Abstract
Purpose
Genetic associations and the presence of complement components within pathological structures of age-related macular degeneration (AMD) have generated the hypothesis that AMD is caused by chronic local complement activation. Since the majority of activity in the common terminal pathway results from engagement of the amplification loop, the alternative pathway has been proposed as a logical therapeutic target. We recently generated a factor H (fH)-based complement inhibitor (CR2-fH) with the capacity to be “targeted” to sites of complement C3 activation. We asked whether the human therapeutic (TT30) is effective in a mouse model of AMD.
Methods
Choroidal neovascularization (CNV) was induced by argon laser photocoagulation of Bruch's membrane. Every other day, mice received intravenous injections of TT30 or vehicles, and after 6 days, the presence or absence of CNV and CNV-related changes were evaluated. Area of CNV, photoreceptor cell function, gene expression for complement components and cytokines, vascular endothelial growth factor (VEGF) protein levels, and TT30 bioavailability were determined.
Results
CNV development, which has previously been shown to require local complement activation, could be reduced by intravenous TT30 delivery. Specific inhibition of the alternative pathway not only reduced angiogenesis in CNV, but also ameliorated changes in several associated disease-related biomarkers, including diminished retinal function and molecular events known to be involved in AMD such as VEGF production. After intravenous injection, TT30 localized to CNV lesion sites in the retinal pigmented epithelium-choroid.
Conclusion
Systemic administration of TT30 was found to reduce CNV pathology. These data may open new avenues for novel systemic AMD treatment strategies.
Introduction
Pathology in age-related macular degeneration (AMD) results in progressive loss of central vision due to damage to the photoreceptor cells in the central area of the retina, the macula. This loss of photoreceptors occurs via 2 distinct mechanisms, which has led to the terms atrophic (dry) AMD and neovascular or exudative (wet) AMD. The dry form is more prevalent, accounting for up to 90% of all cases; however, wet AMD causes the most severe acute visual loss, as it is associated with choroidal neovascularization (CNV) in the area of the macula.1 The mechanisms by which photoreceptors degenerate in the macula is not well understood in dry AMD; however, in wet AMD, leakage of the new vessels, which invade the subretinal space, leads to fluid accumulation and retinal detachment, resulting in rapid photoreceptor loss. Despite the differences in clinical presentation, both forms of AMD have in common one of the primary target tissue sites, that being the retinal pigmented epithelium (RPE)/choroid interface. Structural changes at this site include the deposition of extracellular material between the RPE and Bruch's membrane (designated basal laminar sub-RPE deposits and drusen), and loss of integrity of Bruch's membrane as well as the blood-retina barrier.
AMD is a complex multifactorial disease (see Retnet.org for genes associated with autosomal dominant, autosomal recessive, or X-linked macular degeneration) that is influenced by age and hormonal status as well as environmental factors such as smoking, dietary oxidants, and levels of sunlight exposure.2 It is proposed that oxidative stress,3 as well as complement activation and the resultant inflammatory response, each play important roles as mediators of all forms of AMD.4 Several of the main genetic risk factors are polymorphisms occurring in complement genes, including the complement alternative pathway (CAP) control protein fH [complement factor H5–8], fB (complement factor B9), C2 (complement component 29), and C39,10.
Complement components have been found to be associated with the pathological features of AMD. Drusen, which form at the choroid/RPE interface,4,11–13 contain complement components, in addition to beta-amyloid, coagulation factors, and other inflammation-associated proteins. Bruch's membrane and the RPE have been shown to be immunopositive for complement regulatory proteins, C3 (complement component 3) activation fragments, and the membrane attack complex (MAC) proteins.4,11,14,15 It is worth noting that the macula shows the highest levels of MAC deposition, and MAC staining intensity is correlated with AMD severity and the loss of RPE cells.4 While it is unclear whether the complement components present in the eye are systemically derived or locally produced, Hageman has shown that in human AMD, local synthesis of CAP components occurs in AMD, just as we have demonstrated local upregulation of CAP components in mouse CNV.16,17 While drusen appear to lack immunoglobulins (which would activate the classical pathway),18 the involvement of the classical and/or lectin pathway cannot be excluded.19 In the absence of an identified ligand (or ligands) to initiate the complement cascade in AMD, a common or essential component of the cascade should be investigated as a potential therapeutic target in AMD. We have investigated the alternative pathway as a logical therapeutic target based on the observation that regardless of the pathway by which the complement cascade is initiated, the majority of C3 activated on cell surfaces appears to be due to activity of the CAP amplification loop (i.e., 80%–90% for CP20; for LP21).
A novel strategy of directing complement inhibitors to sites of complement activation has been developed based on the concept that the iC3b/C3dg/C3d fragments remain covalently attached to these sites for prolonged periods of time.22 To accomplish this tissue targeting, the recombinant iC3b/C3dg/C3d ligand binding site of complement receptor type 2 (CR2/CD21) has been fused to regulatory domains of complement inhibitors in an amino-to-carboxy orientation.22 One such targeted human inhibitor, designated CR2-fH or TT30, has recently been described and shown to regulate the CAP in vitro on red blood cells and other target surfaces.23 We have evaluated the ability of systemically administered TT30 to block local complement activation in the retina and ameliorate tissue damage and subsequent neovascularization in the murine CNV model.
