SUMMARY
mRNAs encoding polarity and secretion factors (POLs) target the incipient bud site in yeast for localized translation during division. In pheromone-treated cells, we now find that these mRNAs are also localized to the shmoo tip. However, in contrast to the budding program, neither the She2 nor She3 proteins are involved. Instead, the Scp160 RNA-binding protein binds POL and mating pathway mRNAs and regulates their spatial distribution in a Myo4- and cortical ER-dependent fashion. RNA-binding by Scp160 is stimulated by activation of Gpa1, the G-protein α-subunit regulated by the pheromone receptor, and is required for pheromone gradient sensing, as well as subsequent chemotropic growth and cell-cell mating. These effects are incurred independently of obvious changes in translation, thus, mRNA trafficking is required for chemotropism and completion of the mating program. This is the first demonstration of ligand-activated RNA targeting in the development of a simple eukaryote.
Keywords: mRNA localization, Scp160, She2, RNA-binding protein, Gpa1, pheromone, Sro7, Sec3, exocyst, Myo4, polarity, exocytosis, chemotropism, yeast
INTRODUCTION
Polarity establishment in eukaryotes involves asymmetric organization of the cytoskeleton and secretory pathway, and leads to the polarized distribution of new membrane along a given axis (Bretscher, 2003; Drubin and Nelson, 1996). This developmental program is important for cellular processes, such as differentiation, motility, chemotaxis, cell division, and morphogenesis (Affolter and Weijer, 2005; Betschinger and Knoblich, 2004; Drubin and Nelson, 1996; Pruyne and Bretscher, 2000; Wodarz, 2002). In the yeast, Saccharomyces cerevisiae, polarized growth leads to the creation of daughter cells during cell division, the asymmetric distribution of cell-fate determinants, and the mating of haplotypes (Bretscher, 2003; Casamayor and Snyder, 2002). Polarization in yeast is dependent upon the activation and asymmetric distribution of polarity factors, principally belonging to the Rho family of small GTPases (e.g. Cdc42, Rho1-3), at the site of incipient bud or mating projection (i.e. shmoo) formation (Park and Bi, 2007). These factors regulate organization of the actin cytoskeleton to deliver new proteins (e.g. landmark proteins, cell wall components and remodeling enzymes, secretory machinery, etc.) and membrane to the site of polarized growth. Moreover, RNA-protein complexes localize to the sites of polarization in yeast and mammalian cells, indicating that mRNA trafficking may contribute to polarization (Aronov et al., 2007; Bassell et al., 1994; Zhang et al., 1999), probably by allowing for the local translation of polarity and secretion factors (Gerst, 2008).
Long et al (1997) and Takizawa et al. (1997) demonstrated that asymmetric localization of ASH1 mRNA, which encodes a transcriptional repressor essential for mating-type switching (Jansen et al., 1996), confers cell-fate determination in daughter cells (Long et al., 1997; Takizawa et al., 1997). ASH1 mRNA is trafficked in an actin- and type-V myosin (Myo4/She1)-dependent fashion, which necessitates both the She2 RNA-binding protein (RBP) and She3 adaptor that form a complex with Myo4. Together, ASH1 and a number of other mRNAs undergo polarization in a She-dependent manner (Lange et al., 2008; Shepard et al., 2003; Takizawa and Vale, 2000). We previously examined whether polarized mRNA trafficking plays a role during budding, particularly in regards to how polarity factors (e.g. Cdc42, Rho3) and components of the exocytic machinery (e.g. Sec1, Sec3, Sec4; collectively termed “POLs”) localize to the incipient bud site during cell division (Aronov et al., 2007). We found that POL mRNAs are trafficked to the bud, like ASH1 mRNA, resulting in the enrichment of their respective proteins therein. Moreover, POL mRNA trafficking depends upon the same factors that facilitate ASH1 mRNA localization (e.g. the 3′-untranslated region (3′UTR), She proteins, Puf6, and actin cytoskeleton). POL mRNA polarization precedes POL protein enrichment and subsequent bud emergence, indicating that mRNA localization might facilitate cell polarization, although the She proteins are neither essential for budding nor for viability. Importantly, POL mRNAs co-traffic with cortical ER (cER) and mutations that affect cortical ER inheritance and anchoring in the bud (e.g. she3Δ, myo4Δ, sec3Δ, and srp101; (Estrada et al., 2003; Prinz et al., 2000; Wiederkehr et al., 2003) alter ASH1 and POL mRNA localization (Aronov et al., 2007). Moreover, these mRNAs associate with ER membranes and, together, undergo co-trafficking in a She2- and She3-dependent fashion. mRNA and cER co-trafficking probably reflects a conserved mechanism by which mRNAs and the translocation apparatus are moved to distal areas of the cell to more effectively control the local concentration of protein (Gerst, 2008).
Interestingly, the She proteins and polarized mRNA trafficking are not essential during budding, probably since essential POLs (e.g. Cdc42, Rho3, Sec4) bear lipid anchors and can access the bud via the secretory pathway. However, we assumed that polarized mRNA trafficking could be important for other developmental processes. Thus, we examined whether mRNA trafficking is necessary for pheromone sensing and the yeast mating response. Haploid yeast (MATa or α) treated with the opposite mating factor form polarized membrane extensions in the direction of the pheromone gradient. These extensions (called shmoos) are larger, more elongated, projections than buds and are analogous to membrane extensions (i.e. dendrites, axons, lamellipodia) seen in higher eukaryotes. Moreover, their ability to sense and respond rapidly to gradients (chemotropism) requires the continual regulation and deposition of polarity factors and mating components at the shmoo tip in order for efficient cell pairing and mating to occur. Recent studies have shown that local protein synthesis may play a role in the chemotropic responses of higher cell types (e.g. neuronal dendrites and axons/growth cones) induced by certain extracellular stimuli (Bramham and Wells, 2007; Lin and Holt, 2008; Yao et al., 2006; Yoon et al., 2009). Moreover, some mRNAs are locally translated in the growth cone in response to different guidance cues, allowing for spatial and temporal sensing of the stimuli (Lin and Holt, 2008; Rosoff et al., 2004).
