Abstract
Purpose
Dry eye syndromes affect a significant proportion of the population worldwide with reported prevalence ranging from 6% to more than 34%. Patients with dry eye can experience intense pain due to eye irritation, gritty/scratchy feeling in the eyes, blurry vision, and light sensitivity. Available treatments for dry eye syndromes remain mainly palliative. The purpose of the present study was to test the hypothesis that inhibiting sodium absorption via the epithelial sodium channel (ENaC) will increase ocular hydration in both normal as well as in animals with experimentally induced dry eye.
Methods
ENaC inhibitors were dissolved in an aqueous buffer that mimics the composition of tears and were applied topically to the ocular surface of isoflurane-anesthetized mice. The effect of ENaC inhibitors was compared with that of the secretagogue uridine triphosphate (UTP; 1%), a purinergic receptor agonist which was shown to increase tear volume in animals. Tear production was measured for 10 s using phenol red-impregnated cotton threads. Fluorescein staining that assesses ocular surface damage was performed at baseline and then at days 1, 2, and 3 after the induction of dry eye in mice.
Results
Our data show that the inhibition of ENaC led to a time- and concentration-dependent increase in tear volume in normal mice. The effect of ENaC inhibition after a single application outperformed UTP, as it was long-lasting with tear volume still above baseline values 8 h postdosing. ENaC inhibition, which led to increased tear production, improved fluorescein scores in our dry eye model, when compared with nontreated or animals treated with buffer or UTP.
Conclusion
We conclude that the inhibition of ENaC provides long-lasting increases in ocular surface hydration and that ENaC blockers could provide an effective new therapy for chronic dry eye.
Introduction
The tear film is the interface between the external environment and the ocular surface.1,2 It forms a smooth refractive surface over the corneal surface, lubricates the eyelids, and maintains the optimal extracellular environment for the epithelial cells of the cornea and conjunctiva.1,2 The tear film is a hydrated mucus gel covered by a lipid layer. Mucins are secreted by the cornea and conjunctiva.3 The aqueous component of the tear film is secreted by the main and accessory lacrimal glands,4 whereas the meibomian glands secrete the outermost lipid layer.5 The lipid layer is thought to play a major role in retarding evaporation of the aqueous components of tears.5
The production of tears in an inadequate quantity or of an inadequate quality leads to symptoms of dry eye.6–9 Dry eye disease is divided into 2 major categories: aqueous deficient dry eye and evaporative dry eye.7 Aqueous deficient dry eye is mainly due to failure of lacrimal gland secretion and is further divided into Sjögren's syndrome dry eye and non-Sjögren's dry eye.8,9 Sjögren's syndrome is a systemic inflammatory disease affecting primarily the lacrimal and salivary glands.10 It may either exist as a primary disorder or can be associated with other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, or systemic sclerosis.10 Evaporative dry eye is due to excessive evaporation of the aqueous layer of the tear film and is mainly caused by meibomian gland dysfunction or posterior blepharitis.8,11
The prevalence of dry eye from various large epidemiological studies reveals a range of about 6% to more than 34%.12–15 Four U.S. studies reported a range of about 8% to 15%; 2 Australian studies reported a range of about 6% to 17%; whereas 2 Asian studies reported a range of 28% to 34%.12 The variation in reported dry eye prevalence between these studies is probably due to differences in the definition of disease used.12 Nevertheless, patients with dry eye can experience intense pain due to eye irritation, gritty/scratchy feeling in the eyes, blurry vision, and light sensitivity. If left unmanaged, dry eye can have devastating consequences on the ocular surface such as corneal abrasion leading to scarring or ulceration and potentially to vision loss.7
The treatment for dry eye remains mainly symptomatic.15–20 Treatments include the use of artificial tears, topical autologous serum eye drops, or punctual plug occlusion.15–20 Although oral pilocarpine and cevimeline, 2 cholinergic muscarinic agonists, were shown to stimulate salivation in Sjögren's syndrome patients, their effect on tear production is still unclear.21 Topical cyclosporine A was shown to suppress ocular inflammation and restore tear production in severe cases of Keratoconjunctivitis sicca (KCS) and received FDA approval.22