Methods
Animals
C57BL/6 mice were generated from breeding pairs (Harlan Laboratories). Animals were housed under a 12:12 h, light:dark cycle with access to food and water ad libitum.
For CNV lesions, 3-month-old mice were anesthetized (xylazine and ketamine, 20 and 80 mg/kg, respectively), and pupils were dilated (2.5% phenylephrine HCl and 1% atropine sulfate). Argon laser photocoagulation (532 nm, 100 μm spot size, 0.1 s duration, 100 mW) was used to generate 4 laser spots in each eye surrounding the optic nerve, using a handheld coverslip as a contact lens.16 Bubble formation at the laser spot indicated the rupture of Bruch's membrane.24
For tail-vein injections, the vein was vasodilated by heat, a 25-G needle was inserted, and a volume of 50 μL was injected [250 μg of TT30 in phosphate-buffered saline (PBS), or PBS only]. Dosing and treatment schedules are outlined in the Results section. All experiments were approved by the University Animal Care and Use Committee and performed in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Expression and purification of TT30
A plasmid incorporating full-length TT30 cDNA was synthesized by using sequences encoding short consensus repeats (SCRs) 1–4 of human CR2 (fused 5′ to the sequence encoding SCRs 1–5 of human complement fH as previously described.23 The production cell line was developed using retroviral (GPEx®) technology. TT30 was expressed and secreted into the protein-free Hyclone culture medium containing L-glutamine and 0.1% pluronic F68, and purified by sequential gel filtration chromatography.
Assessment of CNV lesions
Relative CNV size was determined in flat-mount preparations of RPE-choroid stained with isolectin B (which binds to terminal β-D-galactose residues on endothelial cells and selectively labels the murine vasculature).24 In brief, eyes were collected and immersion fixed in 4% paraformaldehyde (PFA) for 2 h at 4°C, after which the anterior chamber, lens, and retina were removed. The eyecups were incubated in blocking solution (3% bovine serum albumin, 10% normal goat serum, and 0.4% Triton-X in tris-buffered saline) for 1 h. Isolectin B (1:100 of 1 mg/mL solution; Sigma-Aldrich) was applied to eyecups overnight at 4°C in blocking solution. After extensive washing, eyecups were flattened using 4 relaxing cuts, cover-slipped using Fluoromount (Southern Biotechnology Associates, Inc.), and examined by confocal microscopy (Leica TCS SP2 AOBS, Leica Bannockburn). Fluorescence measurements, taken from 2 μm sections using confocal microscopy (40× oil lens), were used for size determination. A Z-stack of images through the entire depth of the CNV lesion was obtained using the same laser intensity setting for all experiments. For each slice, the overall fluorescence was determined to obtain pixel intensity against depth, from which the area under the curve (indirect volume measurement) was calculated.16 Data are expressed as mean±standard error of the mean (SEM) per eye. Individual CNV lesions were also photographed using a microscope (Zeiss) equipped for fluorescence and digital microscopy (Spot camera; Diagnostic Instruments).
Electroretinography
ERG recordings and data analyses were performed as previously detailed16 using the EPIC-2000 system (LKC Technologies, Inc.). Stimuli to determine overall retinal responsiveness consisted of 10 μsec single flashes at a fixed intensity (2.48 cd–s/m2) under scotopic conditions. Measurements were performed before performing the CNV lesion (baseline ERG) and after the CNV lesion period. Peak a-wave amplitude was measured from baseline to the initial negative-going voltage, whereas peak b-wave amplitude was measured from the trough of the a-wave to the peak of the positive b-wave. ERG responses were found to decline with increasing size of the CNV lesion, affecting a- and b-waves equally.
Quantitative RT-PCR
RPE-choroid fractions were isolated from control and CNV eyes and stored at −80°C until they were used. Quantitative RT-PCR analyses were performed as previously described in detail.25 The primers used were β-actin, forward: 5′-AGCTGAGAGGGAAATCGTGC-3′ and reverse: 5′-ACCAGACAGCACTGTGTTG-3′; C3 forward: 5′-GGAAACGGTGGAGAAAGC-3′ and reverse: 5′-CTCTTGACAGGAATGCCATCGG-3′; fH forward: 5′-TGGACTTCCTTGTGGACCTC-3′ and reverse: 5′-CCATCAATTCCAAAGCCTGT-3′; CD55 forward: 5′-TAATGCGAGGGGAAAGTGAC-3′ and reverse: 5′-GTGGACTGCTCCATTGTCCT-3′; CD59 forward: 5′-CTGCTTCTGGCTGTGTTCTG-3′ and reverse: 5′-TCCTGGTCAGGAGAGCAAGT-3′; VEGF forward: 5′-CAGGCTGCTGTAACGATGAA-3′ and reverse: 5′-GCATTCACATCTGCTGTGCT-3′; PEDF forward: 5′-CCTCAGCATCCTTCTCCTTG-3′ and reverse: 5′-TGACATCATGGGGACTCTCA-3′. Real-time PCR analyses were performed in triplicate in a GeneAmp® 5700 Sequence Detection System (Applied Biosystems) using standard cycling conditions. Quantitative values were obtained by the cycle number.
