Abstract
Despite the importance of telomere maintenance in cancer cell survival via the elongation of telomeres by telomerase reverse transcriptase (TERT) or Alternative Lengthening of Telomeres (ALT), it had not been tested directly whether telomere maintenance is dispensable for human tumorigenesis. We engineered human tumor cells containing loxP-flanked hTERT to enable extensive telomere elongation prior to complete hTERT excision. Despite unabated telomere erosion, hTERT-excised cells formed tumors in mice and proliferated in vitro for up to one year. Telomerase reactivation or ALT was not observed, and the eventual loss of telomeric signal coincided with loss of tumorigenic potential and cell viability. Crisis was averted via the reintroduction of active but not inactive hTERT. Thus, telomere maintenance is dispensable for human tumorigenesis when telomere reserves are long. Yet, despite telomere instability and the presence of oncogenic RAS, human tumors remain susceptible to crisis induced by critically short telomeres.
Keywords: telomerase reverse transcriptase, alternative lengthening of telomeres (ALT), telomere length maintenance, human tumorigenesis, crisis
Introduction
The limited in vitro lifespan of normal human cells, referred to as the Hayflick limit, cellular senescence, or mortality stage 1 (M1), was first described in 1961 (Hayflick, 1973). The temporal onset of senescence is correlated tightly to telomere length (Allsopp et al., 1992; Harley et al., 1990), and is bypassed by expression of the telomerase reverse transcriptase hTERT (Bodnar et al., 1998; Vaziri and Benchimol, 1998). Transformation via factors such as SV40 T antigen lead to lifespan extension beyond M1, however cells acquire genetic instability and eventually undergo apoptosis, referred to as M2 or crisis (Wright et al., 1989). Further, the discovery that tumor cells possessed shorter telomeres compared with normal tissues suggested that telomere maintenance was required to avert crisis during tumorigenesis (de Lange et al., 1990). This hypothesis was borne out in SV40-transformed human cells, in which rare clones that acquired telomerase activity survived the genetic instability and cell death that accompany crisis (Counter et al., 1992). In fact, enforced expression of TERT in combination with oncogenic RAS and the SV40 early region (ER) elicits tumorigenic conversion of fibroblast, kidney epithelial and mammary epithelial cells (Elenbaas et al., 2001; Hahn et al., 1999a; Hahn et al., 2002). Thus, the acquisition of telomerase activity appears essential for immortality in many normal and cancer cell types.
While mice have proven a useful model system in which to study cancer, the response to a critically short telomere differs markedly between mice and humans (reviewed in Smogorzewska and de Lange, 2002). Another critical difference between mice and humans is that many human tumor cell types possess a subset of telomeres that are already critically short (Capper et al., 2007; Xu and Blackburn, 2007), whereas laboratory murine strains typically possess much longer average telomere lengths (Hemann and Greider, 2000). For example, inhibition of TERT in human tumor lines induces cell death almost immediately, confounding the ability to distinguish the role of TERT in cell viability independent of telomere maintenance (Hahn et al., 1999b; Zhang et al., 1999). Thus, an important unresolved question is whether TERT, or indeed any mechanism of telomere maintenance, is essential for human tumorigenesis.
To address this question, we engineered a human tumor line in which telomere length and hTERT expression could be controlled genetically and temporally. We employed the Cre-loxP system, which enables stringent and reversible control of hTERT in primary human cells to generate human tumor cells with long telomeres from which hTERT could be excised (Cascio, 2001; Jaiswal et al., 2007; Steinert et al., 2000; Ungrin and Harrington, 2006). The results demonstrated unequivocally that TERT is dispensable for human tumorigenesis and cell viability when telomeres are long. However, despite the continuous presence of RAS and SV40, induction of endogenous telomerase or other telomere maintenance mechanisms (e.g. ALT) was not observed, and the cells eventually succumbed to telomere-induced crisis.
