitamin D status in childhood is related to cortical bone development by examining prospective relationships between plasma concentrations of 25(OH)D2 and 25(OH)D3 at 7.6, 9.9 or 11.8 years and peripheral quantitative computed tomography (pQCT) measurements of the mid-tibia at age 15.5 years, in children from the Avon Longitudinal Study of Parents and Children.
Methods
Relationships between vitamin D status and pQCT outcomes were analysed by bootstrap linear regression, adjusted for age, sex, body composition, socioeconomic position and physical activity, in 2,247 subjects in whom all covariates were available. 25(OH)D3 was also adjusted for season and 25(OH)D2, and 25(OH)D2 for 25(OH)D3.
Results
25(OH)D3 was positively related to BMCC [0.066(0.009,0.122), P = 0.02], whereas no association was seen with 25(OH)D2 [−0.008(−0.044,0.027), P = 0.7] [beta (with 95% CI) represents SD changes per doubling of vitamin D], P = 0.03 for difference in associations of 25(OH)D2 and 25(OH)D3 with BMCC. There were also differences in associations with cortical geometry, since 25(OH)D3 was positively related to cortical thickness [0.11(0.04, 0.19), P = 0.002], whereas no association was seen with 25(OH)D2 [−0.04(−0.08,0.009), P = 0.1], P = 0.0005 for difference. These relationships translated into differences in biomechanical strength as reflected by buckling ratio, which was positively related to 25(OH)D2 [0.06(0.01,0.11), P = 0.02] indicating less resistance to buckling, but inversely related to 25(OH)D3 [−0.1(−0.19,-0.02), P = 0.03], P = 0.001 for difference.
Conclusions
In contrast to 25(OH)D2, 25(OH)D3 was positively related to subsequent cortical bone mass and predicted strength. In vitamin D-deficient children in whom supplementation is being considered, our results suggest that D3 should be used in preference to D2.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-011-1813-9) contains supplementary material, which is available to authorized users.
Keywords: Adolescence, ALSPAC, Cortical bone, pQCT, Vitamin D
Introduction
Severe vitamin D deficiency, caused by reduced sun exposure, leads to osteomalacia (adults)/rickets (children) resulting from defective skeletal mineralisation. Milder vitamin D deficiency, termed ‘insufficiency’, may also affect skeletal health in the elderly by reducing bone mineral density (BMD) and increasing fracture risk due to secondary hyperparathyroidism, in the absence of mineralisation defects [1]. If also applicable in childhood, vitamin D requirements in children would need to be set to prevent insufficiency rather than vitamin D deficiency and rickets [2]. Vitamin D insufficiency in children may be relatively common. For example, in Maine, USA, 48% of girls aged 9–11 had a total 25-hydroxyvitamin D (25(OH)D) level below the 50 nmol l-1 (20 ng ml-1) cutoff commonly used to indicate D insufficiency at least once over 3 years [3].
To ensure adequate vitamin D status, recommended dietary allowances of vitamin D have recently been proposed across different age groups including children [4]. However, a recent Cochrane review concluded that vitamin D supplementation in healthy children had limited effects, but more trials are required to confirm the efficacy of supplementation in deficient children [5]. Whereas three studies in children reported modest improvements in bone outcomes following treatment with cholecalciferol (D3) [6–8], ergocalciferol (D2) was without effect in one study [9]. A possible explanation is that D2 may be less potent than D3, since D3 and its metabolites have a higher affinity than D2 for hepatic 25-hydroxylase and vitamin D receptors [10]. Furthermore, in one such study, effects of D3 supplementation on BMD were suggested to be due to changes in lean mass [6], consistent with observations that levels of vitamin D metabolites and sunlight exposure are related to height and body composition [11–13], which are in turn strongly related to bone parameters [14].
Observational studies of the relationship between plasma concentration of total 25(OH)D and bone outcomes in childhood have yielded conflicting findings [15–17]. These differences may have arisen from confounding, which is difficult to adjust based on results of total 25(OH)D levels, since D2 and D3 are derived from different sources. For example, as the majority of D3 is derived from skin synthesis following the action of UVR, 25(OH)D3 levels are affected by factors influencing sun exposure such as outdoor physical activity which is known to affect bone development [18]. Whereas dietary fish intake and fortification of certain foods contribute to D3, D2 is mainly derived from fungi, plants and dietary supplements, implying that dietary patterns affect levels of 25-hydroxyvitamin-D2 [25(OH)D2] and, to a lesser extent, 25-hydroxyvitamin-D3 [25(OH)D3]. This represents another source of confounding since dietary patterns may affect bone development [19], possibly through coassociation with socioeconomic position (SEP) which is also related to bone development in childhood [20].
We examined whether vitamin D status influences cortical bone development in childhood, based on 25(OH)D2 and 25(OH)D3 concentrations measured at age 7.6, 9.9 or 11.8, and results of peripheral quantitative computed tomography (pQCT) scans of the mid-tibia performed at age 15.5, in the Avon Longitudinal Study of Parents and Children (ALSPAC). Specifically, we wished to determine (i) whether vitamin D status is related to subsequent cortical bone development, (ii) to what extent are its associations which we find are independent of confounders including physical activity and body composition (based on contemporaneous measures of fat and lean mass by DXA), and (iii) whether there is any evidence that 25(OH)D3 has stronger associations with cortical bone development than 25(OH)D2, suggesting supplementation regimes based on D3 are likely to be more effective compared to those using D2.
Methods
Study population
All pregnant women resident within a defined part of the former county of Avon in South West England with an expected date of delivery between April 1991 and December 1992 were eligible for recruitment, of whom 14,451 were enrolled [21] (http://www.alspac.bristol.ac.uk). Written informed consent was provided by the mothers, and informed assent was obtained from the children at the time of assessment. Ethical approval was obtained from the ALSPAC Law and Ethics Committee (internal) and the Central and South Bristol Research Ethics Committee (external). Data in ALSPAC is collected by self-completion postal questionnaires sent to main caregivers and the children themselves, by abstraction from medical records, and from examination of the children at research clinics. All children with available data were included in the analyses.