ELISA for VEGF measurement
To measure production of vascular endothelial growth factor (VEGF), we utilized our previously published protocol.26 In short, eyecups were solubilized in CellLytic MT (mammalian tissue lysis/extraction reagent; Sigma) and centrifuged at 20,000g for 5 min. Microplates were coated with the VEGF capture antibody (Antigenix America, Inc.), and 100 μL of the concentrated supernatant was added. The captured proteins were detected with the same VEGF-specific antibody conjugated to horseradish peroxidase, followed by development with chromogenic substrate OPD (Sigma). Product development was assayed by measuring absorbance at 492 nm. Aliquots were assayed in duplicate, and values were compared with a VEGF dose-response curve.
Immunohistochemistry
Immunohistochemistry was performed as previously described with minor modifications.27 For localization of TT30, the CR2-portion of the fusion protein was identified using a specific mAb against human CR2 (10 μg/mL; anti-CD21; BD Pharmigen) that does not cross-react with mouse CR2, and visualized by using Alexa 488-conjugated goat anti-mouse secondary antibody (1:400; Invitrogen). A no-primary antibody condition was used as the negative staining control. Localization was performed in either flat mounts of RPE-choroid lightly fixed in 2% PFA, or fresh-frozen sections postfixed in ice-cold acetone. Staining was performed on more than 3 eyes per condition, and examined by fluorescence microscopy (Zeiss) equipped with a digital black and white camera, using a fixed exposure time per experimental condition (i.e., CR2 staining after CR2-fH or PBS injection and negative control). Images were false-colored using Adobe® Photoshop (Adobe Systems).
Statistics
For data consisting of multiple groups, Repeated Measures analysis of variance (ANOVA) followed by Fisher post hoc test (P<0.05) was used; single comparisons were analyzed by t-test analysis (P<0.05; Statview; SAS Institute Inc.).
Results
Effect of alternative pathway inhibition using TT30 on CNV development
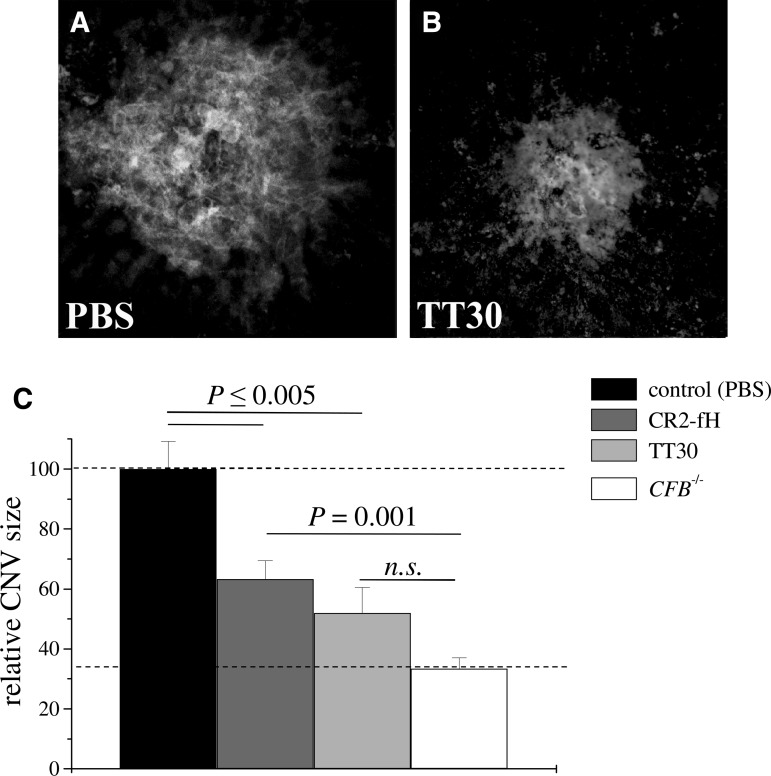
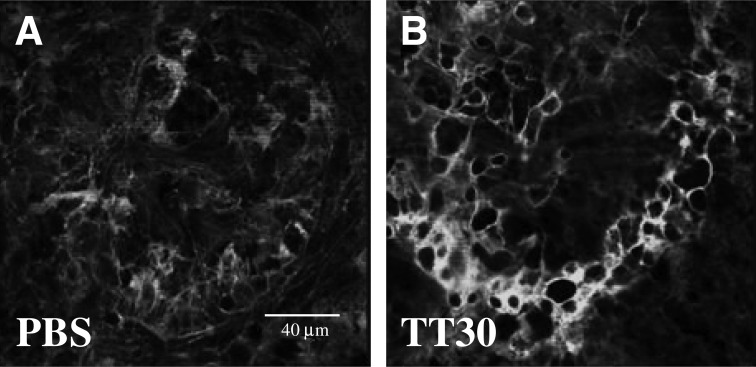
Mouse CR2-fH, a murine targeted inhibitor that specifically inhibits CAP, has previously been found to effectively reduce CNV in a mouse model of laser photocoagulation.16 Here, we investigated the development of CNV as well as retina function in mice intravenously treated with human TT30 or vehicle (PBS). Since a dose of 250 μg of mouse CR2-fH had been found to be effective in our previous publication, the same dose of TT30 was tested herein; animals received tail-vein injections on day 0 immediately after laser photocoagulation and on days 2 and 4. On day 6, mice were dark-adapted, followed by ERG analysis, after which they were sacrificed. The isolectin B4 staining of RPE-choroid flatmounts revealed that TT30 significantly reduced the size of the CNV lesions (∼50%; Fig. 1B) when compared with PBS injections (Fig. 1A; P<0.01). The effects were compared with those previously obtained from mice injected with murine CR2-fH and mice in which the alternative pathway16 had been completely eliminated (fB−/−; Fig. 1C). There was no difference in CNV between TT30- and CR2-fH-treated mice (P=0.1). CNV development was significantly different after mouse CR2-fH-treatment when compared with CFB−/− mice (P<0.001); however, that difference was eliminated when compared with TT30-treatment and CFB−/− mice (P>0.05).