In classic models of ocular fluid balance, the lacrimal glands are viewed as the source of all tear volume. However, in a more current view of tear volume regulation, the lacrimal glands are believed to dominate reflex (or stimulated) tearing, while the corneal and conjunctival epithelia are important modulators of basal tear volume and composition.23–26 Similar to the epithelia of the lung, gastrointestinal tract (GI), and kidneys, the ocular surface epithelium regulates mucosal hydration through a coupled process involving Na+ absorption and Cl− secretion. The transport of electrolytes provides an osmotic gradient entraining water through aquaporin channels, which either decreases (Na+ absorption) or increases (Cl− secretion) tear volume.26–29
Experiments in primary cultures of corneal/conjunctival epithelium,30 ex vivo tissue preparations,27,31 and in vivo bioelectric studies28,29 have identified many of the ocular surface ion channels and signal transduction pathways capable of modifying tear volume. In a series of studies evaluating the electrical potential difference (ePD) on the mouse ocular surface in vivo, Levin et al. identified the cystic fibrosis transmembrane conductance regulator and the calcium-activated chloride channel (CaCC) as the major ocular surface Cl− channels; while sodium absorption was primarily mediated by the epithelial sodium channel (ENaC).28,29
While targeting pathways that increase chloride secretion is a well-described approach for increasing ocular hydration, the inhibition of absorptive pathways has not been as well characterized. The available data suggest that inhibiting sodium absorption via ENaC will increase ocular hydration: (1) ENaC is expressed in corneal and conjunctival epithelia24,32; (2) measuring the ePD across the mouse cornea in vivo, it was shown that ENaC was one of the dominant channels mediating salt transport on the ocular surface in vivo28; and (3) a single instillation of amiloride (0.1%) was associated with an increase in tear volume (as measured by Schirmer's I test) for up to 1 h postdosing.24 Taken together, these studies suggest that ENaC plays a role in regulating the volume of tears on the ocular surface and suggest that the inhibition of ENaC will increase ocular hydration. The purpose of the present study was to test this hypothesis. Our data demonstrate that ENaC blockers can provide long-lasting increases in ocular surface hydration and suggest that ENaC blockers could provide an effective new therapy for chronic dry eye.
Methods
Chemicals and animals
The novel ENaC blockers, P-301 and P-365, were provided by Parion Sciences. Amiloride was purchased from Sigma Aldrich, and uridine triphosphate (UTP) was purchased from CalBiochem.
Female BALB/c mice (10–12 weeks old) were purchased from Taconic. The animals were maintained in constant temperature rooms with fixed light/dark intervals of 12 h length and were fed ad libitum. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Tufts Medical Center Animal Care and Use Committee.
Assessment of ENaC blocker potency
We evaluated the ability of P-301 or amiloride to inhibit the transport of sodium through ENaC in a primary cell culture system as previously described.33 Epithelial cells were isolated from excised canine bronchial tissue and cultured under conditions that promote a polarized, well-differentiated monolayer. Cultures were mounted in modified Ussing Chambers and under voltage-clamped conditions, and short-circuit current (ISC) was measured as an index of transepithelial sodium transport. P-301 or amiloride were added to the apical side of the chambers at increasing concentrations, and the corresponding changes in short-circuit current were measured. Using standard curve-fitting methods and goodness-of-fit assessments, the concentration of the compound needed to inhibit a short circuit current by 50% (IC50 concentration) was calculated as the index of potency.
Measurement of tear volume
Tear production was measured on lightly anesthetized (Isoflurane) mice using phenol red-impregnated cotton threads (Zone-Quick; Lacrimedics), as previously described.34 The threads were held with jeweler forceps and applied to the ocular surface, on both eyes, in the lateral canthus for 10 s. Wetting of the thread (which turns red in contact with tears) was measured in millimeters under a dissecting microscope. All tested compounds were dissolved in a buffer that mimics the composition of tears, containing in mM 106.5 NaCl, 26.1 NaHCO3, 18.7 KCl, 1.0 MgCl2, 0.5 KH2PO4, 1.1 CaCl2, 10 HEPES, and pH 7.4. Tear volume was measured at baseline and at various time points after the application of vehicle (tear buffer) or the tested compound in a 2 μL drop to both eyes.
Measurement of drug levels in the preocular tear film
To determine the actual concentration of P-301 in the preocular tear film, a single drop (2 μL) of P-301 at a concentration of 1 mM was applied. Tear output was measured at baseline and at 1, 2, and 6 h after the application of P-301. Immediately after each measurement, tear buffer (3×3 μL) was applied, and the animals were forced to blink. The concentration of P-301 in the combined “eye washes” (preocular tear film) was determined using a Waters ultra performance liquid chromatography (UPLC) by comparing the peak areas from tears to known standards.