Blood measurements
The primary exposures for this study were circulating concentrations of 25(OH)D2 and 25(OH)D3 as measured on nonfasting blood samples collected at the age 9.9 research clinic. If no samples were available from the 9.9 clinic, samples from the 11.8 clinic were used, or from the age 7.6 year clinic if neither the 9.9 or 11.8 were available. Following collection samples were immediately spun, frozen and stored at −80°C. Assays were performed in 2010 after a maximum of 12 years in storage with no previous freeze–thaw cycles during this period. 25(OH)D2, 25(OH)D3 and deuterated internal standard were extracted from serum samples, following protein precipitation, using Isolute C18 solid phase extraction cartridges. Potential interfering compounds were removed by initial elution with 50% methanol followed by elution of the vitamins using 10% tetrahydrofuran in acetonitrile. Dried extracts were reconstituted prior to injection into a high performance liquid chromatography tandem mass spectrometre in the multiple reaction mode (MRM). The MRM transitions (m/z) used were 413.2 > 395.3, 401.1 > 383.3 and 407.5 > 107.2 for 25(OH)D2, 25(OH)D3 and hexa-deuterated(OH)D3, respectively. Coefficients of variation (CVs) for the assay were <10% across a working range of 1 to 250 ng ml-1 for both 25(OH)D2 and 25(OH)D3. Intact parathyroid hormone [iPTH(1–84)] [1] was measured by electrochemiluminescence immunoassay on an Elecsys 2010 immunoanalyzer (Roche, Lewes, UK). Inter-assay CV was less than 6% from 2 to 50 pmol l-1. The assay sensitivity (replicates of the zero standard) was 1 pmol l-1.
pQCT variables
At the age 15.5 research clinic, pQCT scans at the 50% mid-tibia were also performed using the Stratec XCT2000L (Stratec, Pforzheim, Germany). Cortical bone area, cortical bone mineral content (BMCC), cortical bone mineral density (BMDC), periosteal circumference, endosteal circumference and cortical thickness were recorded. Strength parametres comprised section modulus, cross-sectional moment of inertia, strength strain index and buckling ratio. A density of >650 mg cm-3 was used to define cortical bone. Endosteal and periosteal circumference were derived using a circular ring model. 4502 pQCT scans were performed, of which 88 were excluded due to major motion artifacts. Coefficients of variation for pQCT scans, based on 139 subjects scanned a mean of 31 days apart, were 2.7%, 1.3% and 2.9% for BMCC, BMDC and cortical bone area, respectively.
Other variables
At 15.5 years research clinics, standing height (mm) was measured using the Harpenden Stadiometer (Holtain, Crymych, Wales, UK), and weight using the Tanita Body Fat Analyzer (model TBF 305; Tanita, Arlington Heights, IL, USA). Whole body DXA scans were performed using a Lunar Prodigy scanner with paediatric scanning software (GE Lunar Prodigy, Madison, WI, USA), providing measures of total body fat and lean mass. Maternal SEP was recorded at 32 weeks gestation by questionnaire and categorised according to the Office of Population Censuses and Surveys. Maternal education was assessed at the same time by questionnaire. Pubertal stage was assessed using a Tanner stage (pubic hair domain) questionnaire completed at age 14.7 years [22]. Moderate and vigorous physical activity was assessed by actigraph accelerometre at age 11, and subsequently found to be related to BMD in ALSPAC [23]. Date of birth and sex was obtained from birth notification, and date of the scan was recorded automatically, allowing age at scan to be calculated.
Statistical analyses
Descriptive statistics show means, standard deviation (SD), medians and lower and upper quartiles. Analyses were performed using seasonally adjusted 25 (OH)D3, which was modelled according to date of blood sampling using linear regression with trigonometric sine and cosine functions. 25(OH)D3 was loge transformed to reduce heteroscedasticity. The residual was used as the primary 25(OH)D3 exposure variable in subsequent regression analyses. All analyses were performed on standardised variables, i.e. subtracting the mean and dividing by the SD. To include all participants on whom a 25(OH)D2 was assayed, those with a value below the detectable limit of the assay (0.5 ng ml-1) were assigned a binary variable indicating whether an individual was at or below the lower limit, which was used as a covariable in all regression models. No individuals had 25(OH)D3 below the detectable limit of the assay. Models were checked for linearity by adding higher-order terms into the linear predictor and by comparing the likelihood of nested models.
Further analyses were performed using a nonparametric bootstrap procedure in conjunction with OLS linear regression, based on 5,000 replications. Beta (β) estimates and standard errors were calculated from the mean and SD of the bootstrap distribution, respectively. All P values were calculated using bootstrap means and standard errors, compared to a Z-distribution and 95% percentile confidence intervals calculated. Beta estimates were multiplied by loge(2) and interpreted as per doubling in 25(OH)D2 or 25(OH)D3. Estimates were calculated separately for males and females, and the difference investigated using a bootstrap Wald test. Combined (male and female) associations were also investigated following adjustment for sex. The difference between the effect of 25(OH)D2 and 25(OH)D3 was calculated from the bootstrap replicate distribution, and the P values using a Wald test.
Minimally adjusted analyses (model 1), which were based on seasonally adjusted 25(OH)D3 levels or 25(OH)D2, were adjusted for sex, age at pQCT scan, and adjusted for 25(OH)D2 and seasonally adjusted 25(OH)D3, respectively. In model 2, we additionally adjusted for loge-transformed fat mass, lean mass and height. In the final model (model 3), we also adjusted for physical activity and social economic factors (maternal or paternal social class, maternal education). Analyses with endosteal circumference were adjusted for periosteal circumference throughout (endosteal adjusted for periosteal circumference). Sensitivity analyses were performed based on model 2 by: (a) adjusting for parathyroid hormone (PTH); (b) restricting those with available puberty information and then, in this subgroup, examining the impact of adjusting for pubertal status (tanner stages IV/V versus earlier stages); and (c) restricting those with 25(OH)D assays collected at age 9.9 years. All analyses were conducted using STATA 11.2(College Station, TX, USA), and data is assumed to be missing at random.