FIG. 1.
Choroidal neovascularization in TT30-treated mice. CNV development was evaluated 6 days post laser photocoagulation using isolectin–B4 staining. Representative images of TT30 and PBS-treated mice are shown in (A, B), with corresponding quantification of data (size of the lesion; integrated pixel intensity) in (C). C57BL/6 mice (n=8 per group) treated with intravenous tail-vein injections of (A) PBS or (B) TT-30 (250 μg) on days 0, 2, and 4 post laser photocoagulation. Isolectin–B4 staining intensity is reduced in TT30-treated mice compared with PBS-treated controls. (C) The size of the lesions were compared with published results of animals in which the complement alternative pathway (CAP) was removed (CFB−/−; equivalent to maximal attainable effect) or that were treated with CR2-fH.16 CR2-fH and TT-30 reduced CNV size by 40% and 50%, respectively, whereas eliminating CAP reduced CNV by ∼65%, when compared with the PBS controls. CNV, choroidal neovascularization; PBS, phosphate-buffered saline.
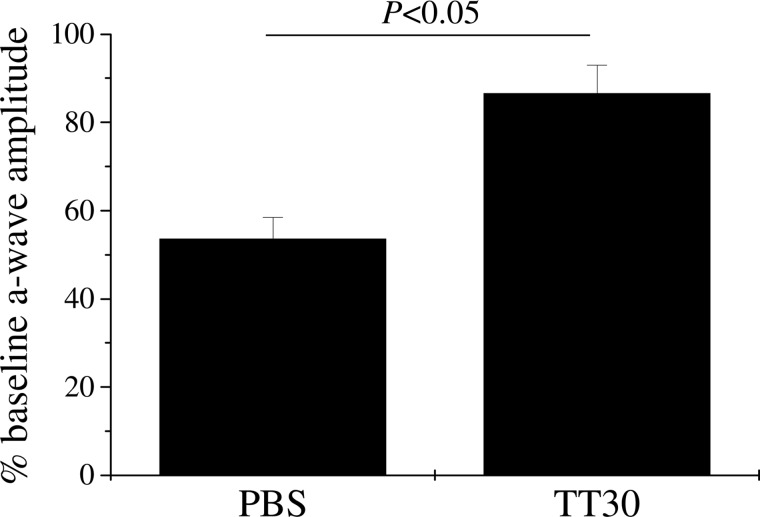
Retina function was assessed using ERG recordings as previously described.16 Impairment of retina function is presumed to be due to photoreceptor cell death in the region of the CNV lesion,28 as well as reduced oxygen supply to the outer retina resulting from the disrupted structure of the RPE/choroid (e.g.,29,30). We have previously shown that the extent of the reduction in ERG amplitudes, and in particular that of the a-wave amplitudes (direct reflection of the photoreceptor currents generated by the absorption of light; reviewed by31), correlates very well with the extent of the lesion.16 Here, we found partial reversal using TT30 of the impairment of a-wave amplitudes. As previously shown, the a-wave amplitudes were reduced by ∼50% in response to the 4 lesions (Fig. 2), whereas blocking AP activation by TT30 significantly preserved retinal function (P<0.05).
FIG. 2.
Retina function in TT30-treated mice. ERG recordings were performed before laser photocoagulation (baseline) and at the end of the experiment (after 6 days of CNV) using single-flash ERG recordings at a single light intensity of 2.48 photopic cd–s/m2. ERG data are expressed as a percentage of baseline a-wave amplitude for the 2 treatment groups. PBS-treated mice exhibited a significant loss of ERG amplitudes after CNV, whereas ERG amplitudes were relatively preserved in TT30-treated mice.