Aqueous-deficient dry eye animal model
Aqueous-deficient dry eye was induced via an injection of recombinant human interleukin 1 into the exorbital lacrimal glands of female BALB/c mice, as described earlier.34 Dry eye symptoms in this animal model are transient and last about 3 days, due to recovery of the lacrimal gland via tissue repair/regeneration, as previously reported.34,35 Before surgery, tear production was measured in all groups of mice followed by fluorescein staining. After surgery and once the animals had regained consciousness, vehicle or the active compound was applied topically in a 2 μL drop. Thereafter, tear production was measured in the morning (starting at 9 a.m.) followed by fluorescein staining examination. Buffer or the active compound was applied twice daily, once immediately after the morning tear measurement/fluorescein staining and then again in the afternoon (∼8 h later).
Fluorescein staining
A fluorescein sodium ophthalmic strip (Fluorets, 1 mg fluorescein in 10 mL tear buffer) was used. A 1 μL drop was applied to the ocular surface, and the mouse was induced to “blink” thrice. After 2 min, the degree of fluorescein staining was determined using a slit-lamp biomicroscope. The following 0 to 4 scaling system was adopted: 0: no staining; 1: minimal staining (scattered staining); 2: mild staining (few areas stained); 3: staining is visible in ∼2/3 of the corneal surface; 4: intense staining—large areas are stained.
Data presentation and statistical analysis
Data are expressed as means±standard error of the mean. The data were statistically analyzed using the Student's t-test for paired or unpaired values. Values of P<0.05 were considered significant.
Results and Discussion
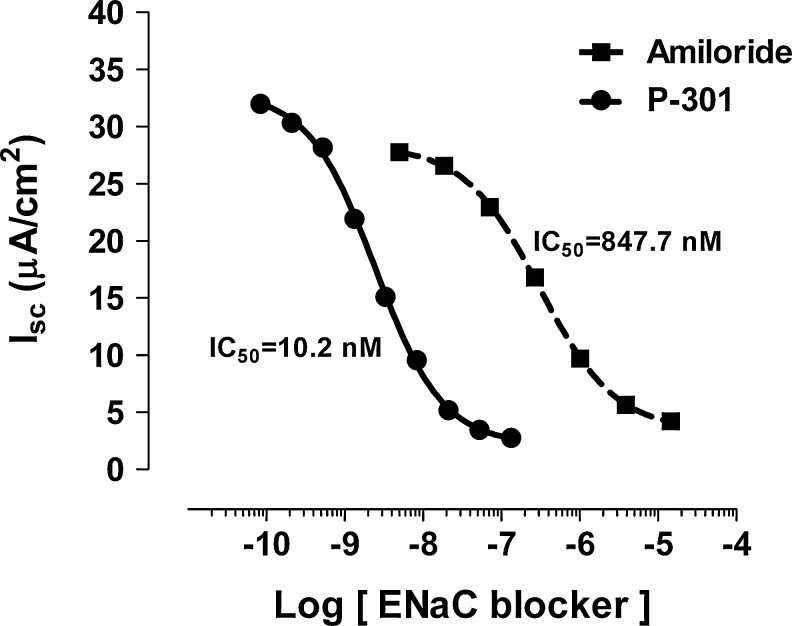
The potency of the novel sodium channel blocker P-301 on ENaC in primary cultures of canine bronchial epithelia (CBE) was determined by recording the change in short circuit currents (Isc) in response to increasing concentrations of drugs (10−11–10−4 M) in the apical bath and after apical wash. As previously reported,33 active sodium transport was the dominant component of Isc in CBE. Amiloride and P-301 produced sigmoidal concentration-effect curves as expected for drug-receptor binding and exhibited saturable binding (Fig. 1). The calculated IC50 for amiloride and P-301 were 847.7 and 10.2 nM, respectively, demonstrating that P-301 is >80 times more potent than amiloride (Fig. 1).
FIG. 1.
Effect of P-301 and amiloride on epithelial sodium channel (ENaC) in primary cell culture system. Epithelial cells were isolated from excised canine bronchial tissue and cultured under conditions that promote a polarized monolayer. Cultures were mounted in modified Ussing Chambers and under voltage-clamped conditions, and short-circuit current (ISC) was measured as an index of transepithelial sodium transport. P-301 or amiloride was added to the apical side of the chambers at increasing concentrations, and the corresponding changes in ISC were measured. Using standard curve-fitting methods and goodness-of-fit assessments, the concentration of the compound needed to inhibit ISC by 50% (IC50 concentration) was calculated (n=40 for amiloride, n=11 for P-301).