Here we demonstrate mRNA and cER co-trafficking in the stochastic enrichment of proteins, like Sro7 and Fus3, at the shmoo tip during pheromone signaling and chemotropic growth. We find that SRO7 and FUS3 mRNA targeting to the shmoo tip is dependent upon Scp160, an RBP containing 14 K homology (KH) domains shown previously to associate with ER-bound polyribosomes and proteins implicated in translation initiation or signal transduction, as well as RNAs, to form large ribonucleoprotein (RNP) complexes (Baum et al., 2004; Frey et al., 2001; Weber et al., 1997). Guo et al. (2003) previously identified Scp160 as a potential effector of the mating factor-activated G-protein α-subunit, Gpa1, however the basis for this interaction remained unknown (Guo et al., 2003). We show that Scp160-mediated RNA-binding is essential for the correct sensing of pheromone gradients and successful culmination of the mating response. Thus, pheromone signaling controls mRNA trafficking to enrich POL and mating pathway components (e.g. Sro7, Ste7, Fus3, Scp160) at the shmoo tip and confer chemotropic directional growth. Moreover, it utilizes some of the machinery involved in the asymmetric localization of mRNA during budding (e.g. type V Myosin 4, cER delivery, but neither She2 nor She3). Importantly, this work suggests that simple eukaryotes coordinate mRNA targeting and localized protein synthesis to respond precisely to the signals from the extracellular environment. Such a mechanism may have evolved to the cells (e.g. neurons) of higher eukaryotes to allow for attractive and repulsive directional growth.
RESULTS
SRO7 mRNA and protein localize to the shmoo tip during its formation
We previously demonstrated that POL mRNAs (e.g. CDC42, SEC4, SRO7) localize to the tip of the incipient bud prior to nuclear division and in a manner that precedes RFP-tagged POL protein enrichment and subsequent bud emergence (Aronov et al., 2007). To determine whether polarized mRNA trafficking plays a role in the formation of mating projections (shmoos) in mating factor-treated cells, we employed the functional RFP-POL gene fusions used previously and which bear binding sites for the bacteriophage MS2 coat protein (MS2-CP) downstream of the coding region and upstream to the 3′UTR. These constructs allow for the simultaneous localization of both mRNA, upon co-expression with MS2-CP fused with GFP, and the RFP-tagged translation product using fluorescence microscopy. Moreover, the localization of mRNAs (e.g. SRO7) expressed from these plasmids was identical to that observed upon expression from the genome (Haim et al., 2007).
We examined the localization of POL mRNAs, such as SRO7 mRNA, which encodes a SNARE regulator and tomosyn ortholog, in α-factor-treated MATa wild-type cells and found that it localized to the shmoo tip (e.g. 93% shmoo localization for RFP-SRO7 mRNA, n=129 cells; Figure 1A). In contrast, mRNAs encoding the Sec4 and Cdc42 small GTPases localized to the cell body and not to the shmoo tip (e.g. 23% and 20% shmoo localization for RFP-SEC4 and RFP-CDC42; n=98 and 118 cells, respectively; Figure 1A and Table S1). Despite these differences in mRNA localization, the RFP-tagged Sec4, Cdc42, and Sro7 proteins generated from these mRNAs concentrated at the shmoo tip (e.g. 96%, 93%, and 87% shmoo localization for Sec4, Sro7, and Cdc42; n=68, 69, and 59 cells, respectively; Figure 1A and Table S1). Thus, POL mRNAs may have specific patterns of localization in mating factor-treated cells (as opposed to budding cells wherein these mRNAs co-localize), although their translation products still reach sites of polarized growth. Moreover, membrane-anchored POL proteins, like Sec4 and Cdc42, localize independently of targeted mRNA trafficking during the mating program. Interestingly, a similar result was observed in budding cells, whereby mutations in the SHE genes abolished mRNA trafficking, but not the delivery of Sec4 and Cdc42 protein to the bud tip (Aronov et al., 2007). In contrast, a soluble (i.e. non-anchored) POL (e.g. Sro7) became mislocalized in the absence of the She machinery, indicating that mRNA trafficking is required for its enrichment at the site of budding (Aronov et al., 2007). Therefore, it would appear that membrane-anchored POL proteins access the sites of polarization via the secretory pathway, while soluble POLs necessitate mRNA trafficking and local translation. Thus, the growth processes of budding and mating yeast might employ both common and distinct mechanisms for POL mRNA transport and localization.
Figure 1. SRO7 mRNA and protein localize to the shmoo tip in mating factor-treated yeast.
A. Wild-type yeast expressing the RFP-SEC4 or RFP-SRO7 genes bearing MS2-CP binding sites upstream to their 3′UTRs and MS2-CP-GFP from plasmids were grown to early log phase on selective medium and either treated with α-factor (5μM; +) or maintained in the absence of mating factor (−) for 1.5hrs, and then examined by fluorescence microscopy. mRNA is indicated by GFP fluorescence; protein by RFP fluorescence; and merge combines the fluorescence and transmitted light windows. White arrowheads indicate co-localization of the mRNA granule and RFP protein at the shmoo (in treated cells) or bud (in untreated cells) tip. B. Wild-type yeast expressing RFP-SRO7 bearing MS2-CP binding sites upstream to its 3′UTR and MS2-CP-GFP from plasmids were treated with α-factor (5μM) and examined by time-lapse fluorescence microscopy. Time is given in minutes. White arrowheads indicate the localization of mRNA granules. See corresponding Movie S1.
We previously found that POL mRNA placement at a specific site precedes POL protein enrichment and bud emergence, suggesting that mRNA trafficking may participate in polarity establishment and maintenance (Aronov et al., 2007). To verify if this is true for shmooing cells, we examined POL mRNA and protein localization as a function of time in MATa wild-type cells treated with α-mating factor (Figure 1B and Movie S1). Importantly, we found that SRO7 mRNA placement at a given site was essentially concomitant with both protein enrichment (i.e. enhanced RFP fluorescence) and shmoo formation at that site in all cells examined. Thus, mRNA placement might be important to facilitate shmoo formation during the mating response.