Results
Establishment of hTERT-excisable human tumor cells
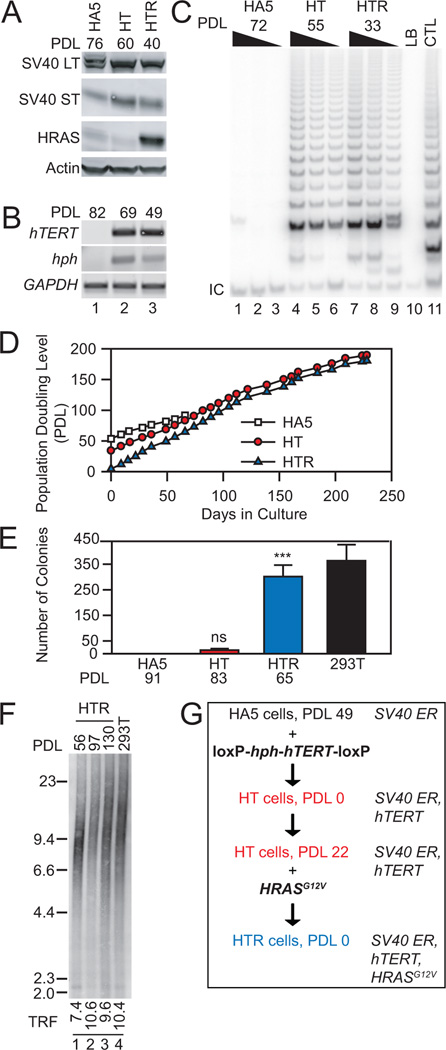
The human TERT cDNA (Harrington et al., 1997) and an E. coli phosphotransferase gene encoding resistance to hygromycin B (hph) (Gritz and Davies, 1983) were flanked by loxP sites and introduced into human HA5 embryonic kidney cells (HA5) containing the SV40 early region (ER) (Stewart and Bacchetti, 1991) (Figure 1). Upon hTERT introduction, HT (HA5 + hTERT) cells became telomerase-positive and immortal but could not support anchorage-independent growth in 0.6% w/v agar. However, after infection with a retrovirus encoding HRASG12V (HT + RAS = HTR) (Hahn et al., 1999a), HTR cells formed colonies in 0.6% w/v agar and gave rise to tumors in immuno-compromised mice when injected sub-cutaneously or beneath the kidney capsule epithelium (Figure 1, Figure 2H). In this tumor cell model, we chose to use an SV40-transformed cell line (HA5) that cannot escape crisis spontaneously (Counter et al., 1992), and hTERT was introduced as the second (rather than first) step in the tumorigenic conversion process (Elenbaas et al., 2001; Hahn et al., 1999a; Hahn et al., 2002). Thus, immortalization is not an obligate first step for human tumorigenesis.
Figure 1. An hTERT-excisable tumorigenic cell line.
(A) Western analysis of whole cell lysates (50 µg) from HA5, HT (HA5 + TERT) and HTR (HT + RAS) cells at indicated population doubling level (PDL). (B) RT-PCR analysis of hTERT, hph, and GAPDH at indicated PDL. (C) Analysis of telomerase activity of cell lysates (200, 100, 50 ng) at indicated PDL. LB, negative buffer control; CTL, HeLa cell lysate positive control; IC, internal control PCR product. (D) Replicative lifespan of HA5, HT and HTR cells. HT or HTR cells were immortal. (E) Anchorage-independent colony growth at indicated PDL (n=3). 293T cells were a positive control for colony formation. Statistical significance between HA5 (no colonies formed) and HT or HTR cell lines as indicated (n=3, *** p<0.001; ns, p>0.05, power(1-β err prob)>0.99, αactual=0.05, two-tailed). (F) TRF analysis of average telomere length at increasing PDL. 293T cells were included as a control. Weighted mean telomere lengths (kbp) are indicated below each lane. (G) Schematic of elements introduced into HA5 cells, at indicated PDL.
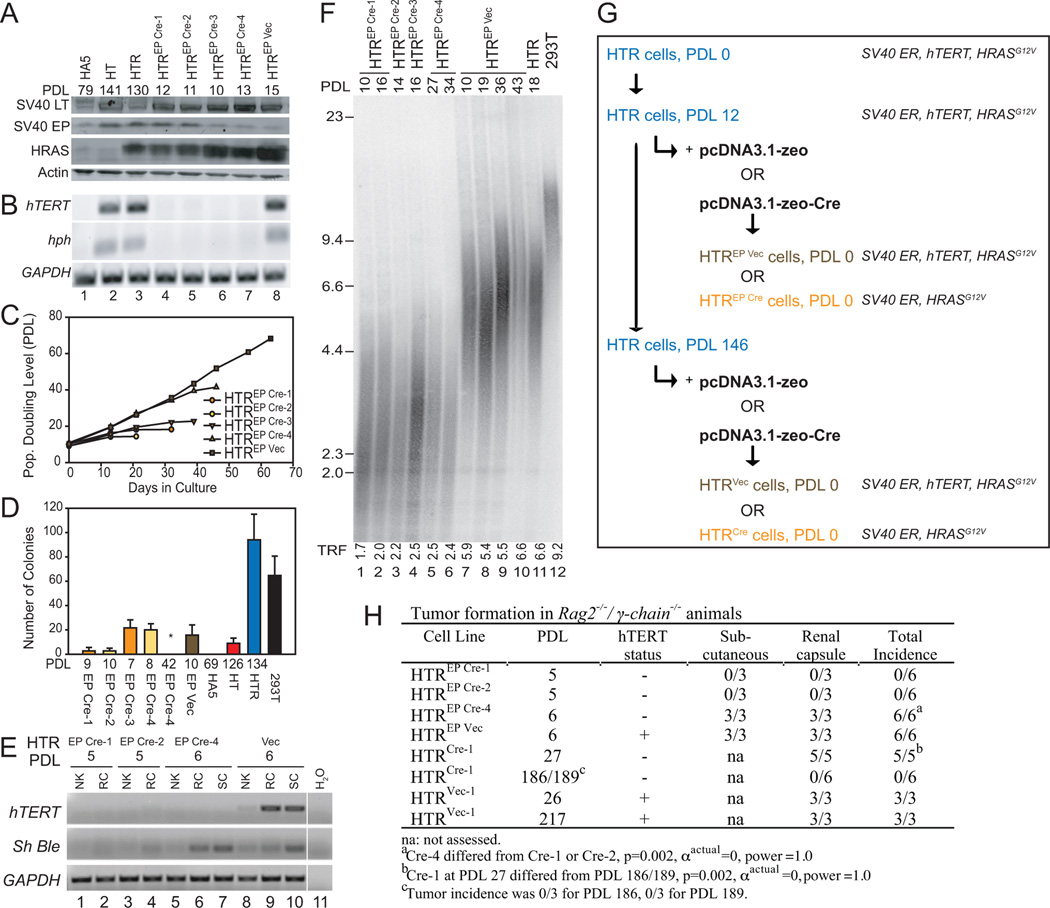
Figure 2. Excision of hTERT from tumor cells with short telomeres.