In another series of experiments, we tested the effect of P-301 as well as another ENaC inhibitor P-365, both used at a single concentration (100 μM), on tear output when applied topically to normal BALB/c mice. The response was compared with that elicited by UTP (1%), a P2Y agonist previously reported to increase tear output when applied topically.25 As shown in Fig. 2, compared with vehicle (tear buffer), all compounds increased tear output at 15 min postdosing. The response to UTP declined by 30 min, and tear output returned to basal levels by 60 min postdosing (Fig. 2). In contrast, although tear output declined with time after application of the ENaC inhibitors, it was still above baseline levels after 120 min (Fig. 2). Since P-301 seemed to perform better than P-365, it was chosen for further analyses.
FIG. 2.
Effect of P-301 and P-365 on tear output. Tear volume was measured at baseline (0) and at 15, 30, 60, and 120 min after application of the vehicle (tear buffer), uridine triphosphate (UTP; 1%), P-301 (100 μM), or P-365 (100 μM). Compounds were added in a 2 μL drop to both eyes, and tear volume was measured using phenol red-impregnated cotton threads for 10 s. Wetting of the thread was measured in millimeters under a dissecting microscope. Data are means±standard error of the mean (SEM) (n=5–8). *Denotes a statistically significant difference from baseline (0 min).
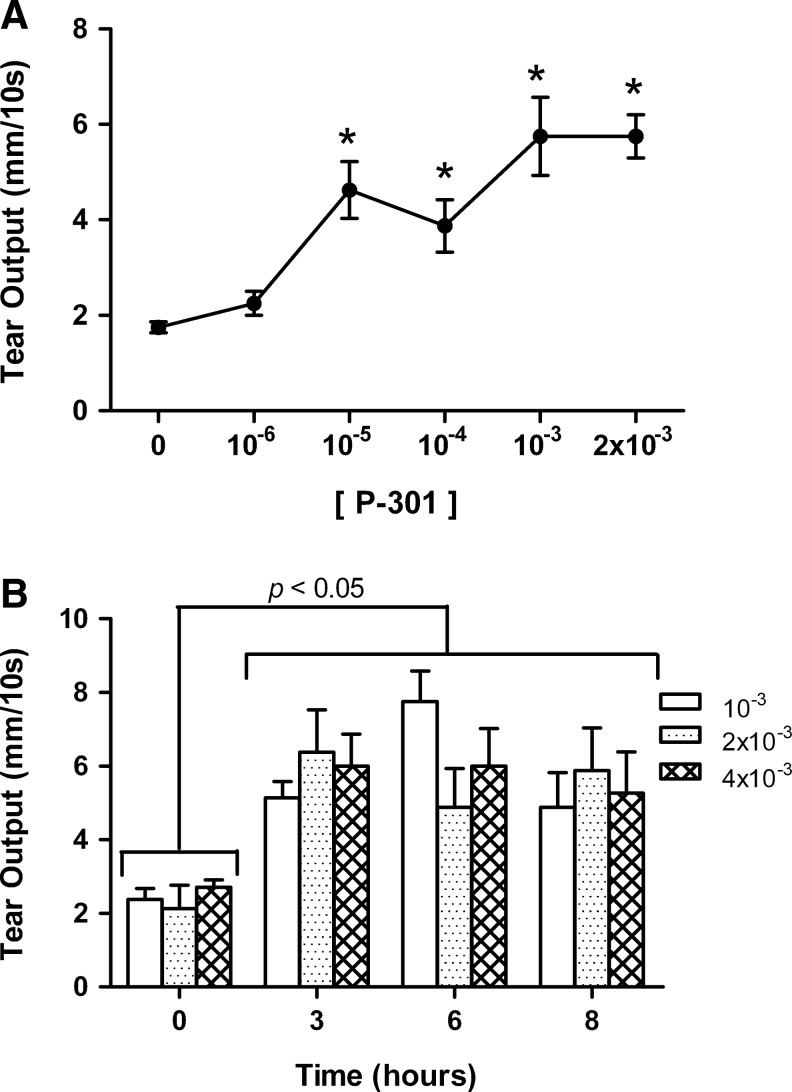
We next evaluated the concentration dependency of P-301 on tear output over a 60 min period. As shown in Fig. 3A, P-301 increased tear output in a concentration-dependent manner with a maximum 5.6-fold increase over basal reached with the 1 mM concentration. We next evaluated the effect of 3 concentrations of P-301 over a longer, clinically relevant 8 h time course. Tear output was measured at baseline and then at 3, 6, and 8 h postdosing following a single 2 μL drop of P-301 formulated at 1, 2, and 4 mM. As shown in Fig. 3B, at all concentrations tested, P-301 produced an increase in tear output that was still significantly above the baseline values at 8 h postdosing.
FIG. 3.