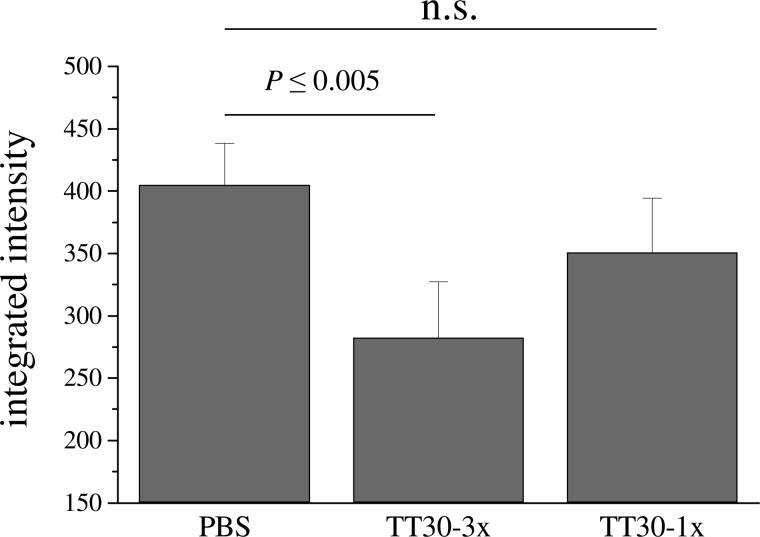
In our previous publication, we showed that delayed CR2-fH treatment after injury (treatments on days 3, 5, and 7 after laser injury; representing time points coincident with and beyond the peak of C3 deposition and VEGF expression) still slowed CNV progression.16 Here, we have asked an additional question: is a single dose of TT30 on day 3 (the peak of C3 deposition and VEGF expression) sufficient to reduce the size of CNV. While a single dose of 250 μg TT30 did not reach statistical significance, a trend toward being effective was noted (Fig. 3).
FIG. 3.
Effect of treatment frequency on CNV progression. Two treatment frequencies of TT30 treatment were compared for efficacy in reducing CNV size. All animals were exposed to laser photocoagulation on day 0. For repeated treatments, the animals were injected at the time of laser injury, followed by two more treatments every 48 h; for the single treatment, the treatment with TT30 was administered on day 3 (the peak of VEGF and C3 expression16) post laser injury. CNV size measurements were normalized to the maximum size obtained with PBS treatment using the 3-treatment paradigm. While the single treatment provided some protection, the effect was not significant, suggesting that repeated treatments are required for optimal protection. Eight animals per treatment group were evaluated. VEGF, vascular endothelial growth factor.
Effect of AP inhibition on CNV-associated changes in markers of complement activation and angiogenesis
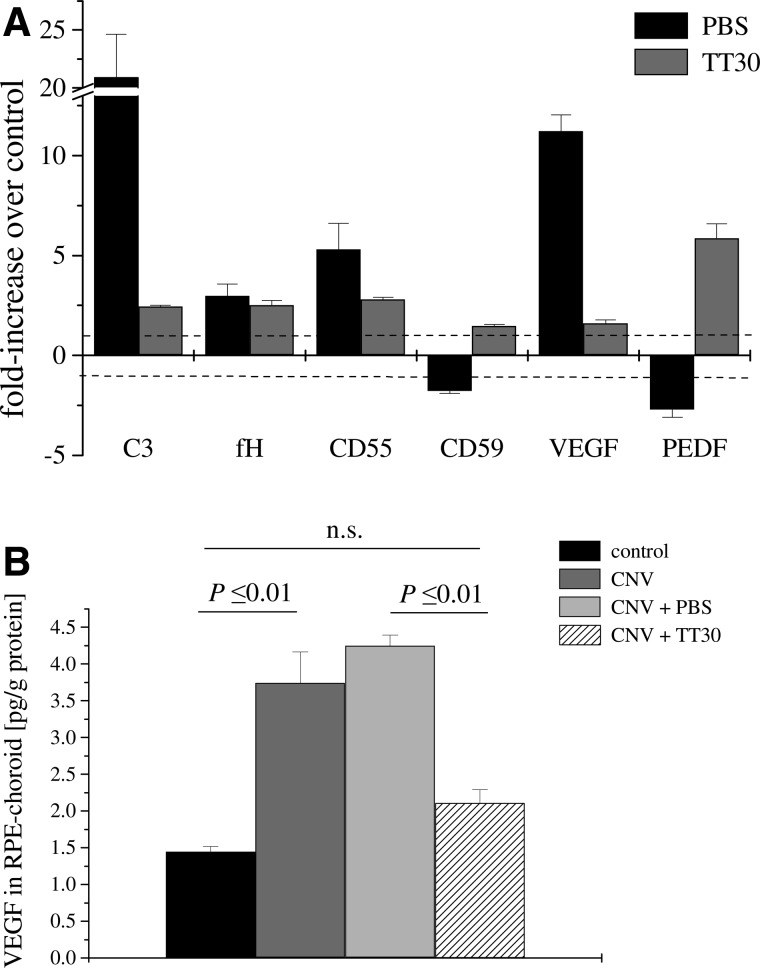
We have previously investigated the local expression of mRNA encoding C3, the complement inhibitors CFH, CD55, and CD59 as well as the angiogenic protein VEGF and the anti-angiogenic protein pigment epithelial derived factor (PEDF).17 In that study, which analyzed wild-type mice with CNV, we found that C3 and VEGF mRNA levels were significantly upregulated, PEDF was downregulated, whereas levels of the complement inhibitors were minimally affected. The control mice in the current study, which received PBS injections only after the induction of CNV, showed the same gene expression profile for the markers of complement activation and angiogenesis - that is, an increase in VEGF and a concomitant decrease in PEDF as well as an increase in C3 without alterations in complement inhibitors. Conversely, TT30 treatment reversed the laser treatment-induced changes (Fig. 4A). We verified that the levels of VEGF protein were increased in control CNV mice whether they received PBS injections or not (Fig. 4B). Notably, TT30 treatment also reduced VEGF protein levels in CNV animals to levels indistinguishable to mice without lesions (P>0.05).