Results
Descriptive analyses
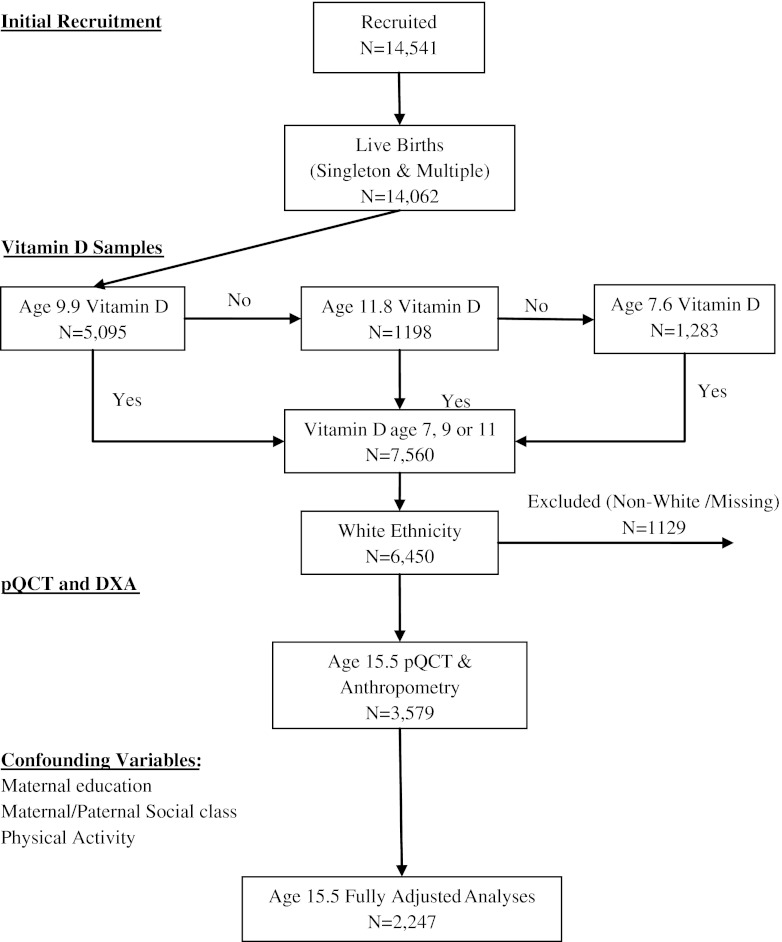
There were 1,709 boys and 1,870 girls with pQCT scans (age 15.5 years), and plasma 25(OH)D2 and 25(OH)D3 (age 7.6, 9.9 or 11.8 years; see Fig. 1). Those who were included in the analysis were of higher maternal and paternal social class compared to those who were not. Boys were taller, heavier and had greater lean mass compared to girls, whereas fat mass was higher in girls (Table 1). BMCC, cortical bone area, periosteal circumference, endosteal circumference and cortical thickness were greater in boys compared to girls, whereas BMDC was higher in girls. 25(OH)D3 levels were slightly higher in boys and 25(OH)D2 levels slightly higher in girls. PTH levels were slightly higher in girls. There was evidence of weak inverse associations between 25(OH)D2 and height LM and FM, which appeared somewhat stronger in girls compared to boys, e.g. P = 0.06 for gender interaction test for association with height (Table 2). There was little association between 25(OH)D3 and height, and LM P > 0.75, and weak evidence of an association with FM P = 0.06. 25(OH)D3 was inversely related to PTH, whereas no association was seen for 25(OH)D2. There was a very weak association between seasonally adjusted 25(OH)D3 and 25(OH)D2, r = −0.0298 P = 0.155, excluding those subjects in whom 25(OH)D2 was below the assay detection limit.
Fig. 1.
Summary of data collection
Table 1.
Descriptive statistics
| [N (male)=1,709; N (female)=1,870] | |||||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | 25th | 50th | 75th | |||
| Age [year] | Male | 15.5 | (0.26) | 15.3 | 15.4 | 15.6 | |
| Female | 15.5 | (0.28) | 15.3 | 15.4 | 15.6 | ||
| Anthropometry | Height [cm] | Male | 174.3 | (7.5) | 169.7 | 174.5 | 179.4 |
| Female | 164.8 | (6.1) | 160.7 | 164.7 | 168.6 | ||
| Weight [kg] | Male | 63.5 | (11.4) | 56.0 | 61.9 | 69.3 | |
| Female | 58.8 | (10.3) | 51.9 | 57.0 | 63.9 | ||
| BMI [kg m-2] | Male | 20.8 | (3.1) | 18.8 | 20.2 | 22.2 | |
| Female | 21.6 | (3.5) | 19.3 | 21.0 | 23.2 | ||
| Fat mass-Total body [kg] | Male | 10.8 | (7.8) | 5.7 | 8.3 | 12.9 | |
| Female | 18.6 | (7.9) | 13.2 | 17.1 | 22.1 | ||
| Lean mass-Total body [kg] | Male | 49.8 | (6.6) | 45.7 | 49.9 | 54.1 | |
| Female | 37.1 | (3.9) | 34.5 | 36.8 | 39.5 | ||
| pQCT | BMDC [mg cm-3] | Male | 1,074.2 | (34.3) | 1,053.1 | 1,077.1 | 1,099.2 |
| Female | 1,124.6 | (22.3) | 1,111.2 | 1,126.3 | 1,139.8 | ||
| BAC [mm2] | Male | 329.1 | (46.8) | 297.1 | 329.3 | 359.6 | |
| Female | 275.1 | (36.6) | 250.0 | 273.6 | 298.7 | ||
| BMCC [mg] | Male | 353.8 | (53.2) | 318.8 | 353.7 | 388.3 | |
| Female | 309.3 | (41.0) | 281.1 | 308.0 | 335.9 | ||
| PC [mm] | Male | 76.2 | (5.3) | 72.8 | 76.1 | 79.6 | |
| Female | 69.5 | (4.9) | 66.3 | 69.2 | 72.6 | ||
| EC [mm] | Male | 40.9 | (5.9) | 37.1 | 40.4 | 44.1 | |
| Female | 37.0 | (5.4) | 33.6 | 36.5 | 39.7 | ||
| CT [mm] | Male | 5.63 | (0.7) | 5.2 | 5.7 | 6.1 | |
| Female | 5.17 | (0.6) | 4.8 | 5.2 | 5.6 | ||
| Plasma measures | 25(OH)D3 [ng ml-1] | Male | 24.1 | (9.0) | 18.1 | 23.0 | 28.5 |
| Female | 22.8 | (8.2) | 17.1 | 22.1 | 27.4 | ||
| 25(OH)D2 [ng ml-1] | Male | 1.80 | (1.9) | 0.5 | 1.2 | 2.6 | |
| Female | 1.89 | (1.9) | 0.5 | 1.4 | 2.7 | ||
| PTH [pmol l-1] | Male | 4.53 | (1.8) | 3.2 | 4.2 | 5.5 | |
| Female | 5.11 | (2.3) | 3.5 | 4.6 | 6.1 | ||
Table shows descriptive characteristics of anthropometric parametres, 50% tibia pQCT parametres, and plasma measures in males and females at age 15.5 years. Statistics are presented as means, SDs, medians, and upper and lower quartiles
Table 2.