(A) Western analysis of cell lysates (50 µg) in HTREP (Early Passage) cells transfected with Cre recombinase or empty vector control at indicated population doubling level (PDL). (B) RT-PCR analysis of hTERT, hph, and GAPDH at indicated PDL. (C) Replicative lifespan of indicated cell lines. HTREP Vec remained immortal. (D) Anchorage-independent colony formation at indicated PDL. 293T cells were included as a positive control, and HA5 as a negative control. HTREP Cre-4 at PDL 42 (no colonies) differed significantly from HTREP Cre-4 at PDL 8 (n=3, * p<0.05, power(1-β err prob)=1.0, αactual=0.05, two-tailed). (E) RT-PCR analysis of hTERT, Sh Ble (zeocin) and GAPDH in tissue extracted from renal capsule (RC) or subcutaneous (SC) injection sites, or normal adjacent kidney (NK). (F) TRF analysis of average telomere length at increasing PDL. Weighted mean telomere lengths (kbp) are indicated below each lane. (G) Schematic of elements introduced into HT cells, at indicated PDL. (H) Incidence of tumor formation of indicated cell lines in immunodeficient mice (see Experimental Procedures for details).
TERT-excised tumor cells with short telomeres capable of transient tumor formation
After a short period of propagation in culture (PDL 12, mean TRF <6 kbp, e.g. Figure 2F, lane 12), Cre recombinase or the appropriate empty vector control encoding zeocin resistance (Sh Ble) was introduced into this HTR ‘early passage’ population (HTREP) (Figure 2A, G), and after transient selection of clonal populations, the excision of hTERT (and hph) was queried via RT-PCR analysis (Figure 2B, lanes 4–8). Cell crisis ensued in hTERT-excised populations soon thereafter (Figure 2C), however the two longest-lived cell lines supported anchorage-independent growth immediately after hTERT excision (Figure 2D, HTREP Cre-3 and HTREP Cre-4). HTREP Cre-4 cells, although hTERT-negative (Figure 2E, lanes 6, 7), formed tumors in mice at an incidence indistinguishable from hTERT-positive HTR cells (HTREP Vec) (Figure 2H). This controlled hTERT genetic excision is consistent with the transient survival observed upon telomerase suppression in human cancer lines with short telomeres (Hahn et al., 1999b; Zhang et al., 1999).