FIG. 4.
Analysis of biomarker mRNA and protein expression in CNV. (A) Quantitative RT-PCR was performed on RPE-choroid samples removed after visual function analysis. Quantitative values were obtained by cycle number (Ct value), determining the difference between the mean experimental (e.g., VEGF) and control (β-actin) ΔCt values. VEGF and C3 mRNA expression was significantly increased in animals treated with PBS, PEDF was downregulated, whereas complement regulatory protein mRNA expression showed marginal changes without a consistent trend. Fold differences for QRT-PCR on RPE-choroid samples removed from TT-30 treated animals were normalized (VEGF, C3) or reversed (PEDF). Eyes from 4 animals were evaluated per group. (B) Quantitative ELISA assays were performed on RPE-choroid samples removed post-laser photocoagulation from PBS- and TT30-treated mice. Quantitative values were obtained by using a standard curve of purified mouse protein (VEGF). Protein levels for VEGF followed the mRNA levels; they were normalized in TT30-treated mice when compared with the control. Tissues from 3 animals per group were evaluated. PEDF, pigment epithelial derived factor; RPE, retinal pigmented epithelium.
Tissue localization of TT30
Mouse CR2-fH has previously been shown to localize to sites of C3d deposition (i.e., at the edge of the CNV lesion) when injected at a therapeutically relevant dose (250 μg) on day 3 (the peak of C3d deposition in the lesion) and imaged within 24 h.16 Here, we visualized the tissue localization of TT30 by anti-human CR2 immunofluorescence (Fig. 5). Similar to the analysis of CR2-fH, some staining was localized to the CNV lesion in the PBS-treated animals (Fig. 5A); however, human CR2 staining was significantly higher in TT30-treated mice, in particular at the edge of the lesion (Fig. 5B).
FIG. 5.
Localization of TT30 to the CNV lesion. TT30 was detected in RPE/choroid flatmounts using anti-human CR2 immunofluorescence staining to detect the CR2 portion of TT30. Animals were injected with a therapeutic dose of TT30 (250 μg) on day 3 post-laser photocoagulation, and eyes were collected 24 h later. (A) Whole-mount immunofluorescent microscopy revealed the presence of CR2-positive material in the TT30-injected eyes (B), which was significantly reduced in the PBS-injected eyes; no staining was observed when the primary antibody was omitted (negative control; data not shown).
Discussion
CNV induced by argon laser photocoagulation has been the most studied animal model for wet AMD. The laser damage ruptures Bruch's membrane and damages the RPE, which triggers CNV and results in the loss of underlying photoreceptors. This injury model is particularly useful to evaluate particular pathways using pharmacological interventions, as it rapidly reproduces some of the pathology seen in human AMD. In particular, in the mouse model of CNV, the involvement of the complement system has been well documented (e.g.,16,17,24,32–34). In addition, VEGF levels have been found to correlate with pathology,17 and VEGF inhibition decreases CNV size.35 In a previous study, we examined which pathway is essential in mouse laser-induced CNV17; in particular, we asked whether the CAP is required or sufficient in CNV. To answer this question, CNV was analyzed in fB−/− mice (no CAP) and in double-knockout mice in which the CP and LP are disabled (C1q−/− MBL−/−). Interestingly, while single-knockout mice for the CP or LP (C1q−/− or MBL−/−) did develop CNV similar to WT mice, C1q−/− MBL−/− were as protected as the fB−/− mice. This would suggest that in mouse CNV, multiple independent ligands can initiate the complement cascade in AMD, and that the CAP amplification loop is also required whatever the initiating pathway. Therefore, we have argued that the CAP, as a common or essential component of the cascade, should be investigated as a potential therapeutic target in AMD. The logical targets are either increasing inhibition (by supplying exogenous fH) or decreasing activation (by blocking fB or fD). Here, we have continued our investigation by using our novel complement therapeutic that has the capacity to be “targeted” to sites of complement activation,36 thus inhibiting the progression of mouse CNV16 as well as other complement-related processes.22,26 This therapeutic inhibitor consists of the CR2 domain (which encodes the iC3b/C3dg/C3d binding-site from CR2), followed by a linker and the 5 amino-terminal SCRs of fH (which contain the CAP-inhibitory domain of fH). Herein, we have evaluated the effectiveness of the human version of mouse CR2-fH, TT30, in reducing CNV and ameliorating CNV-related pathological changes.
The main results of the current study are as follows: (1) TT30 significantly reduces CNV as well as the detrimental changes to retinal function; (2) the molecular changes associated with CNV (i.e., the imbalance between the angiogenic and anti-angiogenic factors) as well as the increase in complement activation products was found to be normalized by use of TT30; and (3) the therapeutic effect of TT30 was achieved with intravenous injections and did not require intraocular applications.