SRO7 mRNA localization to the shmoo tip is independent of SHE2 and SHE3, but correlates with localization to the ER in a MYO4- and SEC3-dependent manner
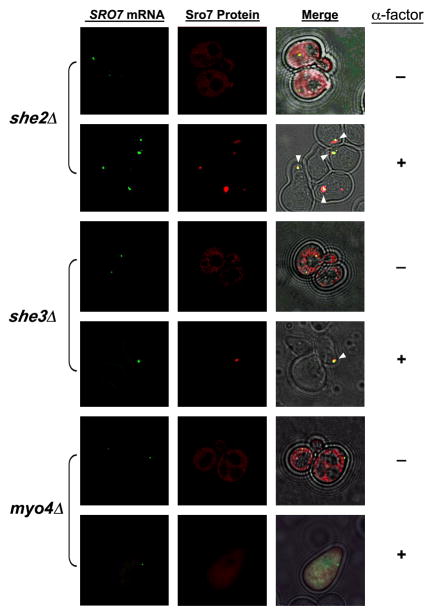
As SRO7 mRNA is targeted to the shmoo tip (Figure 1A), we determined whether proteins involved in ASH1 and POL mRNA transport to the bud tip play a role in this process. We examined POL mRNA localization in α-mating factor-treated MATa myo4Δ/she1Δ, she2Δ, and she3Δ yeast. Shmoo formation was not blocked in the absence of these proteins and SRO7 mRNA was mislocalized only in myo4Δ cells and did not reach the shmoo tip therein (e.g. 9% shmoo localization for RFP-SRO7 mRNA, n=86 cells; Figure 2). In contrast, SRO7 mRNA localized properly to the shmoo tip in both she2Δ and she3Δ cells (e.g. 95% and 98% shmoo localization, n=65 and 59 cells, respectively; Figure 2 and see she3Δ cells shown in Movie S2). This contrasts with a requirement for all She components in either ASH1 or POL mRNA localization in budding cells.
Figure 2. SRO7 mRNA localization is SHE2- and SHE3-independent in mating factor-treated yeast.
she2Δ, she3Δ, and myo4Δ cells expressing RFP-SRO7 bearing MS2-CP binding sites upstream to the 3′-UTR and MS2-CP-GFP from plasmids were either treated with α-factor (5μM; +) or left untreated (−), and examined using a DeltaVision imaging system. The white arrowheads indicate co-localization of the mRNA granule and RFP-Sro7 protein at the shmoo tip. Merge combines the fluorescence and transmitted light windows. See corresponding movie for SRO7 mRNA and protein in she3Δ cells in Movie S2.
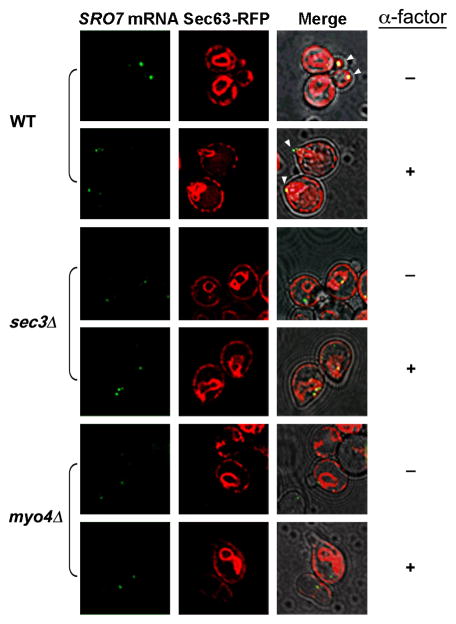
We previously demonstrated that POL mRNAs associate with cER and are co-trafficked to the bud tip in wild-type cells, but not in myo4Δ or sec3Δ cells (Aronov et al., 2007). By examining ER (visualized using Sec63-RFP) and SRO7 mRNA by time-lapse, we again noted the delivery of both cER and mRNA to the incipient bud tip and newly forming bud in wild-type cells during budding (Figure 3, Movie S3). In contrast, SRO7 mRNA was not delivered to the bud and ER inheritance was delayed in sec3Δ cells (Figure 3, Movie S4) and myo4Δ cells (Figure 3). We then examined ER and SRO7 mRNA localization in α-factor treated cells and found similar results; both reached the shmoo tip in wild-type cells (Figure 3, Movie S5), but not in myo4Δ or sec3Δ cells (Figure 3 and Movie S6). Under these conditions, SRO7 mRNA remained juxtaposed to ER present in the cell body, whereas the cER appeared unable to localize correctly to the shmoo tip. This result suggests that mRNA localization and ER inheritance are interconnected during both the budding and shmooing programs.
Figure 3. SRO7 mRNA localization correlates with ER localization in a MYO4- and SEC3-dependent manner.
Wild-type (WT), sec3Δ, and myo4Δ yeast all expressing SRO7 bearing MS2-CP binding sites (upstream of the 3′-UTR), SEC63-RFP, and MS2-CP-GFP from plasmids were either treated with α-factor (5μM; +) or left untreated (−) and examined by fluorescence microscopy using a DeltaVision imaging system. White arrowheads indicate localization of the mRNA granule to either the shmoo or bud tips. Merge combines the fluorescence and transmitted light windows. See corresponding movie for SRO7 mRNA and ER localization in: untreated wild-type cells (Movie S3); untreated sec3Δ cells (Movie S4); α-factor-treated wild-type cells (Movie S5); and α-factor-treated sec3Δ cells (Movie S6).