TERT-excised tumor cells exhibit robust tumor formation until telomere crisis
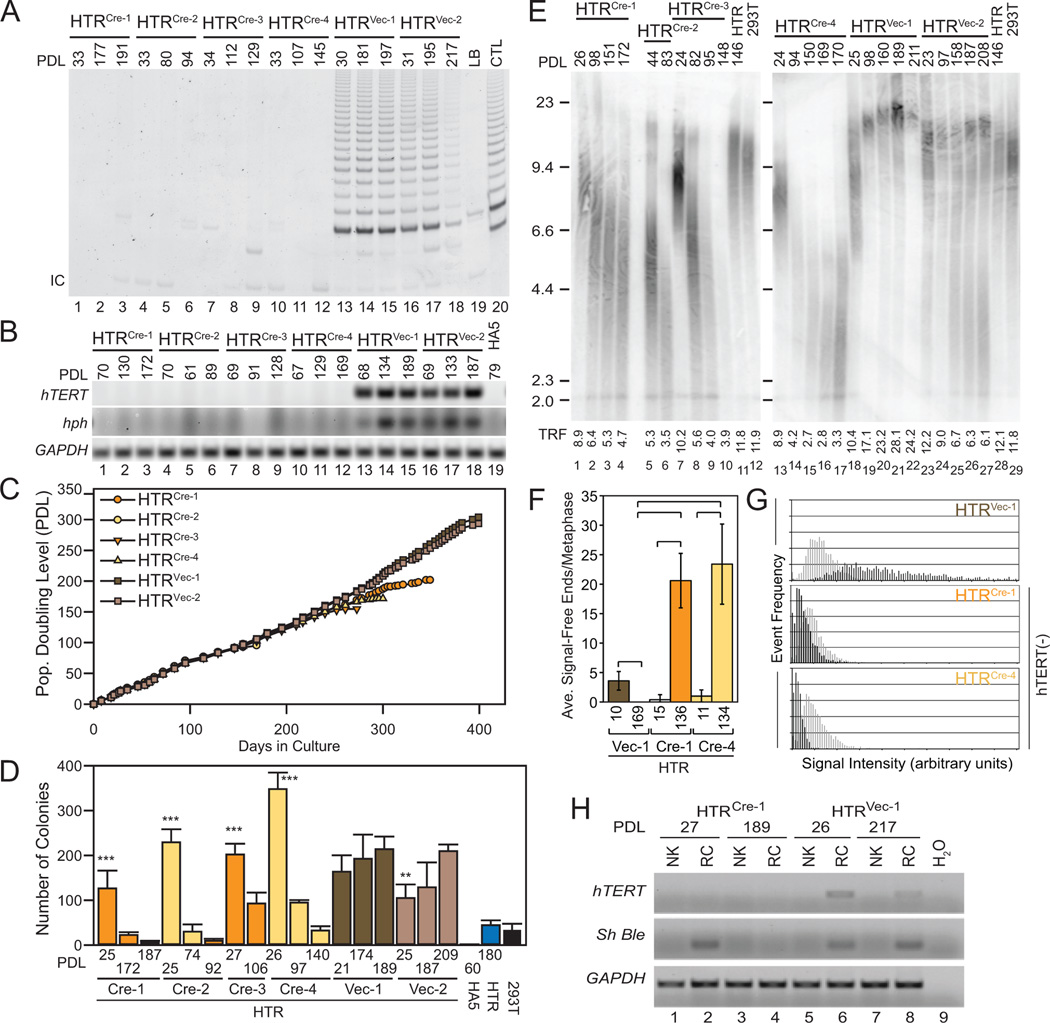
To create hTERT-negative human tumor populations with long telomeres, the HTR population was propagated in culture for more than 240 days (PDL 146) until average telomere length reached 12 kbp (Figure 3E, lane 11) prior to hTERT excision. Control cell clones in which an empty vector (HTRVec) was introduced retained hTERT and hph expression, and exhibited telomere elongation and colony forming potential in 0.6% w/v agar (Figure 3A–E). In clones selected for Cre recombinase expression (HTRCre), loss of hTERT expression was confirmed by RT-PCR and measurement of telomerase activity (Figure 3A, B, lanes 1–12). The maximum lifespan of these hTERT-excised clones exceeded 250 days, and one clone survived for 1 year (Figure 3C). Telomerase activity remained absent, and telomere attrition continued unabated with no evidence of the telomere length heterogeneity typical of telomerase-negative tumor cells that undergo telomere recombination (ALT) (Figure 3A, E, G). Even in the complete absence of hTERT, HTRCre lines retained a significant initial capacity for anchorage-independent growth (Figure 3D). Upon injection into the sub-renal capsule, which in some instances is more permissive for tumor formation than sub-cutaneous injection (Liang et al., 2008; Sun et al., 2005), HTRCre lines exhibited a tumor incidence of 100% even after more than 1 month in culture (PDL 27) (Figure 2H). This difference was indistinguishable from the 100% tumor incidence of telomerase-positive HTRVec cells, and exhibited statistical significance at high probability (α=0, power=1.0) compared with a tumor incidence of zero percent in HTRCre lines at late passages (PDL 186/189) (Figure 2H). Analysis of HTRCre tumor explants confirmed the absence of hTERT and retention of Sh Ble expression specific to HTRCre cells (Figure 3H, lane 2). The eventual loss of tumor-forming capability and anchorage-independent growth at later passages was coincident with the appearance of chromosome ends with no detectable telomeric DNA (Figure 3D, F, G, Figure 2H). These results demonstrate that longer telomere reserves permit human tumor formation for prolonged periods in the absence of telomere maintenance and hTERT, but that the eventual loss of telomeric DNA leads to crisis and an inability to support tumor formation.
Figure 3. Excision of hTERT from tumorigenic cells with elongated telomeres.