Associations between plasma concentration of 25(OH)D2 and 25(OH)D3 and anthropometry variables
| Vitamin 25(OH)D2 | Vitamin 25(OH)D3 | P value (D2D3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimally adjusted, N = 3,579 (males=1,709) | Minimally adjusted, N = 3,579 (males=1,709) | |||||||
| Beta | 95% CI | P value (sex) | Beta | 95% CI | P value (sex) | |||
| Height | Male | −0.026 | (−0.072, 0.021) | 0.06 | −0.070 | (−0.169, 0.026) | 0.04 | 0.42 |
| Female | −0.070 | (−0.107, -0.028) | 0.056 | (−0.016, 0.131) | 0.01 | |||
| ALL | −0.050 | (−0.085, -0.011) | 0.000 | (−0.061, 0.061) | 0.17 | |||
| Lean mass | Male | −0.021 | (−0.059, 0.017) | 0.17 | −0.027 | (−0.112, 0.060) | 0.22 | 0.90 |
| Female | −0.040 | (−0.073, -0.017) | 0.034 | (−0.012, 0.081) | 0.01 | |||
| ALL | −0.030 | (−0.063, -0.006) | 0.007 | (−0.040, 0.054) | 0.14 | |||
| Fat mass | Male | −0.017 | (−0.066, 0.031) | 0.30 | −0.048 | (−0.160, 0.066) | 0.72 | 0.61 |
| Female | −0.040 | (−0.081, -0.001) | −0.070 | (−0.140, -0.003) | 0.44 | |||
| ALL | −0.030 | (−0.069, 0.007) | −0.060 | (−0.124, 0.002) | 0.40 | |||
| Ln PTH | Male | −0.010 | (−0.064, 0.045) | 0.55 | −0.260 | (−0.367, -0.148) | 0.65 | 0.01 |
| Female | −0.026 | (−0.076, 0.024) | −0.290 | (−0.392, -0.189) | 0.01 | |||
| ALL | −0.019 | (−0.064, 0.027) | −0.270 | (−0.346, -0.200) | 0.01 | |||
Table shows associations between plasma concentration of 25(OH)D2 and 25(OH)D3 and height, total body lean mass, loge fat mass and loge parathyroid hormone (PTH), adjusted for sex, age at scan and 25(OH)D3 and 25(OH)D2 respectively, in 1709 males and 1870 females at age 15.5 years. Beta coefficients represent SD change in height, lean mass, loge fat mass, and loge PTH, per doubling of vitamin 25(OH)D2 and 25(OH)D3. 95% confidence intervals are presented for beta coefficients, P value (sex) shows the difference in associations between males and females, and P value (D2D3) is the probability of a difference in associations between 25(OH)D2 and 25(OH)D3
Associations between 25(OH)D concentrations and pQCT variables
There was a weak inverse association between 25(OH)D2 and BMDC in both minimally and more fully adjusted analyses, to a similar extent in boys and girls (Table 3). For example, in boys and girls combined, in our most completely adjusted model, a doubling in 25(OH)D2 was associated with a 0.05SD decrease in BMDC. Whereas 25(OH)D2 was unrelated to periosteal circumference in minimally adjusted analyses, there was a weak positive association in more fully adjusted models, to a similar degree, in boys and girls. In minimally adjusted analyses, 25(OH)D2 was inversely related to cortical bone area, BMCC, endosteal adjusted for periosteal circumference and cortical thickness in females, but this was not seen after more complete adjustment. There was a positive association between 25(OH)D2 levels and buckling ratio in all models, to a similar extent, in boys and girls (Table S1).
Table 3.
Associations between plasma concentration of 25(OH)D2 and pQCT parametres
| Vitamin 25(OH)D2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimally adjusted, N = 3,579 (males=1,709) | Anthropometry-adjusted, N = 3,579 (males=1,709) | Anthropometry-, SES- and PA-adjusted, N = 2,247 (males=1,203) | ||||||||
| Beta | 95% CI | P value (sex) | Beta | 95% CI | P value (sex) | Beta | 95% CI | P value (sex) | ||
| Cortical bone mineral density | Male | −0.055 | (−0.099, -0.010) | 0.74 | −0.048 | (−0.091, -0.005) | 0.89 | −0.049 | (−0.101, 0.004) | 0.98 |
| Female | −0.048 | (−0.082, -0.014) | −0.046 | (−0.078, -0.014) | −0.048 | (−0.089, -0.008) | ||||
| ALL | −0.051 | (−0.084, -0.017) | −0.047 | (−0.079, -0.015) | −0.048 | (−0.087, -0.011) | ||||
| Cortical bone area | Male | −0.006 | (−0.055, 0.043) | 0.04 | 0.013 | (−0.021, 0.047) | 0.32 | 0.006 | (−0.038, 0.049) | 0.90 |
| Female | −0.054 | (−0.095, -0.013) | −0.003 | (−0.030, 0.025) | 0.003 | (−0.033, 0.039) | ||||
| ALL | −0.033 | (−0.071, 0.006) | 0.004 | (−0.022, 0.031) | 0.004 | (−0.029, 0.037) | ||||
| Cortical bone mineral content | Male | −0.021 | (−0.074, 0.031) | 0.05 | 0.000 | (−0.035, 0.036) | 0.37 | −0.007 | (−0.053, 0.039) | 0.93 |
| Female | −0.069 | (−0.113, -0.026) | −0.015 | (−0.044, 0.015) | −0.009 | (−0.047, 0.028) | ||||
| ALL | −0.048 | (−0.089, -0.007) | −0.008 | (−0.036, 0.020) | −0.008 | (−0.044, 0.027) | ||||
| Periosteal circumference | Male | 0.018 | (−0.026, 0.062) | 0.08 | 0.035 | (0.002, 0.067) | 0.82 | 0.037 | (−0.007, 0.080) | 0.86 |
| Female | −0.021 | (−0.061, 0.021) | 0.031 | (0.000, 0.063) | 0.041 | (0.003, 0.079) | ||||
| ALL | −0.004 | (−0.040, 0.032) | 0.033 | (0.005, 0.060) | 0.039 | (0.006, 0.075) | ||||
| Endosteal adjusted for periosteal circumference | Male | 0.026 | (−0.012, 0.063) | 0.14 | 0.01 | (−0.024, 0.044) | 0.22 | 0.017 | (−0.025, 0.058) | 0.71 |
| Female | 0.052 | (0.021, 0.082) | 0.029 | (0.001, 0.057) | 0.024 | (−0.009, 0.059) | ||||
| ALL | 0.040 | (0.011, 0.069) | 0.021 | (−0.007, 0.047) | 0.021 | (−0.010, 0.053) | ||||
| Cortical thickness | Male | −0.031 | (−0.085, 0.025) | 0.07 | −0.018 | (−0.065, 0.030) | 0.24 | −0.029 | (−0.088, 0.029) | 0.73 |
| Female | −0.078 | (−0.123, -0.033) | −0.044 | (−0.082, -0.006) | −0.039 | (−0.089, 0.007) | ||||