Scp160 and its intact RNA-binding domain are required for SRO7 mRNA localization
SRO7 mRNA localizes to the shmoo tip in a SHE2 and SHE3-independent manner in α-factor treated cells (Figure 2), although the mechanism of transport is not clear. Interestingly, Guo et al. (2003) identified a potential G-protein signaling pathway in yeast dependent upon the G-protein α subunit, Gpa1, and a novel RBP, Scp160. It was also shown previously that ASH1 mRNA is partially delocalized in scp160Δ mutants (Irie et al., 2002). Therefore, we examined the localization of SRO7 mRNA in MATa scp160Δ cells with or without added α-factor (Figure 4A).
Figure 4. Scp160 is required for SRO7 mRNA localization.
A. SRO7 mRNA and protein are mislocalized in cells lacking SCP160. scp160Δ and scp160Δ cells over-expressing SCP160 (+SCP160) from a multi-copy plasmid were transformed with single-copy plasmids expressing SRO7 bearing MS2-CP binding sites upstream of the 3′-UTR, and MS2-CP-GFP. Cells were grown to mid-log phase and either treated with α-factor (5μM; +) or left untreated (−) and then examined by fluorescence microscopy. Merge combines the fluorescence and transmitted light windows. B. Scp160-GFP also localizes to cER present at the bud and shmoo tips. Cells expressing SCP160-GFP from the SCP160 locus were grown and treated either with (+) or without 5μM α-factor (−), and examined using fluorescence microscopy after 1.5hrs. C. Scp160 associates with Myo4. Cells expressing FLAG-SCP160 from its chromosomal locus were grown to mid-log phase, either treated with 5μM α-factor for 1.5hrs or left untreated, lysed, and incubated with anti-Flag M2 affinity gel to immunoprecipitate proteins. Untreated wild-type control cells were grown and processed in parallel. Samples of the total cell lysate (TCL) and each immunoprecipitate (IP) were separated on a 7% SDS-PAGE gel, blotted, and probed with anti-Flag and anti-Myo4 antibodies.
Unlike in untreated MATa wild-type cells, we observed SRO7 mRNA localization to the bud tip in only 14% of untreated scp160Δ cells (n=121 cells; Figure 4A, Table S1). Moreover, SRO7 mRNA remained mislocalized (3% localization to shmoo tip, n=110 cells; Figure 4A) in mating factor-treated scp160Δ cells. Accordingly, Sro7 protein enrichment in either the bud or shmoo tip was observed in only 7% and 9% of scp160Δ cells (n=109 and 98 cells, respectively; Figure 4A and Table S1). A similar mislocalization of SRO7 RNA and Sro7 protein was observed in untreated MATα scp160Δ cells, as well (Figure S1). In contrast, scp160Δ cells over-expressing SCP160 completely relocalized SRO7 mRNA and protein to either the bud or shmoo tip (84% and 88% mRNA and protein localization to bud, respectively; n=98 and 95 cells; and 86% and 91% localization mRNA and protein localization to shmoo, respectively; n=91 and 88 cells; Figure 4A). Thus, Scp160 mediates SRO7 mRNA localization in both budding and shmooing cells. Moreover, our results imply that Scp160 confers SHE-independent SRO7 mRNA transport in α-factor treated cells.
We next examined the localization of FUS3 mRNA, which encodes a mitogen-activated kinase involved in mating, and found that both FUS3 mRNA and protein are targeted to the shmoo tip in the α-factor-treated cells (Figure S2A, Table S1). Like SRO7, both FUS3 mRNA and protein were significantly mislocalized in the absence of SCP160 (Table S1). This effect appears to be independent of changes in translation as similar levels of Fus3 protein were detected in Westerns (Figure S2B) using WT control cells and cells either lacking SCP160 or bearing a deletion of the 14th KH domain (SCP160KH14Δ), which results in a mutant deficient in both RNA binding and RNP formation(Brykailo et al., 2007; Lang and Fridovich-Keil, 2000; Li et al., 2003).
We also examined the localization of SRO7 mRNA in SCP160KH14Δ cells and found it localized to the shmoo tip in only 43% of cells upon treatment with mating factor (n=76 cells; Table S1). This suggests that the intact RNA-binding function of Scp160 is required for normal RNA localization. The remaining KH domains may account for the residual level of SRO7 mRNA localization observed with this mutant.
Scp160 is an ER protein, however, as it regulates mRNA placement at the bud/shmoo tip, we hypothesized that it might concentrate there. We expressed Scp160-GFP from its genomic locus and observed a typical pattern for ER labeling in both untreated and α-factor-treated (Figure 4B). However, strong Scp160-GFP labeling was also observed at the tips of buds and shmoos.
Since SRO7 mRNA is mislocalized in myo4Δ cells treated with α-factor (Figure 3), we investigated whether Scp160 and Myo4 interact. We immunoprecipitated FLAG- tagged Scp160 expressed from its genomic locus and found that endogenous Myo4 co-immunoprecipitated with Scp160 from both untreated and α-factor-treated cells (Figure 4C). This interaction was reproducible and was not significantly altered in the presence or absence of pheromone (data not shown). Thus, Myo4 interacts (at least indirectly) with Scp160 and is involved in Scp160-mediated mRNA transport.