(A) Telomerase activity in cell lysates (200 ng) from HTRCre and HTRVec clonal cell lines at indicated PDL, controls as specified in Figure 2. (B) RT-PCR analysis of hTERT, hph, and GAPDH at indicated PDL. HA5 cells were included as a negative control. (C) Replicative lifespan of each clonal line, as indicated. HTRVec cells remained immortal. (D) Anchorage-independent colony growth at increasing PDL, including HA5 and HTR cells as controls (n=4 each), and 293T cells (n=3). Difference between the latest and earliest PDL within each line as indicated (**, p<0.01; ***, p<0.001, power(1-β err prob)=1.0, αactual=0.05, two-tailed). (E) TRF analysis of average telomere length at indicated PDL. Weighted mean telomere lengths (kbp) are indicated below each lane. (F) Analysis of telomere integrity. X-axis, individual lines and respective PDL; y-axis, average number of telomere signal-free ends (SFE) per metaphase (n=10). Brackets indicate a statistically significant difference (p<0.001, power(1-β err prob)=1.0, αactual=0.038–0.044). HTRVec at PDL 169 possessed no SFE. (G) Relative telomere length of the lines depicted in (F). X-axis, telomere fluorescence intensity in arbitrary units; y-axis, frequency of events. Early PDL (light grey), late PDL (dark grey). Graphs are scaled equivalently. (H) RT-PCR analysis of hTERT, Sh Ble (zeocin resistance) and GAPDH in normal adjacent kidney (NK) or renal capsule (RC). The water control (H2O) is the same as in Figure 2E, lane 11.
Crisis in TERT-excised cells is rescued by catalytically active TERT
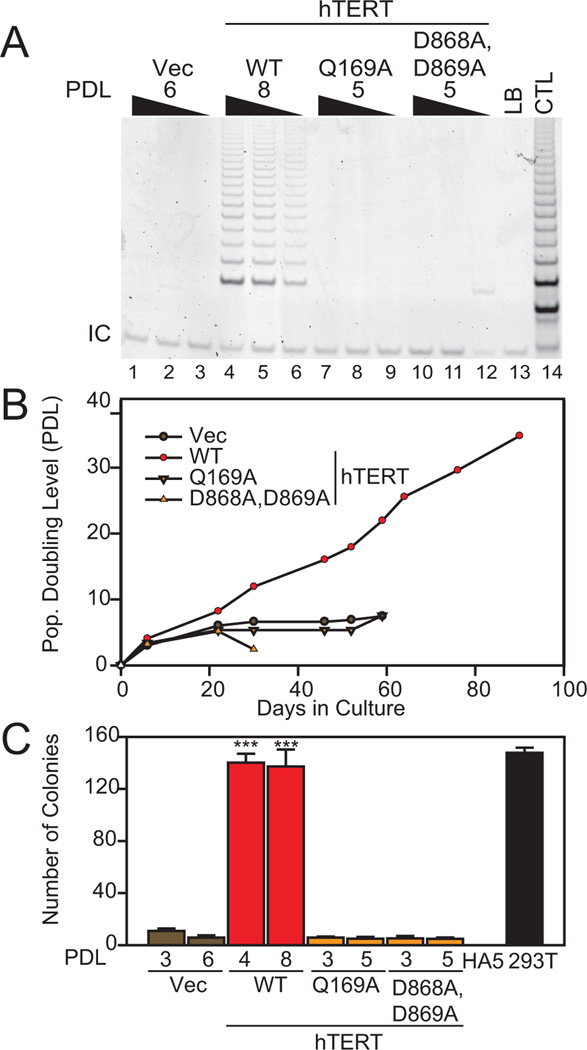
To confirm that crisis was induced by critically short telomeres and not via unrelated genetic events, wild-type hTERT or inactive hTERT mutants Q169A (Sealey et al., 2010; Wyatt et al., 2009) or D868A/D869A (Harrington et al., 1997) were introduced into HTREP Cre-4 cells at PDL 6 (Figure 4). Only wild-type hTERT restored telomerase activity (Figure 4A), extended cellular lifespan (Figure 4B), and conferred anchorage-independent growth (Figure 4C). HA5 cells without HRASG12V also depend on the catalytic activity of hTERT to avoid crisis (Sealey et al., 2010). The fact that telomerase catalytic activity was essential to avert crisis and promote anchorage-independent growth supports the critical role of hTERT-mediated telomere extension activity in tumor cell survival when telomeres are short.
Figure 4. Ability of hTERT to rescue crisis in hTERT-excised cells.
(A) Wild-type (WT) or mutant (Q169A; D868A, D869A) hTERT or empty vector (Vec) were introduced into HTREP Cre cells and analyzed for telomerase activity (200, 100, 50 ng lysate). (B) Replicative lifespan of cell lines as indicated above. hTERT WT cells remained immortal. (C) Anchorage-independent growth of cell lines as indicated (n=4). Statistical significance compared with vector controls as indicated (***, p<0.001, power(1-β err prob)=1.0, αactual=0.05, two-tailed). Controls and axis labels as in Figure 2.