Concentration- and time dependency of P-301 on tear output. (A) Increasing concentrations of P-301 (10−6−2×10−3 M) were applied, and tear volume was measured 60 min postdosing. Data are means±SEM (n=4). *Denotes a statistically significant difference from baseline (0). (B) P-301 was applied at 3 concentrations (10−3, 2×10−3, and 4×10−3 M), and tear volume was measured at baseline (0) and then, at 3, 6, and 8 h postdosing. Data are means±SEM (n=4).
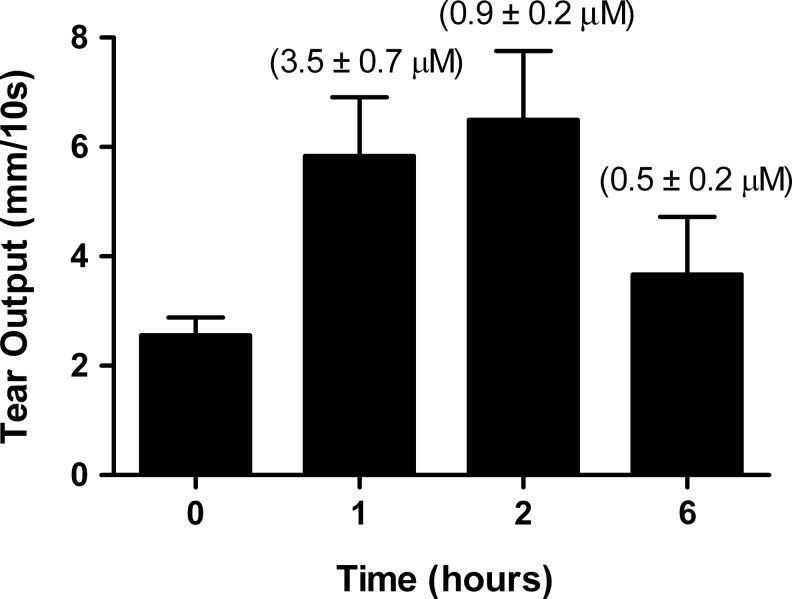
To determine the actual concentration of P-301 in the preocular tear film, a single drop (2 μL) of P-301 at a concentration of 1 mM was applied. Tear output was measured at baseline and at 1, 2, and 6 h after application of P-301. Immediately after each measurement, tear buffer (3×3 μL) was applied, and the animals were forced to blink. The concentration of P-301 in the combined “eye washes” (preocular tear film) was determined. As shown in Fig. 4, even at 6 h postdosing, there was sufficient P-301 (520 nM, ∼50-times the IC50) present in the ocular surface to fully block ENaC, hence accounting for the sustained effect of P-301 on tear output.
FIG. 4.
Concentration of P-301 in the preocular tear film. A single drop (2 μL) of P-301 (10−3 M) was applied, and tear volume was measured at baseline and at 1, 2, and 3 h after the application of P-301. Immediately after each measurement, tear buffer (3×3 μL) was applied, and the animals were forced to blink. The concentration of P-301 in the combined eye washes was determined by UPLC. Data are presented as means±SEM (n=3). The concentration of P-301 is shown above each bar.
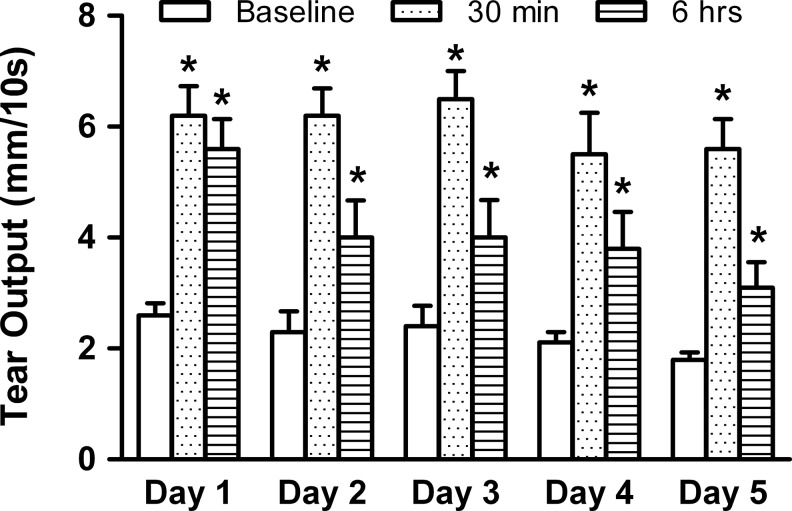
So far, our data suggest that P-301 have long-lasting effects on tear output and imply that continual application of P-301 to the ocular surface will not result in “desentizisation” of ENaC and will still result in increased tear output. To test this hypothesis, we evaluated the effect of repeated dosing of P-301, on the same group of animals, over a 5-day period. During that period, tear output was measured daily at baseline, and then at 30 min and 6 h postdosing with P-301 (1 mM). As shown in Fig. 5, daily application of P-301 elicited the same tear output measured 30 min postdosing. Although the 6-h tear output response seemed to diminish with repeated application, it remained above baseline values after completion of the 5-day dosing regimen (Fig. 5).