Scp160 binds specific mRNAs upon mating factor treatment
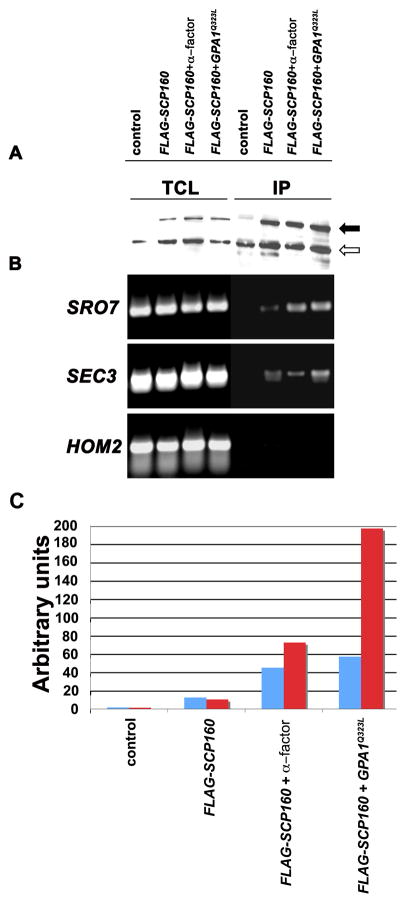
Scp160 is an RBP whose functional significance is unclear. As SRO7 mRNA is polarized in an Scp160-dependent manner, we examined whether other mRNAs bind to Scp160. We performed immunoprecipitation (IP) with endogenously expressed FLAG-tagged Scp160 (Figure 5A) and examined the precipitates for the presence of mRNAs encoding POL (e.g. SRO7, SEC3) and non-POL (e.g. HOM2) proteins (Figure 5B). We found that the SRO7 and SEC3 mRNAs were present in precipitates from cells expressing FLAG-Scp160, but not in precipitates from control (untagged) cells (Figure 5B). In contrast, HOM2 mRNA, which has been used as a non-localized control mRNA in live-cell imaging experiments, was not detected in precipitates from cells expressing FLAG-Scp160. Thus, Scp160 binds to specific mRNAs.
Figure 5. mRNA binding by Scp160 is modulated by the mating pheromone response.
A. Precipitation of Flag-tagged Scp160. Untreated and α-factor-treated wild-type cells expressing FLAG epitope-tagged Scp160, as well as untreated cells expressing both FLAG-Scp160 and Gpa1Q323L, and a wild-type control were grown to mid-log phase, lysed, and incubated with anti-Flag M2 affinity gel to immunoprecipitate proteins. Samples of the total cell lysate (TCL) and each immunoprecipitate (IP) were separated on a 9% SDS-PAGE gel, blotted, and probed with anti-Flag antibody. The filled arrow indicates Scp160; the open arrow indicates a ~100 kDa anti-Flag cross-reacting protein (Lang and Fridovich-Keil, 2000) that served as an internal control for loading. B. and C. SRO7 and SEC3 mRNA binding to Scp160 is increased upon pheromone treatment or Gα activation. RNA was extracted from the TCL samples and immunoprecipitates, and used as a template for RT in either semi-quantitative (B) or real-time PCR (C). Specific primer pairs were used to detect the SRO7, SEC3, and HOM2 mRNAs (as labeled) in (B); and the SRO7 (blue) and SEC3 (red) mRNAs in (C).
As Scp160 has been proposed to be an effector of pheromone signaling through the Gα subunit, we determined what influence Gα subunit signaling (i.e. mimicked by over-expression of an activated form, Gpa1Q323L) has upon on mRNA binding to Scp160 in mating factor-treated cells. By employing real-time PCR, we found that SRO7 and SEC3 POL mRNAs were significantly enriched (~4-fold more than untreated cells; Figures 5B and C) in immunoprecipitates derived from MATa wild-type cells treated with α-mating factor. Importantly, Scp160 binding to these messages was even more dramatic in untreated cells over-expressing Gpa1Q323L (i.e. Scp160 bound 5-fold more SRO7 mRNA and ~20-fold more SEC3 mRNA than in untreated wild-type cells; Figure 5C). We also examined whether Scp160 binds transcripts encoding proteins involved in functions downstream of signaling from the mating factor receptor (e.g. STE7, FUS3, KAR3, SCP160, FAR1). In contrast to She2, Scp160 bound many of these messages both in response to mating factor treatment, as well as upon stimulation by Gpa1Q323L (Figures S3 and S4A). RNA binding was dependent on the KH14 domain, as its removal resulted in an inability to pull down these messages (Figure S4B). These results support the contention that cell signaling through the Gα subunit and Scp160 function are connected, and suggest that RNA binding by Scp160 could be an effector of the signal.
A vertebrate ortholog of Scp160, vigilin, was shown to bind to the 3′UTR of vitellogenin mRNA (Dodson and Shapiro, 1994). Thus, we examined importance of the SRO7 3′UTR in binding to Scp160. We IP’d FLAG-tagged Scp160 from either wild-type cells or the same cells expressing Sro7 tagged at the C-terminus with GFP, and examined the immunoprecipitates for the presence of SRO7 mRNA (Figure S4C). As GFP integration at the SRO7 locus results in removal of the 3′UTR from the ORF (Huh et al., 2003), endogenously expressed SRO7-GFP lacks its 3′UTR. Importantly, we found that the amount of SRO7 mRNA precipitated from the GFP-tagged strain was dramatically reduced in comparison to that obtained from wild-type cells (Figure S4C).
Finally, in order to verify a requirement for the 3′UTR in mRNA targeting to the shmoo tip, we expressed chimeras between the RFP gene and the 3′UTR of SRO7 and the mCHERRY gene and the 3′UTR of FUS3. Both chimeric mRNAs were found to localize to the shmoo tip (91% and 96% respectively, Table S1) in contrast to either the RFP or mCHERRY mRNAs that lacked the 3′UTRs (3% and 1% respectively, Table S1). Thus, the 3′UTRs of SRO7 and FUS3 are probably sufficient to confer localization of the native transcripts, which we have shown to be Scp160-dependent.