| ALL | −0.057 | (−0.100, -0.014) | −0.032 | (−0.069, 0.005) | −0.035 | (−0.081, 0.009) | ||||
Table shows associations between plasma concentration of 25(OH)D2 and 50% tibial pQCT parametres at age 15.5 years. Beta coefficients represent SD change in pQCT parametre per doubling of vitamin 25(OH)D2. 95% Confidence intervals are presented with respect to the beta coefficients, P value (sex) shows the difference in associations between males and females. Results are also shown for the following adjustments: minimally adjusted=sex and age at scan; anthropometry adjusted=minimally adjusted+height, loge fat mass and lean mass; anthropometry, SES, PA adjusted=anthropometry-adjusted+maternal and paternal social class, maternal education, and physical activity. All analyses were adjusted for vitamin 25(OH)D3
Positive associations were observed between 25(OH)D3 and cortical bone area and BMCC in anthropometry adjusted and fully adjusted analyses (Table 4). In all models, 25(OH)D3 was positively related to cortical thickness and inversely related to endosteal adjusted for periosteal circumference. For example, in our most fully adjusted model, a doubling in 25(OH)D3 was associated with a 0.11 SD increase in cortical thickness. There was also an inverse association between 25(OH)D3 and buckling ratio in both minimally and more fully adjusted analyses (Table S2), suggesting a protective effect on the skeleton since buckling ratio is inversely related to bone strength. These associations tended to be stronger in boys, in whom beta coefficients were two to three times higher than in girls, and P values for gender-specific regression equations were only below the P < 0.05 significance threshold in boys. However, formal gender interaction tests were consistently P> = 0.1, and so evidence that these associations were stronger in boys compared to girls is not compelling.
Table 4.
Associations between plasma concentration of 25(OH)D3 and Pqct parametres
| Vitamin 25(OH)D3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimally adjusted, N = 3,579 (males=1,709) | Anthropometry-adjusted, N = 3,579 (males=1,709) | Anthropometry-, SES- and PA-adjusted, N = 2,247 (males=1,203) | ||||||||
| Beta | 95% CI | P value (sex) | Beta | 95% CI | P value (sex) | Beta | 95% CI | P value (sex) | ||
| Cortical bone mineral density | Male | −0.028 | (−0.124, 0.066) | 0.52 | −0.020 | (−0.110, 0.070) | 0.53 | 0.018 | (−0.103, 0.137) | 0.94 |
| Female | 0.010 | (−0.054, 0.072) | 0.015 | (−0.047, 0.077) | 0.013 | (−0.065, 0.089) | ||||
| ALL | −0.007 | (−0.064, 0.047) | −0.001 | (−0.054, 0.052) | 0.016 | (−0.054, 0.082) | ||||
| Cortical bone area | Male | 0.062 | (−0.043, 0.163) | 0.45 | 0.091 | (0.023, 0.162) | 0.05 | 0.100 | (0.015, 0.191) | 0.22 |
| Female | 0.013 | (−0.064, 0.087) | 0.006 | (−0.047, 0.058) | 0.031 | (−0.034, 0.096) | ||||
| ALL | 0.036 | (−0.028, 0.099) | 0.045 | (0.003, 0.087) | 0.061 | (0.008, 0.116) | ||||
| Cortical bone mineral content | Male | 0.057 | (−0.056, 0.170) | 0.55 | 0.089 | (0.019, 0.162) | 0.08 | 0.105 | (0.014, 0.198) | 0.23 |
| Female | 0.015 | (−0.067, 0.093) | 0.008 | (−0.049, 0.064) | 0.034 | (−0.036, 0.103) | ||||
| ALL | 0.035 | (−0.034, 0.104) | 0.045 | (0.002, 0.090) | 0.066 | (0.009, 0.122) | ||||
| Periosteal circumference | Male | 0.002 | (−0.095, 0.096) | 0.65 | 0.034 | (−0.039, 0.104) | 0.13 | 0.004 | (−0.090, 0.099) | 0.96 |
| Female | −0.027 | (−0.111, 0.054) | −0.038 | (−0.096, 0.021) | 0.001 | (−0.069, 0.068) | ||||
| ALL | −0.013 | (−0.077, 0.047) | −0.005 | (−0.052, 0.039) | 0.002 | (−0.056, 0.059) | ||||
| Endosteal adjusted for periosteal circumference | Male | −0.083 | (−0.161, -0.007) | 0.43 | −0.097 | (−0.164, -0.031) | 0.17 | −0.127 | (−0.214, -0.045) | 0.13 |
| Female | −0.044 | (−0.100, 0.014) | −0.036 | (−0.087, 0.018) | −0.043 | (−0.106, 0.020) | ||||
| ALL | −0.062 | (−0.108, -0.015) | −0.064 | (−0.105, -0.022) | −0.080 | (−0.132, -0.029) | ||||
| Cortical thickness | Male | 0.117 | (0.006, 0.228) | 0.39 | 0.131 | (0.039, 0.226) | 0.21 | 0.180 | (0.061, 0.306) | 0.13 |
| Female | 0.054 | (−0.029, 0.137) | 0.054 | (−0.021, 0.126) | 0.061 | (−0.029, 0.151) | ||||
| ALL | 0.084 | (0.014, 0.152) | 0.089 | (0.031, 0.148) | 0.114 | (0.041, 0.190) | ||||
Table shows associations between plasma concentration of 25(OH)D3 and 50% tibial pQCT parametres at age 15.5 years. Beta coefficients represent SD change in pQCT parametre per doubling of vitamin 25(OH)D3. 95% Confidence intervals are presented with respect to the beta coefficients, P value (sex) shows the difference in associations between males and females. Results are also shown for the following adjustments: minimally adjusted=sex, season of 25(OH)D3 measurement and age at scan; anthropometry-adjusted=minimally adjusted+height, loge fat mass and lean mass; anthropometry-, SES- and PA-adjusted=anthropometry adjusted+maternal and paternal social class, maternal education, and physical activity. All analyses were adjusted for vitamin 25(OH)D2