Discussion
In the presence of sufficiently long telomeres, telomere erosion or the absence of hTERT did not impede human tumorigenesis. Only when telomeric DNA was lost from chromosome ends did cells resume dependence upon the telomere elongation activity of hTERT. Other examples of tumor-forming capability in cells that do not express hTERT are known, for example in ALT cells or primary tissues transformed with oncogenic RAS (Liang et al., 2008; Sun et al., 2005), but these examples did not permit the ability to test the compatibility of ongoing telomere erosion with cell survival. Examples of tumors that lack in vitro telomerase activity have been correlated with clinical regression (e.g. retinoblastoma or neuroblastoma) (Gupta et al., 1996; Hiyama et al., 1995), however these studies preceded the cloning of hTERT or identification of ALT and in many cases these tumor types are now known to exhibit ALT-like characteristics or low hTERT expression (reviewed in Cesare and Reddel, 2010). Here, we showed in a defined genetic system that telomerase-negative human tumor cells are capable of tumor formation and cell viability in the absence of endogenous hTERT expression or ALT.
Although tumorigenic potential has not been examined in mice lacking Tert, its absence has no phenotypic consequences in normal murine tissues while telomere reserves remain intact (Erdmann and Harrington, 2009; Meznikova et al., 2009; Strong et al., 2011; Vidal-Cardenas and Greider, 2010). The fact that TERT is dispensable for human tumor formation was not expected. For example, deletion of one subunit of the Ku heterodimer, a complex also important in maintaining telomere integrity, is lethal in human tumor cells but is dispensable in other organisms (Fattah et al., 2008; Li et al., 2002). Once telomeres became critically short, however, aversion of tumor cell crisis depended upon active TERT. In contrast, when TERT is over-expressed, its ability to stimulate proliferation does not always depend on catalytic activity, e.g. in ALT cells (Stewart et al., 2002) or murine hair follicles (Flores et al., 2005; Parkinson et al., 2008; Sarin et al., 2005).
Human tumor cells retained their susceptibility to telomere-induced crisis even after prolonged growth periods. This delayed dependence upon telomerase function differs from the ‘addiction’ to oncogenic factors such as MYC or RAS, in which cell survival remains reliant on these factors (Greider, 1999; Weinstein and Joe, 2008). Thus, human tumor cells are reliant upon telomere integrity rather than hTERT or telomerase activity. Although such dependence was well established for normal cell growth, it was not possible to predict whether tumor cells might somehow subvert telomere-induced crisis via induction of endogenous telomerase, ALT, or another mechanism. For example, S. cerevisiae lacking telomerase and the recombination factor RAD52 can escape senescence indefinitely via activation of RAD52-independent telomere maintenance mechanisms, provided the strain possesses long telomeres initially (Grandin and Charbonneau, 2009; Lebel et al., 2009). In contrast, our results suggest that human tumor cells with initially long telomeres can only temporarily avert the requirement for telomere maintenance.
These results have implications for telomerase inhibition in cancer therapy. Telomerase-negative pediatric cancers such as ependymoma possess a better long-term prognosis than telomerase-positive cancers (Tabori and Dome, 2007), and low telomerase expression or ALT correlates with a better outcome in histiocytoma and colorectal cancer (Matsuo et al., 2009; Tatsumoto et al., 2000). Our finding that telomerase-negative tumors do not invoke ALT and remain mortal may provide a mechanism to explain the more favorable prognosis for a subset of telomerase-negative tumor types in vivo. Thus, even in telomerase-positive tumors with long telomeres, telomerase inhibition combined with adjunct treatments that limit tumor progression could prove effective as an anti-cancer therapy.
Experimental Procedures
Cell culture
Cell culture and PDL determination was performed as described (Hayflick, 1973; Sealey et al., 2010). hTERT was introduced via electroporation and clonal populations selected in 200 µg/mL hygromycin (Invitrogen), followed by retroviral infection with pBABE- puro-HRASG12V (Addgene) (Hahn et al., 1999a) and selection in 2 µg/mL puromycin (Invitrogen). Transfection with pcDNA3.1-zeo-Cre (Cre recombinase cDNA provided by Dr. Michael Reth) or pcDNA3.1-zeo (Invitrogen) was performed using Fugene6 (Roche) with transient selection in 200 µg/mL zeocin (Invitrogen). Inactive hTERT variants were introduced as described (Sealey et al., 2010).
Protein and RNA analysis
Western blots, RT-PCR mRNA analysis, and the telomere repeat amplification protocol (TRAPeze, Millipore) were performed as described (Sealey et al., 2010). RT-PCR analysis of mRNA encoding zeocin resistance (Sh Ble) was conducted using the following DNA primers: 5′-GACTTCGTGGAGGACGACTT-3′ and 5′-GACACGACCTCCGACCACT-3′. Primary antibodies employed were anti-SV40 T Ag (Pab-108) (Santa Cruz), anti-HRAS (C-20) (Santa Cruz) and anti-actin (Sigma).