As highlighted in the introduction, the complement cascade, and, in particular, the alternative pathway, is a logical target for intervention in AMD. Testing of this hypothesis is highly dependent on the availability of an appropriate animal model and relevant biomarkers or biological readouts. Laser-induced CNV, the mouse model of wet AMD, despite relying on physical injury for the triggering of the response, is an extensively utilized animal model for wet AMD. In murine CNV, VEGF expression in the RPE/choroid is high after CNV induction, but can be reduced by blocking complement activation.16,24,32 Histopathological studies of CNV membranes from patients with AMD have demonstrated the presence of VEGF and its receptors (e.g.,37). Most importantly, CNV progression is responsive to anti-VEGF-based therapy; that is, monoclonal antibodies against VEGF are successful in reducing the amount of damage in both mouse35 and man (e.g.,38). Since complement dysregulation is a hallmark of both mouse and human CNV, complement-based therapy could also succeed as a novel therapy for human wet AMD.39
Relevant clinical readouts to determine the amount of damage or recovery in wet AMD include measures of leakage or CNV area, and visual function. We have confirmed that TT30 reduces the area of CNV in RPE/choroid flatmounts stained with a marker that identifies newly formed blood vessels,24 and improves retinal function as measured by full-field electroretinography. In the clinic, the most commonly used electrophysiological tests are the full-field ERG such as we employed and multifocal ERG. Most studies analyzing wet AMD patients have used multifocal ERG40 presumably due to its ability to examine local areas on the retina. However, it is particularly intriguing that Skaat and coworkers41 have reported that patients treated with intravitreal bevacizumab have significantly improved a-wave amplitudes in full-field ERGs, similar to the findings reported here for TT30. Biomarkers for AMD include elevated levels of VEGF (e.g.,37) as well as activated complement components in RPE/choroid4 and serum (e.g., 42,43), although alterations in response to treatment have not yet been reported. Here, we report that VEGF and complement C3 are significantly elevated in mouse CNV tissues, an effect that is blunted by TT30 therapy. Imaging techniques that monitor VEGF and levels of complement activation in vivo are likely to provide a sensitive readout for future clinical trials examining therapeutics for AMD.
Finally, when designing potential therapeutics for AMD, 2 additional important considerations are the route of application and the frequency of dosing. For intravitreal injections, molecules that target the RPE/Bruch's membrane/choroid need to be small to diffuse through the retina, or to be administered at a higher dose (e.g.,44), and in diseases that do not impair the internal limiting membrane, macromolecules may not penetrate.45 Here, we provide an example of a systemic approach that circumvents the problem of the blood retinal barrier, as the blood vessels in the choroid are fenestrated, allowing passage of large plasma-derived proteins. Systemic administration for ocular delivery, however, has to deliver the active compound to the eye, while overcoming the challenges of rapid washout and potential systemic toxicity. Although both TT30 and its mouse version CR2-fH have a short circulatory half-life,22,23 both of them are present in the target organ, the eye, 24 hours after tail-vein injection (see16 and Fig. 5). Importantly, the CR2 targeting strategy has been confirmed to inhibit complement activation locally, while maintaining host resistance to infection.46 Finally, in mouse CNV, which grows rapidly and linearly for ∼14 days before leveling off,47 frequent dosing is required. Additional experiments are required to examine models with slower growth rates to determine the feasibility of systemic administration. In summary, we have demonstrated for the first time proof-of-concept for the use of human TT30 as a treatment strategy for AMD.
Acknowledgments
The authors thank Kannan Kunchithapautham for his help with QRT-PCR and ELISA assays, and Luanna Bartholomew for critical review. This work was supported in part by a National Institutes of Health grant EY019320 (B.R.), a Department for Veterans Affairs merit award RX000444 (B.R.), a sponsored research agreement by Taligen Therapeutics (B.R.), and an unrestricted grant to MUSC from Research to Prevent Blindness (RPB), New York, NY. B.R. is a RPB Olga Keith Wiess Scholar. Animal studies were conducted in a facility constructed with support from the NIH (C06 RR015455).
Author Disclosure Statement
The authors declare the following financial or proprietary interests in the product mentioned herein. V.M.H. is a co-founder of Taligen Therapeutics, Inc., Cambridge, MA, which developed complement inhibitors for therapeutic use; a consultant to Alexion Pharmaceuticals, Cheshire, CT; and holds licensed patents for CR2-targeted complement inhibitors; B.R. was a consultant to Taligen Therapeutics; and holds licensed patents for CR2-targeted complement fH inhibitor. B.C. has no competing financial interests.