Subsequently, we compared associations between 25(OH)D2 and pQCT parametres as shown in Table 3, with associations between 25(OH)3 and pQCT parametres as shown in Table 4. P values for differences in these associations are shown in Table 5, for minimally and more fully adjusted models. In the case of BMDC and cortical bone area, there was weak evidence of a difference between 25(OH)D2 and 25(OH)D3 in fully adjusted models, P = 0.1 and P = 0.07, respectively, boys and girls combined (Table 5). For BMCC, there was moderate evidence of a difference between 25(OH)D2 and 25(OH)D3 P < 0.05 in all models, boys and girls combined. There was strong evidence of difference between 25(OH)D2 and 25(OH)D3 in CT, endosteal adjusted for periosteal circumference and BR, P < 0.001 in minimal and more completely adjusted models, boys and girls combined. Apart from weak evidence of a difference in girls in our anthropometry-adjusted model (P = 0.04), there was no evidence of a difference between 25(OH)D2 and 25(OH)D3 with respect to periosteal circumference. No difference was observed for any model in respect of associations between 25(OH)D2 and 25(OH)D3 and cross-sectional moment of inertia, section modulus and strength strain index (results not shown).
Table 5.
Differences between associations of plasma concentration of 25(OH)D2 and 25(OH)D3 with pQCT parametres
| Sex | Minimally adjusted P value | Anthropometry-adjusted P value | Anthropometry-, SES- and PA-adjusted P value | |
|---|---|---|---|---|
| N = 3,579 (males=1,709) | N = 3,579 (males=1,709) | N = 2,247 (males=1,203) | ||
| BMDC | Male | 0.62 | 0.58 | 0.31 |
| Female | 0.11 | 0.08 | 0.16 | |
| All | 0.19 | 0.14 | 0.10 | |
| BAC | Male | 0.25 | 0.05 | 0.07 |
| Female | 0.13 | 0.77 | 0.45 | |
| All | 0.06 | 0.10 | 0.07 | |
| BMCC | Male | 0.22 | 0.03 | 0.03 |
| Female | 0.07 | 0.46 | 0.28 | |
| All | 0.04 | 0.04 | 0.03 | |
| PC | Male | 0.77 | 0.98 | 0.53 |
| Female | 0.89 | 0.04 | 0.30 | |
| All | 0.80 | 0.15 | 0.26 | |
| ECPC | Male | 0.01 | 0.01 | 0.01 |
| Female | 0.01 | 0.03 | 0.07 | |
| All | 0.01 | 0.01 | 0.01 | |
| CT | Male | 0.02 | 0.01 | 0.01 |
| Female | 0.01 | 0.02 | 0.05 | |
| All | 0.01 | 0.01 | 0.01 | |
| BR | Male | 0.03 | 0.03 | 0.01 |
| Female | 0.01 | 0.01 | 0.04 | |
| All | 0.01 | 0.01 | 0.01 |
Table shows the P value for differences between the associations of plasma concentration of 25(OH)D2 and 25(OH)D3 with 50% tibial pQCT parametres at age 15.5 years (as shown in Tables 3 and 4, respectively). Results are also shown for the following adjustments: minimally adjusted=sex and age at scan; anthropometry-adjusted=minimally adjusted+height, loge fat mass and lean mass; anthropometry-, SES- and PA-adjusted= anthropometry-adjusted+maternal and paternal social class, maternal education, and physical activity. All results are adjusted for 25(OH)D2 and D3
Sensitivity analyses and exploration of additional models
In view of the biological relationship between vitamin D status and PTH concentrations, we examined whether associations between pQCT parametres and 25(OH)D which we observed were mediated by PTH, but repeating the above analyses including additional adjustment for PTH did not affect the results (see Table S3 for results for buckling ratio, anthropometry-adjusted analyses). In the case of associations between 25(OH)D2 and buckling ratio, β was attenuated by approximately 15% when restricting analyses to those with complete puberty information, but no further change was seen after adjusting for Tanner stage within this subset. β for the association between 25(OH)D2 and buckling ratio increased by approximately 50% on restricting analyses to subjects with blood samples at age 9.9, suggesting some associations may be strengthened when vitamin D samples obtained a longer interval before pQCT measurements are excluded. β values were very similar across all groups for associations between 25(OH)D3 and buckling ratio. We found no evidence of nonlinearity of associations between either seasonally adjusted 25(OH)D3 or 25(OH)D2 in any of the models fitted.
Discussion
We report by far the largest prospective cohort study of relationships between vitamin D status in childhood and subsequent cortical bone outcomes. 25(OH)D3 was positively related to BMCC as measured by pQCT approximately 5 years later, which appeared to be secondary to an increase in CT. This association between 25(OH)D3 and cortical thickness resulted from a decrease in endosteal expansion, since 25(OH)D3 showed an equivalent inverse association with endosteal adjusted for periosteal circumference. This relationship may also have led to greater biomechanical strength, in view of the inverse association observed between 25(OH)D3 and buckling ratio. The latter relationship may have implications in terms of reduced fracture risk in later life, based on associations between buckling ratio as measured at the hip in elderly populations, and subsequent risk of hip fracture [24, 25]. The effect sizes that we observed were similar in magnitude to that of other important external influences on skeletal development such as fat mass, which we have previously reported to influence cortical bone development [14]. In further analyses, based on the same study sample, we found that a doubling in fat mass was associated with a 0.13 SD increase in cortical thickness (analyses adjusted for age and height), which was similar to that seen for 25(OH)D3, of which a doubling was associated with a 0.11 SD increase in cortical thickness.