Anchorage-independent growth assay
Equal cell numbers (5 × 104) were plated onto 0.6% w/v agar and incubated at 37°C (5% v/v CO2) for 21 days as described (Cifone and Fidler, 1980). Colonies were stained with 0.01% w/v crystal violet and images acquired with a Bio-Rad Molecular Imager Gel Doc XR System. Colonies were counted using Imagequant TL (GE Healthcare).
Cell line injections in vivo
A suspension of 5 × 105 cells was injected subcutaneously or under the subrenal capsule space of Rag2−/− / γ-chain−/− immunodeficient mice (Mazurier et al., 1999). After 21–22 days the mice were sacrificed and examined. Explanted tissues were extracted for RNA and analyzed by RT-PCR as described above. Experiments were performed in accordance with protocols approved by the Animal Care Committee.
Telomere Terminal Restriction Fragment (TRF) and Q-FISH analysis
Telomere length was analyzed via TRF (telomere restriction fragment) analysis (Sealey et al., 2010), and average length determined after Southern blotting using Imagequant TL and UTSWTELORUN software first developed by H. Vaziri and C. Harley (Ouellette et al., 2000). Q-FISH was performed as described (Erdmann and Harrington, 2009) on 10 separate metaphases for each PDL indicated.
Statistical analysis
Differences in average colony number were assessed via ANOVA, assuming unequal variance and using a Tukey post-test (Instat3, GraphPad). Statistical significance of tumor incidence was assessed using Fisher’s exact test (Prism5, GraphPad). G*power3 was used to determine power and alpha values where indicated (Faul et al., 2009). Quantification of telomere-signal free ends (SFE) after Q-FISH was compared using ANOVA with a Tukey post-test (Instat3, GraphPad).
Highlights.
Human cells with long telomeres can form tumors without telomerase or ALT
Telomere length maintenance is not strictly required for tumorigenic potential
Human telomerase-negative tumors remain mortal and eventually enter crisis
Acknowledgements
The authors wish to dedicate this publication to our late colleague and friend Dr. Michael A.S. Taboski, whose passionate commitment to science will continue to inspire us. Supported by the CBCF-Ontario Chapter to the late M.A.S.T., the CIHR (MOP-86453) to D.H.B., and the NCIC (15072), NIH (RO1-AG024398), and Wellcome Trust UK (84637) to L.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Capper R, Britt-Compton B, Tankimanova M, Rowson J, Letsolo B, Man S, Haughton M, Baird DM. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio SM. Novel strategies for immortalization of human hepatocytes. Artif. Organs. 2001;25:529–538. doi: 10.1046/j.1525-1594.2001.025007529.x. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- Cifone MA, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc. Natl. Acad. Sci. USA. 1980;77:1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol. Cell. Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann N, Harrington LA. No attenuation of the ATM-dependent DNA damage response in murine telomerase-deficient cells. DNA Rep. 2009;8:347–353. doi: 10.1016/j.dnarep.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattah KR, Ruis BL, Hendrickson EA. Mutations to Ku reveal differences in human somatic cell lines. DNA Rep. 2008;7:762–774. doi: 10.1016/j.dnarep.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- Grandin N, Charbonneau M. Telomerase- and Rad52-independent immortalization of budding yeast by an inherited-long-telomere pathway of telomeric repeat amplification. Mol. Cell. Biol. 2009;29:965–985. doi: 10.1128/MCB.00817-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW. Telomerase activation. One step on the road to cancer? Trends Genet. 1999;15:109–112. doi: 10.1016/s0168-9525(98)01681-3. [DOI] [PubMed] [Google Scholar]
- Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983;25:179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Gupta J, Han LP, Wang P, Gallie BL, Bacchetti S. Development of retinoblastoma in the absence of telomerase activity. J. Nat. Canc. Inst. 1996;88:1152–1157. doi: 10.1093/jnci/88.16.1152. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999a;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 1999b;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harrington L, Zhou W, McPhail T, Oulton R, Yeung DSK, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Subculturing Human Diploid Fibroblast Cultures. In: J Kruse PF, Patterson MK, editors. Tissue Culture Methods and Applications. New York: Academic Press; 1973. pp. 220–223. [Google Scholar]
- Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucl. Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat. Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, et al. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T) Dis. Esoph. 2007;20:256–264. doi: 10.1111/j.1442-2050.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rosonina E, Sealey DC, Pryde F, Lydall D, Maringele L, Harrington LA. Telomere maintenance and survival in saccharomyces cerevisiae in the absence of telomerase and RAD52. Genetics. 2009;182:671–684. doi: 10.1534/genetics.109.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nelsen C, Hendrickson EA. Ku86 is essential in human somatic cells. Proc. Natl. Acad. Sci. USA. 2002;99:832–837. doi: 10.1073/pnas.022649699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Kahlenberg MS, Rousseau DL, Jr, Hornsby PJ. Neoplastic conversion of human colon smooth muscle cells: No requirement for telomerase. Mol. Carcinog. 2008;47:478–484. doi: 10.1002/mc.20405. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Shay JW, Wright WE, Hiyama E, Shimose S, Kubo T, Sugita T, Yasunaga Y, Ochi M. Telomere-maintenance mechanisms in soft-tissue malignant fibrous histiocytomas. J. Bone Joint Surg. Am. 2009;91:928–937. doi: 10.2106/JBJS.G.01390. [DOI] [PubMed] [Google Scholar]
- Mazurier F, Fontanellas A, Salesse S, Taine L, Landriau S, Moreau-Gaudry F, Reiffers J, Peault B, Di Santo JP, de Verneuil H. A novel immunodeficient mouse model--RAG2 x common cytokine receptor gamma chain double mutants--requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res. 1999;19:533–541. doi: 10.1089/107999099313983. [DOI] [PubMed] [Google Scholar]
- Meznikova M, Erdmann N, Allsopp R, Harrington LA. Telomerase reverse transcriptase-dependent telomere equilibration mitigates tissue dysfunction in mTert heterozygotes. Dis. Model Mech. 2009;2:620–626. doi: 10.1242/dmm.004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette MM, Liao M, Herbert B-S, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE. Subsenescent Telomere Lengths in Fibroblasts Immortalized by Limiting Amounts of Telomerase. J. Biol. Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- Parkinson EK, Fitchett C, Cereser B. Dissecting the non-canonical functions of telomerase. Cytog. Gen. Res. 2008;122:273–280. doi: 10.1159/000167813. [DOI] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey DC, Zheng L, Taboski MA, Cruickshank J, Ikura M, Harrington LA. The N-terminus of hTERT contains a DNA-binding domain and is required for telomerase activity and cellular immortalization. Nucl. Acids Res. 2010;38:2019–2035. doi: 10.1093/nar/gkp1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert S, Shay JW, Wright WE. Transient expression of human telomerase extends the life span of normal human fibroblasts. Bioch. Biophy. Res. Comm. 2000;273:1095–1098. doi: 10.1006/bbrc.2000.3080. [DOI] [PubMed] [Google Scholar]
- Stewart N, Bacchetti S. Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology. 1991;180:49–57. doi: 10.1016/0042-6822(91)90008-y. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Hahn WC, O'Connor BF, Banner EN, Lundberg AS, Modha P, Mizuno H, Brooks MW, Fleming M, Zimonjic DB, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA. 2002;99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MA, Vidal-Cardenas SL, Karim B, Yu H, Guo N, Greider CW. Phenotypes in mTERT+/− and mTERT−/− mice are due to short telomeres, not telomere-independent functions of TERT. Mol. Cell. Biol. 2011;31:2369–2379. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Chen M, Hawks CL, Hornsby PJ. Immortal ALT+ human cells do not require telomerase reverse transcriptase for malignant transformation. Cancer Res. 2005;65:6512–6515. doi: 10.1158/0008-5472.CAN-05-1210. [DOI] [PubMed] [Google Scholar]
- Tabori U, Dome JS. Telomere biology of pediatric cancer. Cancer Invest. 2007;25:197–208. doi: 10.1080/07357900701208683. [DOI] [PubMed] [Google Scholar]
- Tatsumoto N, Hiyama E, Murakami Y, Imamura Y, Shay JW, Matsuura Y, Yokoyama T. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin. Cancer Res. 2000;6:2696–2701. [PubMed] [Google Scholar]
- Ungrin MD, Harrington L. Strict control of telomerase activation using Cre-mediated inversion. BMC Biotechnol. 2006;6:10–14. doi: 10.1186/1472-6750-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- Vidal-Cardenas SL, Greider CW. Comparing effects of mTR and mTERT deletion on gene expression and DNA damage response: a critical examination of telomere length maintenance-independent roles of telomerase. Nucl. Acids Res. 2010;38:60–71. doi: 10.1093/nar/gkp855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt HD, Tsang AR, Lobb DA, Beattie TL. Human telomerase reverse transcriptase (hTERT) Q169 is essential for telomerase function in vitro and in vivo. PLoS ONE. 2009;4:e7176. doi: 10.1371/journal.pone.0007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Blackburn EH. Human Cancer Cells Harbor T-Stumps, a Distinct Class of Extremely Short Telomeres. Mol. Cell. 2007;28:315. doi: 10.1016/j.molcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mar V, Zhou W, Harrington L, Robinson MO. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]