FIG. 5.
Effect of repeated dosing of P-301 on tear output. P-301 (10−3 M) was topically applied (2 μL) to the same group of animals over a 5-day period. During that period, tear output was measured daily at baseline, and then, at 30 min and 6 h postdosing with P-301. Data are means±SEM (n=4). *Denotes a statistically significant difference from baseline.
Taken together, our data demonstrate that P-301 produces a long-acting increase in tear volume which corresponds to the retention of an active concentration of drug in the preocular tear film. Furthermore, at the concentrations administered in this study, P-301 appears to be safe, as no obvious corneal damage was observed by slit-lamp examination. In addition, in exaggerated ocular safety studies, repeat ocular dosing of P-301 in New Zealand white rabbits at concentrations exceeding 10 mM did not produce signs of ocular irritation (data not shown). Thus, our data imply that prolonged daily applications of P-301 appear to be safe and that the response of ENaC does not subside with time after repeated dosing.
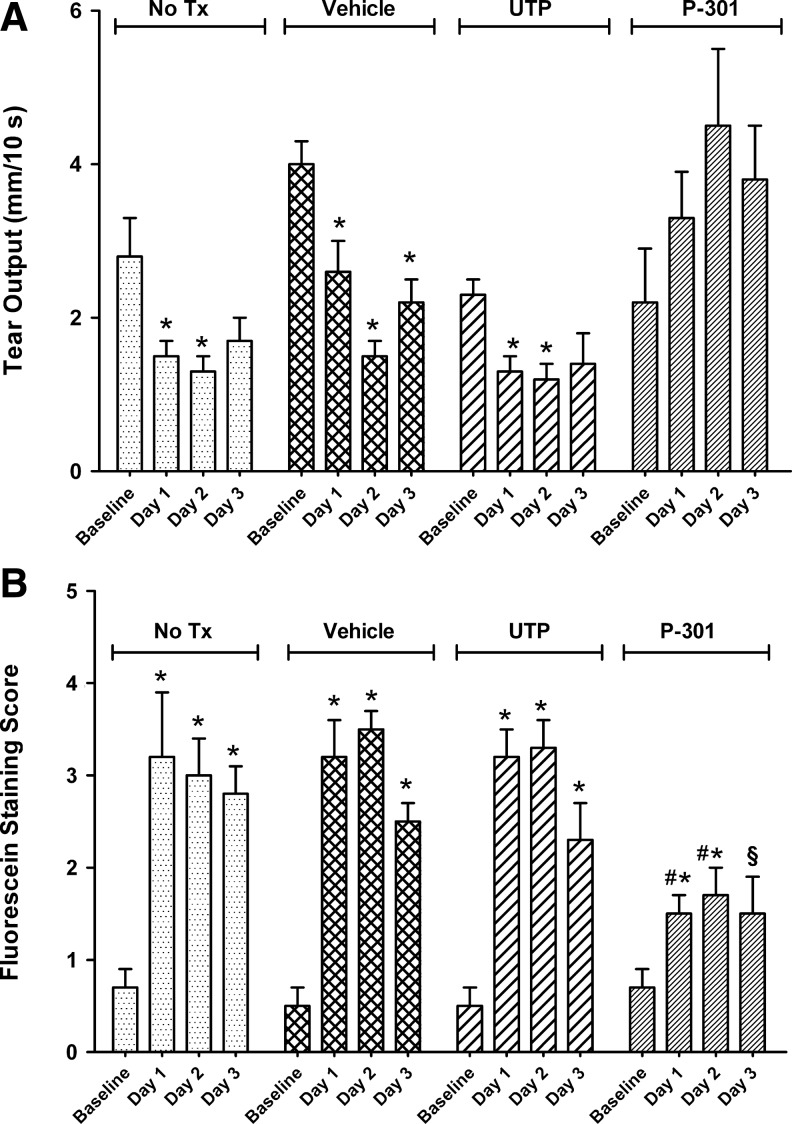
In the next series of experiments, we sought to determine whether topical application of P-301 can diminish corneal damage caused by experimentally induced aqueous-deficient type of dry eye in BALB/c mice. Corneal damage can be visualized and quantified using vital dyes such as fluorescein: Healthy corneas with an intact epithelium do not pick up the dye, whereas damaged corneas would, and the amount of dye retained on the ocular surface is proportional to the degree of epithelial damage. After induction of dry eye, animals were divided into 4 groups: no treatment, tear buffer, UTP (1%), or P-301 (1 mM); applied topically twice a day.