RNA binding by Scp160 modulates mating efficiency
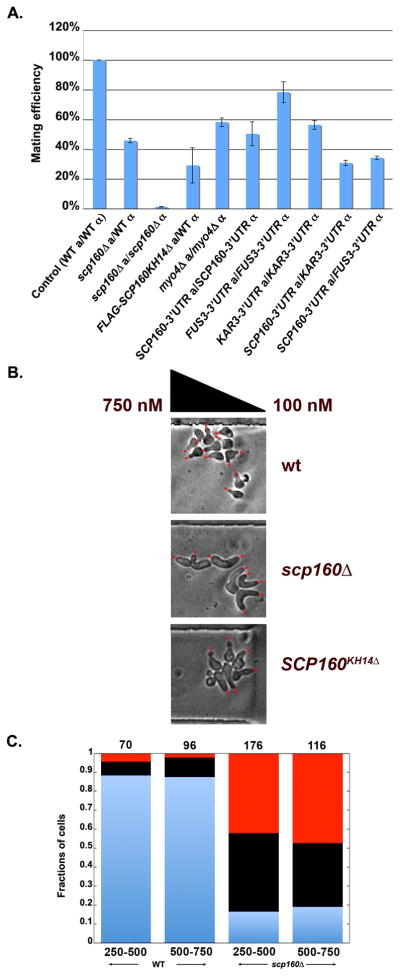
The goal of the pheromone response is to facilitate cell-cell mating. To determine the contribution of Scp160, we compared the mating efficiency of cells lacking SCP160 to that of wild-type cells. As shown in Figure 6A, a heterozygous cross between MATa scp160Δ and MATα wild-type cells showed only 46% mating efficiency, as compared to a homozygous cross between control wild-type cells. Moreover, mating was nearly abolished (1.2 ± 0.02%; n=3 experiments) when homozygous crosses between cells lacking SCP160 were performed (Figure 6A).
Figure 6. RNA-binding by Scp160 modulates mating efficiency and is required for chemotropism.
A. Mating efficiency is affected by the loss of Scp160, its RNA-binding function, or removal of the 3′UTRs belonging to mating pathway components. Mating between: scp160Δ (MATa) and wild-type BY4742 (MATα) cells; scp160Δ (MATa) and scp160Δ (MATα) cells; FLAG-SCP160KH14Δ (MATa) and wild-type BY4742 (MATα) cells; myo4Δ (MATa) and myo4Δ (MATα) cells; SCP160-GFP (MATa) and SCP160-GFP (MATα) cells; FUS3-GFP (MATa) and FUS3-GFP (MATα) cells; KAR3-GFP (MATa) and KAR3-GFP (MATα) cells; SCP160-GFP (MATa) and KAR3-GFP (MATα) cells; and SCP160-GFP (MATa) and FUS3-GFP (MATα) cells was compared in a quantitative fashion with mating between control BY4741 (MATa) and BY4742 (MATα) wild-type cells. Mating efficiency of the wild-type control cells was designated as 100%. B. Cells lacking Scp160 or its RNA-binding function are deficient in chemotropism. Wild-type, scp160Δ, and SCP160KH14Δ-expressing yeast were co-incubated in the same experiment, and exposed to a gradient created between a high (750nM) and low pheromone source (100nM) in a microfluidic chamber for 6hrs. Shmoo orientation relative to the direction of the gradient was determined. Representative photos of cells in the microfluidic chamber for each cell type are shown. Red arrows indicate the direction of shmoo orientation. C. Cells lacking Scp160 show an increased number of projections and reduced viability. Wild-type and scp160Δ cells were compared in terms of the number of projections and cell death. Cells that formed a single projection and sensed gradient are shown in blue, cells that formed multiple projections are shown in black, and cells that underwent cell death in red. The numbers on top reflect the total number of cells scored for each category. The numbers on bottom indicate pheromone concentration (in nM).
To evaluate the importance of Scp160 RNA-binding on mating efficiency, we compared the activity of cells expressing SCP160KH14Δ in the mating assay. Mating efficiency was greatly inhibited (i.e. <30%) in heterozygous crosses between cells expressing SCP160KH14Δ and wild-type cells (Figure 6A). This suggests that the RNA-binding function of Scp160 is critical for penetrance of the pheromone-mediated mating response in yeast. We also examined the mating efficiency of cells lacking MYO4 and saw a significant decrease in mating by >40% in homozygous crosses. Thus, Myo4 is important, but not critical, for normal mating efficiency.
In order to examine contribution of the 3′UTR in the functionality of mRNAs that bind to Scp160 during mating, we performed quantitative mating assays with cells expressing these mRNAs without their 3′UTRs. To do so, we employed wild-type yeast bearing the GFP gene fused downstream of the SCP160, FUS3, and KAR3 ORFs, which results in removal of the 3′UTR from the transcripts (Huh et al., 2003). We found that the mating efficiency of homozygous mating partners decreased to 50%, 78%, and 56%, respectively (Figure 6A), while heterozygous crosses between SCP160-GFP cells and either KAR3-GFP or FUS3-GFP cells (Figure 6A) led to a combinatorial effect upon mating efficiency (i.e. 31% and 34% mating, respectively, Figure 6A). Thus, removal of the 3′UTR (and the Scp160 binding sites) for mating pathway components has deleterious effects upon mating.
Loss of the Scp160 RNA-binding function results in defects in chemotropic growth
Because Scp160 is required for SRO7 and FUS3 mRNA trafficking to the shmoo tip and efficient cell-cell mating, we examined whether its functions are necessary for correct orientation of the shmoo to a mating factor gradient. By employing a microfluidic device (Paliwal et al., 2007; Segall, 1993), we examined the orientation of shmoos formed from MATa wild-type, scp160Δ, and SCP160KH14Δ-expressing cells in response to an α-factor gradient ranging from 750 to 100nM in a micro-chamber (Figure 6B). We found that SCP160KH14Δ cells displayed impaired gradient sensing by forming mating projections that were less oriented in the direction of the gradient, as compared to wild-type cells [e.g. the mean ± SEM angle to the direction of the gradient was 72.3° ± 4.0° for SCP160KH14Δ cells (T=2hrs; n=156 cells) and 63.1° ± 4.0° for wild-type cells (T=2 hrs; n=125 cells)] (Figure 6B; see Figure S5A for polar plots). Cells lacking SCP160 also exhibited defects in gradient sensing, whereby the mean angle to the direction of the gradient was 74.9° ± 4.6° for scp160Δ cells (T=2 hrs; n=105 cells), versus 58.6° ± 6.8° for wild-type cells (T=2 hrs; n=44 cells) (Figure 6B; see Figure S5B for polar plots). These experiments were repeated several times and involved examining ~1000 cells for the analysis of WT vs. SCP160KH14Δ cells and ~700 cells for WT vs. scp160Δ cells. Only cells that displayed one mating projection for the duration of the experiment (T=2hrs) and over the dynamic range of 350–500nM pheromone (wherein chemotropism is constant and no multiple projections form) were included in the final analysis. We also analyzed gradient sensing in cells that did not form multiple projections in the course of 6hrs and noted that WT cells displayed better gradient sensing than scp160Δ cells and SCP160KH14Δ cells during this period (data not shown).