Identification of 25(OH)D concentrations in childhood associated with optimal outcomes for bone and other health outcomes, and how these might translate into public health recommendations, is a matter of controversy [26]. Arguably, the finding that a doubling in 25(OH)D3 is associated with a 0.11 SD increase in cortical thickness is not a strong enough effect to justify widespread vitamin D supplementation in childhood. Since >25% of our study population had insufficient total 25(OH)D based on the 20 ng ml-1 cutoff [26], this conclusion is likely to apply to other, predominantly Caucasian, populations with a similarly high prevalence of vitamin D insufficiency based on this definition. This may represent a contrast with early life exposure in utero, when vitamin D status has been suggested to have major long-term influences on subsequent bone development including periosteal growth [27, 28]. On the other hand, 25(OH)D3 may have a stronger association with cortical outcomes in certain subgroups, in whom supplementation may be more justifiable. For example, beta coefficients were generally higher in boys, in whom a doubling in 25(OH)D3 was associated with a 0.18 SD increase in CT. Moreover, the magnitude of effects that we observed may have been tempered by aspects of the study design (see ‘Limitations’ below). Furthermore, whereas observational studies of this nature provide some information as to the likely benefits of vitamin D supplementation in childhood, evidence from randomized controlled trials is required before definitive conclusions can be drawn.
In those children in whom vitamin D supplementation is being considered, an important question which follows is which form of vitamin D is the most effective. In contrast to the positive associations between 25(OH)D3 and cortical bone outcomes described above, relationships with 25(OH)D2 were null in the case of BMCC and cortical thickness. Whereas a weak positive association was present between 25(OH)D2 and periosteal circumference, there was a weak inverse association with BMDC, as well as a weak positive association with buckling ratio suggesting reduced resistance to buckling. Taken together, these findings suggest that, in contrast to 25(OH)D3, 25(OH)D2, at these concentrations, is not associated with an overall benefit in terms of future cortical bone development, which may have important implications in terms of the choice of vitamin D supplementation in childhood.
To our knowledge, no previous study has examined the separate relationships between 25(OH)D2, 25(OH)D3 and bone outcomes in childhood. Since 25(OH)D3 makes the major contribution to total 25(OH)D, it is relevant to compare our findings with those from these previous studies based on total 25(OH)D. In a prospective study of 171 girls aged 9–15 years, total 25(OH)D was positively associated with gains in femoral neck BMD over the following 3 years which may have reflected an influence of 25(OH)D3 on cortical thickness as we observed [16]. On the other hand, our findings contrast with those of a previous study in which total 25(OH)D was found to be positively related to BMDC of the radius and tibia in a cross-sectional study based on 193 10- to 12-year-old girls [15]. In terms of previous interventional studies, in a recent study in 20 pairs of peripubertal female twins, D3 supplements for 6 months led to an increase in tibial cortical bone area due to reduced endosteal expansion as assessed by pQCT [7]. In contrast, in a recent D2 supplementation trial in 73 girls aged 12–14 years, no effect was observed on pQCT parametres [9]. Although these findings are consistent with our observation of an inverse association between endosteal adjusted for periosteal circumference and 25(OH)D3, but not 25(OH)D2, to our knowledge, no previous study has directly compared the effect of administering these two forms of vitamin D on cortical bone.
In terms of biological explanations for possible distinct effects of 25(OH)D2 and 25(OH)D3 on bone, as suggested by our results, indirect pathways via PTH may be involved. Whereas 25(OH)D3 levels are known to be inversely related to PTH, as confirmed here, an equivalent relationship was not seen for 25(OH)D2, which is consistent with a previous finding that a large dose of D3 decreased PTH in the elderly, whereas D2 was without effect [29]. Any tendency for 25(OH)D2 and 25(OH)D3 to differ in respect of their relationships with PTH may be partly due to the fact that D2 is less potent than D3: D3 and its metabolites have a higher affinity than D2 for hepatic 25-hydroxylase and vitamin D receptors; D3 is not directly metabolised to 24(OH)D as is D2; 25(OH)D2 has a lower affinity for vitamin D binding protein compared to 25(OH)D3, leading to faster metabolism and a shorter half life [10]. However, adjusting our analyses for PTH did not attenuate the observed association between 25(OH)D3 and endosteal adjusted for periosteal circumference, suggesting that differing relationships with PTH are unlikely to explain the distinct associations between 25(OH)D2, 25(OH)D3 and cortical bone parametres which we observed.
Alternatively, due to the observational nature of this study, these different associations with 25(OH)D2 and 25(OH)D3 may have arisen from confounding. For example, 25(OH)D3 levels are determined by sun exposure and diet that may be affected by a range of factors including SEP and outdoor physical activity, which may confound relationships with bone outcomes. Although the association between 25(OH)D3 and endosteal adjusted for periosteal circumference was unaffected by adjusting for observed measurements of these additional factors, unmeasured confounders may be important. For example, D2 intake is related to consumption of fruits and vegetables, which is positively associated with childhood BMD as measured by DXA [30]. In addition, fruit and vegetable intake is related to a ‘prudent’ or ‘healthy’ diet [31], of which intake in pregnancy is positively associated with BMD in subsequent childhood [19].
Limitations
In terms of limitations of this study, our pQCT measurements comprised a single slice, namely, the 50% mid-tibia, which is unable to provide any information about trabecular bone. In the study of 171 girls aged 9–15 years described above, the relationship between baseline total 25(OH)D and subsequent gain in BMD across puberty was particularly strong at the lumbar spine [16] which is rich in trabecular bone. Whereas the present study suggests that 25(OH)D status has minimal effects on cortical bone, it may be that stronger effects exist for trabecular bone which we were unable to evaluate here. A further limitation is the relatively long interval between measurement of 25(OH)D and measurement of cortical bone from pQCT scans, which may have reduced the strength of associations observed between these sets of parametres. Finally, the generalisability of our findings is limited by the fact that the subset of 3,579 subjects forming the basis of the present study is likely to differ in important ways from the original cohort drawn from the general population. For example, maternal social class in the subset on which this paper is based was higher compared with those who were not included (P = 0.0001).