As shown in Fig. 6, the control nontreated animals had reduced tear output (Fig. 6A), which resulted in higher fluorescein staining scores (Fig. 6B). Similarly, animals that received tear buffer or UTP (which only causes short-term (15–30 min, Fig. 2) increase in tear volume) had diminished tear output, which resulted in higher fluorescein staining scores (Fig. 6A, B). In contrast, tear output in animals that were treated with topical applications of P-301 was equivalent or greater than that measured at baseline (Fig. 6A). Concomitant with preserved/enhanced tear output, fluorescein staining scores were significantly lower in the P-301-treated animals (Fig. 6B); albeit, day 3 scores were only statistically significantly different from the no-treatment group. It is worth noting that tear output and fluorescein staining measurements were done before the application of the first dose of medication each day (or ∼16 h postdose), once again demonstrating the long-lasting action of P-301.
FIG. 6.
Effect of P-301 on tear output and corneal fluorescein staining in a dry eye animal model. Eyes of animals with induced dry eye were either left untreated (No Tx) or received twice daily topical applications of tear buffer (vehicle), UTP (1%), or P-301 (10−3 M). Thereafter, tears (A) were measured in the morning followed by fluorescein staining (B) examination. Buffer or the active compound were applied twice daily, once immediately after the morning tear measurement/fluorescein staining and then again in the afternoon (∼8 h later). Data are means±SEM (n=3). *Denotes a statistically significant difference from baseline. #Denotes a statistically significant difference from No Tx, vehicle, and UTP. §Denotes a statistically significant difference from No Tx.
These results suggest that ENaC blockers are good drug targets for chronic dry eye, as both an increase in tear output and a decrease in corneal damage were observed after topical application of P-301, compared with untreated, buffer treated, or UTP-treated controls.
In summary, our data demonstrate that ENaC blockers can provide long-lasting increases in ocular surface hydration, suggesting that ENaC blockers could provide an effective new therapy for treating the underlying cause of chronic dry eye, insufficient tear volume.
Acknowledgments
The authors gratefully acknowledge Dr. Fara Sourie for her invaluable contribution to this work. This study was supported in part by the National Eye Institute Grant RO1-EY12383 and by an unrestricted research grant from Parion Sciences.
Author Disclosure Statement
W.R.T., M.R., and A.J.H. are employees of Parion Sciences. C.K. and D.Z. have no competing financial interest.
References
- 1.Tiffany J.M. The normal tear film. Dev. Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- 2.Tiffany J.M. Bron A.J. Role of tears in maintaining corneal integrity. Trans. Ophthalmol. Soc. U. K. 1978;98:335–338. [PubMed] [Google Scholar]
- 3.Gipson I.K. Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 4.Dartt D.A. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog. Retin. Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bron A.J. Tiffany J.M. Gouveia S.M. Yokoi N. Voon L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee A.J. Lee J. Saw S.M. Gazzard G. Koh D. Widjaja D. Tan D.T. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br. J. Ophthalmol. 2002;86:1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Listed N.A. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul. Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 8.Stern M.E. Gao J. Siemasko K.F. Beuerman R.W. Pflugfelder S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp. Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitali C. Bombardieri S. Jonsson R. Moutsopoulos H.M. Alexander E.L. Carsons S.E. Daniels T.E. Fox P.C. Fox R.I. Kassan S.S. Pillemer S.R. Talal N. Weisman M.H. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan D.A. Sullivan B.D. Evans J.E. Schirra F. Yamagami H. Liu M. Richards S.M. Suzuki T. Schaumberg D.A. Sullivan R.M. Dana M.R. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. N. Y. Acad. Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 12.Listed N.A. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul. Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 13.Schaumberg D.A. Sullivan D.A. Dana M.R. Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 2002;506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 14.Moss S.E. Klein R. Klein B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 15.Gayton J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvao-Santos G. Borges C. Nunes S. Salgado-Borges J. Duarte L. Efficacy of 3 different artificial tears for the treatment of dry eye in frequent computer users and/or contact lens users. Eur. J. Ophthalmol. 2011;21:538–544. doi: 10.5301/EJO.2011.6324. [DOI] [PubMed] [Google Scholar]