Interestingly, scp160Δ cells are less efficient at properly responding to pheromone gradients over extended time intervals, as many such cells formed multiple mating projections in random directions, as compared to the single gradient-oriented projection seen with wild-type cells (Figure 6C), after 4hrs of exposure to pheromone. In addition, we observed cell lysis and significantly higher cell death at intermediate and high pheromone concentrations for scp160Δ cells (Figure 6C), which has been documented previously for cells unable to mount a proper mating response (Zhang et al., 2006). Thus, either the loss of Scp160 or its RNA-binding function results in cells unable to perform normal gradient sensing and to undergo a normal pheromone response.
DISCUSSION
POL mRNAs (e.g. SRO7, SEC4, CDC42) localize to the incipient bud in an ER- and SHE gene-dependent fashion in budding yeast (Aronov et al., 2007). This may allow for the stochastic enrichment of polarity and secretion factors at the site of polarized growth upon local translation, in order to facilitate polarization (Gerst, 2008). Here, we examined whether this mechanism is also involved in the cellular responses to pheromone. Upon α-mating factor treatment, SRO7 mRNA localizes to site of polarized growth (Figure 1A), as during budding, resulting in the subsequent translation and enrichment of Sro7 protein at the shmoo tip (Figure 1B, Movie S1). Likewise, FUS3 mRNA and protein, which confer mating, also concentrate at the shmoo tip in mating factor-treated cells (Figure S2A, Table S1). In contrast, the SEC4 and CDC42 mRNAs were not polarized upon exposure to mating factor, unlike during cell division, although Sec4 and Cdc42 protein became enriched at the shmoo tip (Figure 1A, Table S1). Thus, the different developmental programs (i.e. budding and mating) show differences in mRNA localization to the sites of polarization.
The differences between budding and mating are best illustrated by the finding that there is no requirement for She2 or She3 in order for SRO7 mRNA to reach the shmoo tip (Figure 2, Movie S2), unlike in budding cells where both proteins are necessary for asymmetric mRNA and protein localization. In contrast, however, Myo4/She1 and Sec3 are required for SRO7 mRNA localization in both budding and shmooing cells (Figures 2, 3). This requirement is likely to be related to actin- and exocyst-mediated cER delivery to the shmoo tip since both proteins confer cER inheritance (Reinke et al., 2004; Wiederkehr et al., 2003) and Sec3 confers ASH1 and POL mRNA association with the ER during budding (Aronov et al., 2007). To confirm this idea, we examined the influence of Myo4 and Sec3 on ER distribution in shmooing cells and found that cER reached the shmoo tip in mating factor-treated wild-type cells (Figure 3, Movie S5), but not in either myo4Δ or sec3Δ cells (Figure 3, Movie S6). Correspondingly, SRO7 mRNA did not reach the shmoo tip in myo4Δ or sec3Δ cells, but remained adjacent to the ER present in the cell body (Figure 3, Movie S6). The tight correlation between ER trafficking and SRO7 mRNA localization to the shmoo tip suggests that this POL mRNA is physically associated, if not transported, with cER during the mating program.
mRNA and cER co-trafficking is conserved in evolution (Gerst, 2008) and may confer the localized translation and translocation of secreted/membrane proteins necessary for shmoo formation in mating factor-treated yeast. However, it was unclear how certain POL mRNAs (e.g. SRO7 ) anchor to ER and undergo trafficking to the shmoo in the absence of She2 or She3, which are necessary during budding. Thus, we examined Scp160, an RBP identified as an effector of the Gpa1 Gα subunit during the mating response, although its specific contribution to mating was unclear. While Scp160 and She2 are required for SRO7 mRNA localization to the bud tip (Figures 2, 4A), Scp160 performs this function alone during mating, with no requirement for either She2 or She3 (Figures 2, 4A, Table S1). Moreover, Scp160 binds (either directly or indirectly) to Myo4 (Figure 4C), localizes to cER at the shmoo tip (Figure 4B), and its association with mRNAs is strongly enhanced upon mating factor-treatment or Gpa1 activation (Figures 5B,C, S3, and S4A). Importantly, these mRNAs are involved in cell polarity (e.g. SRO7, SEC3), as well as the pheromone response (e.g. STE7, FUS3, KAR3), indicating the relevance of Scp160 function to mating. Thus, Scp160 substitutes for She2 upon mating factor stimulation, especially in the trafficking of polarity factor and mating pathway mRNAs. We note, however, that no defects in ER delivery were observed in the absence of SCP160 (data not shown) or SHE2 (Estrada et al., 2003; Prinz et al., 2000; Wiederkehr et al., 2003). Thus, neither Scp160 nor She2 regulate ER movement.
Binding of pheromone to the G protein-coupled mating factor receptors activates the Gα-subunit (Gpa1) and Gβγ subunit dimer (Ste4/Ste18) (Blumer and Thorner, 1991). Upon activation, Gα undergoes GDP-GTP exchange and dissociates from Gβγ, which initiates events that precede mating, including gene transcription, cell cycle arrest, and both morphological and cytoskeletal changes (Dohlman, 2002). In addition to signaling via Gβγ, Guo et al. (2003) demonstrated a signaling function for Gα that occurs via Scp160, although this signal was not shown to be connected directly to RNA binding. Based upon our results, we propose that Scp160 binds to, transports, and localizes POL and mating pathway mRNAs to the sites of active growth and gradient sensing located at the shmoo tip. As defects in protein translation were not obvious in cells lacking functional Scp160 (Figures S1, S2), it suggests that Scp160 acts primarily, though perhaps not exclusively, upon mRNA trafficking to facilitate the accumulation of components involved in cell polarization and mating at the site of shmoo formation. This may require other connections to the secretory pathway, in addition to the idea of cER-based RNA transport, since additional Gα effectors include core components of phosphatidylinositol 3-kinase, Vps34 and Vps15 (Slessareva et al., 2006), which are required for protein transport and cell wall integrity (Takahashi et al., 2001). Likewise, a large-scale examination of protein localization in α-mating factor-treated cells demonstrated that proteins involved in exocytosis, including exocyst subunits, localize tightly to the shmoo tip (Narayanaswamy et al., 2009).