References
- 1.Brown M.M. Brown G.C. Stein J.D., et al. Age-related macular degeneration: economic burden and value-based medicine analysis. Can. J. Ophthalmol. 2005;40:277–287. doi: 10.1016/S0008-4182(05)80070-5. [DOI] [PubMed] [Google Scholar]
- 2.Tomany S.C. Wang J.J. Van Leeuwen R., et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Khandhadia S. Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev. Mol. Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 4.Hageman G.S. Luthert P.J. Victor Chong N.H., et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Klein R.J. Zeiss C. Chew E.Y., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hageman G.S. Anderson D.H. Johnson L.V., et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines J.L. Hauser M.A. Schmidt S., et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 8.Edwards A.O. Ritter R., 3rd Abel K.J., et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 9.Gold B. Merriam J.E. Zernant J., et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates J.R. Sepp T. Matharu B.K., et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 11.Johnson L.V. Leitner W.P. Staples M.K. Anderson D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 12.Anderson D.H. Mullins R.F. Hageman G.S. Johnson L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 13.Chong N.H. Keonin J. Luthert P.J., et al. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am. J. Pathol. 2005;166:241–251. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kijlstra A. La Heij E. Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul. Immunol. Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 15.Lommatzsch A. Hermans P. Muller K.D., et al. Are low inflammatory reactions involved in exudative age-related macular degeneration?. Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes. Arch. Clin. Exp. Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 16.Rohrer B. Long Q. Coughlin B., et al. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2009;50:3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrer B. Coughlin B. Kunchithapautham K., et al. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol. Immunol. 2011;48:e1–e8. doi: 10.1016/j.molimm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabb J.W. Miyagi M. Gu X., et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollyfield J.G. Bonilha V.L. Rayborn M.E., et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harboe M. Ulvund G. Vien L., et al. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harboe M. Garred P. Borgen M.S., et al. Design of a complement mannose-binding lectin pathway-specific activation system applicable at low serum dilutions. Clin. Exp. Immunol. 2006;144:512–520. doi: 10.1111/j.1365-2249.2006.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y. Qiao F. Atkinson C., et al. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J. Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridkis-Hareli M. Storek M. Mazsaroff I., et al. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of the human complement alternative pathway-mediated diseases. Blood. 2011;118:4705–4713. doi: 10.1182/blood-2011-06-359646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nozaki M. Raisler B.J. Sakurai E., et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohr H.R. Kuntchithapautham K. Sharma A.K. Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp. Eye Res. 2006;83:380–389. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Thurman J.M. Renner B. Kunchithapautham K., et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrer B. Korenbrot J.I. LaVail M.M., et al. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J. Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caicedo A. Espinosa-Heidmann D.G. Hamasaki D., et al. Photoreceptor synapses degenerate early in experimental choroidal neovascularization. J. Comp. Neurol. 2005;483:263–277. doi: 10.1002/cne.20413. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M.A. Marcus S. Elman M.J. McPhee T.J. Neovascularization in central retinal vein occlusion: electroretinographic findings. Arch. Ophthalmol. 1988;106:348–352. doi: 10.1001/archopht.1988.01060130374025. [DOI] [PubMed] [Google Scholar]
- 30.Sabates R. Hirose T. McMeel J.W. Electroretinography in the prognosis and classification of central retinal vein occlusion. Arch. Ophthalmol. 1983;101:232–235. doi: 10.1001/archopht.1983.01040010234010. [DOI] [PubMed] [Google Scholar]
- 31.Pugh E.N., Jr. Falsini B. Lyubarsky A.L. The origin of the major rod- and cone-driven components of the rodent electroretinogram and the effect of age and light-rearing history on the magnitude of these components. In: Williams T.P., editor; Thistle A.B., editor. Photostasis and Related Topics. New York, NY: Plenum Press; 1998. pp. 93–128. [Google Scholar]
- 32.Bora N.S. Kaliappan S. Jha P., et al. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J. Immunol. 2006;177:1872–1878. doi: 10.4049/jimmunol.177.3.1872. [DOI] [PubMed] [Google Scholar]
- 33.Bora N.S. Kaliappan S. Jha P., et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J. Immunol. 2007;178:1783–1790. doi: 10.4049/jimmunol.178.3.1783. [DOI] [PubMed] [Google Scholar]
- 34.Bora N.S. Jha P. Lyzogubov V.V., et al. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J. Biol. Chem. 2010;285:33826–33833. doi: 10.1074/jbc.M110.153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campa C. Kasman I. Ye W., et al. Effects of an anti-VEGF-A monoclonal antibody on laser-induced choroidal neovascularization in mice: optimizing methods to quantify vascular changes. Invest. Ophthalmol. Vis. Sci. 2008;49:1178–1183. doi: 10.1167/iovs.07-1194. [DOI] [PubMed] [Google Scholar]
- 36.Song H. He C. Knaak C., et al. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Invest. 2003;111:1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhutto I.A. McLeod D.S. Hasegawa T., et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp. Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab. Curr. Med. Res. Opin. 2011;27:1465–1475. doi: 10.1185/03007995.2011.585394. [DOI] [PubMed] [Google Scholar]
- 39.Zarbin M.A. Rosenfeld P.J. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 2010;30:1350–1367. doi: 10.1097/IAE.0b013e3181f57e30. [DOI] [PubMed] [Google Scholar]
- 40.Gerth C. The role of the ERG in the diagnosis and treatment of age-related macular degeneration. Doc. Ophthalmol. 2009;118:63–68. doi: 10.1007/s10633-008-9133-x. [DOI] [PubMed] [Google Scholar]
- 41.Skaat A. Solomon A. Moroz I., et al. Increased electroretinogram a-wave amplitude after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Acta. Ophthalmol. 2011;89:e269–e273. doi: 10.1111/j.1755-3768.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 42.Hecker L.A. Edwards A.O. Ryu E., et al. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum. Mol. Genet. 2010;19:209–215. doi: 10.1093/hmg/ddp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl H.P. Charbel Issa P. Walier M., et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin D.F. Maguire M.G. Ying G.S., et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Short B.G. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 46.Atkinson C. Song H. Lu B., et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espinosa-Heidmann D.G. Suner I. Hernandez E.P., et al. Age as an independent risk factor for severity of experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2002;43:1567–1573. [PubMed] [Google Scholar]