- 17.Friedman N.J. Impact of dry eye disease and treatment on quality of life. Curr. Opin. Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 18.Hamano T. Lacrimal duct occlusion for the treatment of dry eye. Semin. Ophthalmol. 2005;20:71–74. doi: 10.1080/08820530590931133. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien P.D. Collum L.M. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004;4:314–319. doi: 10.1007/s11882-004-0077-2. [DOI] [PubMed] [Google Scholar]
- 20.Foulks G.N. The evolving treatment of dry eye. Ophthalmol Clin North Am. 2003;16:29–35. doi: 10.1016/s0896-1549(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 21.Fox R.I. Konttinen Y. Fisher A. Use of muscarinic agonists in the treatment of Sjögren's syndrome. Clin. Immunol. 2001;101:249–263. doi: 10.1006/clim.2001.5128. [DOI] [PubMed] [Google Scholar]
- 22.Pflugfelder S.C. Integrating restasis into the management of dry eye. Int. Ophthalmol. Clin. 2006;46:101–103. doi: 10.1097/01.iio.0000212137.85298.98. [DOI] [PubMed] [Google Scholar]
- 23.Murakami T. Fujihara T. Horibe Y. Nakamura M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004;36:89–93. doi: 10.1159/000076887. [DOI] [PubMed] [Google Scholar]
- 24.Hara S. Hazama A. Miyake M. Kojima T. Sasaki Y. Shimazaki J. Dogru M. Tsubota K. The effect of topical amiloride eye drops on tear quantity in rabbits. Mol. Vis. 2010;16:2279–2285. [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y. Kuang K. Yerxa B. Wen Q. Rosskothen H. Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl(-) and fluid secretion. Am. J. Physiol. Cell Physiol. 2001;281:C595–C602. doi: 10.1152/ajpcell.2001.281.2.C595. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Ederra J. Levin M.H. Verkman A.S. In situ fluorescence measurement of tear film [Na+], [K+], [Cl−], and pH in mice shows marked hypertonicity in aquaporin-5 deficiency. Invest. Ophthalmol. Vis. Sci. 2009;50:2132–2138. doi: 10.1167/iovs.08-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oen H. Cheng P. Turner H.C. Alvarez L.J. Candia O.A. Identification and localization of aquaporin 5 in the mammalian conjunctival epithelium. Exp. Eye Res. 2006;83:995–998. doi: 10.1016/j.exer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Levin M.H. Kim J.K. Hu J. Verkman A.S. Potential difference measurements of ocular surface Na+ absorption analyzed using an electrokinetic model. Invest. Ophthalmol. Vis. Sci. 2006;47:306–316. doi: 10.1167/iovs.05-1082. [DOI] [PubMed] [Google Scholar]
- 29.Levin M.H. Verkman A.S. CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest. Ophthalmol. Vis. Sci. 2005;46:1428–1434. doi: 10.1167/iovs.04-1314. [DOI] [PubMed] [Google Scholar]
- 30.Chang-Lin J.E. Kim K.J. Lee V.H. Characterization of active ion transport across primary rabbit corneal epithelial cell layers (RCrECL) cultured at an air-interface. Exp. Eye Res. 2005;80:827–836. doi: 10.1016/j.exer.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Midelfart A. The effects of amiloride, ouabain and osmolality on sodium transport across bovine cornea. Pflugers Arch. 1987;408:243–248. doi: 10.1007/BF02181466. [DOI] [PubMed] [Google Scholar]
- 32.Mirshahi M. Nicolas C. Mirshahi S. Golestaneh N. d'Hermies F. Agarwal M.K. Immunochemical analysis of the sodium channel in rodent and human eye. Exp. Eye Res. 1999;69:21–32. doi: 10.1006/exer.1999.0675. [DOI] [PubMed] [Google Scholar]
- 33.Hirsh A.J. Zhang J. Zamurs A. Fleegle J. Thelin W.R. Caldwell R.A. Sabater J.R. Abraham W.M. Donowitz M. Cha B. Johnson K.B. St. George J.A. Johnson M.R. Boucher R.C. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N'-4-[4-(2,3-dihydroxypropoxy) phenyl]butyl-guanidine methanesulfonate (552–02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J. Pharmacol. Exp. Ther. 2008;325:77–88. doi: 10.1124/jpet.107.130443. [DOI] [PubMed] [Google Scholar]
- 34.Zoukhri D. Macari E. Kublin C.L. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp. Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoukhri D. Fix A. Alroy J. Kublin C.L. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest. Ophthalmol. Vis. Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]