In conclusion, we found that in contrast to 25(OH)D2, 25(OH)D3, as measured in childhood, was positively related to BMCC, cortical thickness and resistance to buckling as assessed 5 years later. These different associations suggest that supplementation with vitamin D3 in childhood is likely to prove more beneficial for subsequent cortical bone development compared to vitamin D2, presumably reflecting important differences between the actions of these two isoforms on bone, the basis of which is currently unclear. Interventional studies are justified in which effects of these two forms of vitamin D are directly compared in the same population, in order to test the conclusions from this observational study, given that we are unable to exclude confounding as a possible explanation for our findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Associations between plasma concentration of 25(OH)D2 and pQCT strength parametres (DOC 60.0 kb)
Associations between plasma concentration of 25(OH)D3 and pQCT strength parametres (DOC 59.0 kb)
Sensitivity analysis of the associations of plasma concentration of 25(OH)D2 and 25(OH)D3 with buckling ratio (DOC 61.5 kb)
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
The work presented here is funded by a UK Medical Research Council grant (G0701603). The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC. Salary support for AS is provided by Wellcome Trust grant ref. 079960, which also funded the pQCT scans. DAL works in a centre that receives core funds from the UK Medical Research Council (G G0600705) and University of Bristol. No funding body directed the study or interfered with its conduct and interpretation of results; the views presented here are those of the authors and not necessarily any funding body. This publication is the work of the authors who serve as guarantors for the contents of this paper.
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
A. Sayers, Email: adrian.sayers@bristol.ac.uk
J. H. Tobias, Email: jon.tobias@bristol.ac.uk
References
- 1.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf) 2005;62(3):265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CM. Vitamin D, calcium homeostasis, and skeleton accretion in children. J Bone Miner Res. 2007;22(Suppl 2):V45–V49. doi: 10.1359/jbmr.07s201. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Dietetic Assoc. 2005;105(6):971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Ross AC, Taylor LC, Yaktine AL, Del Valle HB (2010) Committee to review dietary reference intakes for vitamin D and calcium. Institute of Medicine Institute of Medicine
- 5.Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;10:CD006944. doi: 10.1002/14651858.CD006944.pub2. [DOI] [PubMed] [Google Scholar]
- 6.El-Hajj Fuleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91(2):405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 7.Greene DA, Naughton GA (2010) Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: a randomised controlled trial. Osteoporos Int. Jun 11 [DOI] [PubMed]
- 8.Viljakainen HT, Natri AM, Karkkainen M, et al. A positive dose–response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res. 2006;21(6):836–844. doi: 10.1359/jbmr.060302. [DOI] [PubMed] [Google Scholar]
- 9.Ward KA, Das G, Roberts SA, et al (2010) A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. Jul 14 [DOI] [PubMed]
- 10.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 11.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 12.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 13.Waldie KE, Poulton R, Kirk IJ, Silva PA. The effects of pre- and post-natal sunlight exposure on human growth: evidence from the southern hemisphere. Early Hum Dev. 2000;60(1):35–42. doi: 10.1016/S0378-3782(00)00102-X. [DOI] [PubMed] [Google Scholar]
- 14.Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95(2):699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Tylavsky F, Kroger H, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78(3):485–492. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 16.Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr. 2002;76(6):1446–1453. doi: 10.1093/ajcn/76.6.1446. [DOI] [PubMed] [Google Scholar]
- 17.Tylavsky FA, Ryder KM, Li R, et al. Preliminary findings: 25(OH)D levels and PTH are indicators of rapid bone accrual in pubertal children. J Am Coll Nutr. 2007;26(5):462–470. doi: 10.1080/07315724.2007.10719637. [DOI] [PubMed] [Google Scholar]
- 18.McKay HA, MacLean L, Petit M, et al. “Bounce at the bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39(8):521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole ZA, Gale CR, Javaid MK, et al. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J Bone Miner Res. 2009;24(4):663–668. doi: 10.1359/jbmr.081212. [DOI] [PubMed] [Google Scholar]
- 20.Clark EM, Ness A, Tobias JH. Social position affects bone mass in childhood through opposing actions on height and weight. J Bone Miner Res. 2005;20:2082–2089. doi: 10.1359/JBMR.050808. [DOI] [PubMed] [Google Scholar]
- 21.Golding J, Pembrey M, Jones R. ALSPAC — the Avon Longitudinal Study of Parents and Children: 1. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 22.Morris N, Udrey J. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 23.Tobias JH, Steer CD, Mattocks C, Riddoch C, Ness AR. Habitual levels of physical activity influence bone mass in 11 year-old children from the UK: findings from a large population-based cohort. J Bone Miner Res. 2007;22:101–109. doi: 10.1359/jbmr.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892–1904. doi: 10.1359/jbmr.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivadeneira F, Zillikens MC, De Laet CE, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam study. J Bone Miner Res. 2007;22(11):1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 26.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 27.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 28.Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94(3):765–771. doi: 10.1210/jc.2008-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 30.McGartland CP, Robson PJ, Murray LJ, et al. Fruit and vegetable consumption and bone mineral density: the Northern Ireland Young Hearts Project. Am J Clin Nutr. 2004;80(4):1019–1023. doi: 10.1093/ajcn/80.4.1019. [DOI] [PubMed] [Google Scholar]
- 31.Crozier SR, Robinson SM, Borland SE, Inskip HM. Dietary patterns in the Southampton women’s survey. Eur J Clin Nutr. 2006;60(12):1391–1399. doi: 10.1038/sj.ejcn.1602469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations between plasma concentration of 25(OH)D2 and pQCT strength parametres (DOC 60.0 kb)
Associations between plasma concentration of 25(OH)D3 and pQCT strength parametres (DOC 59.0 kb)
Sensitivity analysis of the associations of plasma concentration of 25(OH)D2 and 25(OH)D3 with buckling ratio (DOC 61.5 kb)