As cells lacking SCP160 form morphologically abnormal (i.e. stunted and enlarged) shmoos, it seemed likely that Scp160 facilitates polarization and mating via its RNA binding and trafficking functions. To verify this hypothesis, we compared the mating efficiency of wild-type and scp160Δ cells in hetero- and homozygous crosses (Figure 6A). Importantly, heterozygous crosses between scp160Δ and wild-type cells showed a diminished ability to undergo successful mating (e.g. <50%), whereas homozygous scp160Δ mating pairs had only ~1% mating efficiency. Thus, Scp160 is required for the normal mating response. Moreover, deletion of the KH14 domain alone, which disrupts RNA binding, greatly reduced mating to wild-type cells (e.g. 29% efficiency; Figure 6A). Since this mutant is morphologically normal, it reveals that the RNA-binding function is important for mating. This idea is supported, in part, by crosses made between yeast lacking the 3′UTRs of mRNAs that bind to Scp160 and encode specific mating pathway components (e.g. FUS3, KAR3, SCP160). Homozygous crosses all showed defects in mating, while heterozygous crosses (between mutants) showed even stronger defects (Figure 6A). Since these mRNAs cannot bind to Scp160 without their 3′UTRs, it affirms that Scp160-mediated mRNA trafficking likely controls the mating process. Finally, we examined the contribution of Myo4 to mating, as it is required for normal SRO7 mRNA localization to the shmoo tip (Figure 3). However, homozygous crosses between myo4Δ cells revealed only a partial defect (i.e. 60% mating), indicating that other means might facilitate POL/mating RNA localization to the shmoo in the absence of this non-essential motor.
Importantly, the reduced mating efficiency seen with scp160Δ and SCP160KH14Δ cells correlates with a loss in pheromone gradient sensing, which abolishes the normal directional growth response to the gradient (Figure 6B, 6C, Figure S5). This defect in chemotropism suggests that Scp160-deficient cells cannot find nearby mating partners; hence the strong decrease in mating efficiency. This is the first demonstration of the necessity of mRNA trafficking for the polarized chemotropic growth of a simple eukaryote. Interestingly, scp160Δ cells display an increased sensitivity to pheromone, resulting in multiple projections or lysis-like cell death at intermediate/high pheromone concentrations (Figure 6C). Perhaps the capacity to maintain a wide dynamic range of gradient sensing (i.e. the concentration wherein only one projection is formed to sense pheromone) is as important a determinant of the overall mating response as the actual accuracy of the response (i.e. the angle of the projection with respect to the gradient). Thus, the defects in mating observed for scp160Δ cells (Figure 6A) reflect the sharply reduced range of pheromone concentrations in which these cells sense gradients (Figure 6C).
One advantage to regulating gene expression by controlling mRNA localization is that spatially restricted stimuli can thereby affect protein translation in a local fashion. This avoids signaling to the nucleus and subsequent dependence upon the processes of mRNA export, cytoplasmic translation, and transport of protein to the site of stimulation (Gerst, 2008; Martin and Ephrussi, 2009). As Scp160 binds to mRNAs involved in polarization and pheromone signaling (Figure 5, S3, S4A) and that deficiencies in its function reduce cell polarization, chemotropism, and mating (Figure 6A,B), it is likely that defects in mRNA trafficking in scp160 mutants are the cause of decreased fecundity, although we cannot exclude other possible mechanisms. That shmoo formation and mating occurs at all likely attests to the ability of POL proteins, like Cdc42, to reach the shmoo tip in the absence of mRNA trafficking, much like in budding cells. Why shmooing cells are more sensitive to defects in mRNA trafficking than budding cells is unclear, but could relate to the ability of proteins, such as Sro7 or Cdc42 or mating pathway elements, to diffuse away from the shmoo tip (and, thus, exert less control) than in the daughter cells in which diffusion is restricted by the mother-bud junction. Alternatively, the pheromone response may involve a broader requirement for factors involved in cell growth and mating (i.e. mating factor receptors, Gα, βγ), thus defects in mRNA delivery may reduce/delay the kinetics of protein enrichment at the site of polarization and affect temporal/spatial responsiveness to the pheromone gradient. As directional shmoo formation in yeast may be analogous to axonal turning, which also depends upon gradient sensing, it is not surprising that this developmental program is sensitive to defects in mRNA trafficking.
During the mating response in yeast, shmoo initiation occurs in the direction of the mating-factor concentration gradient and is typified by continuous correction of the growth of mating projection, since temporal and spatial accuracy is required for the successful fusion between mating partners. Our study suggests that Scp160 contributes to the mating response by effecting the targeting of specific mRNAs to the site of polarization, as well as temporal and spatial growth responses to the gradient (see proposed model, Figure 7). This suggests that targeted mRNA transport is an integral component and effector of intracellular signaling pathways involved in cell polarization. That this occurs in both simple eukaryotes (e.g. yeast, wherein the distance from the cell body to site of polarization is small) to extremely polarized cells (e.g. neurons, wherein mRNA trafficking allows cells to respond quickly to distal stimuli through the localized translation of specific transcripts) suggests two possibilities. First, it says that these processes either evolved from lower eukaryotes or are examples of convergence. Second, it suggests that local translation may be critical for the localized assembly and organization of functional cellular domains, such that the distance of a given protein from its site of function will greatly influence its ability to carry out that function. Given the short diffusion distances involved in yeast, it implies that the factors governing protein function or complex assembly act over very limited distances.
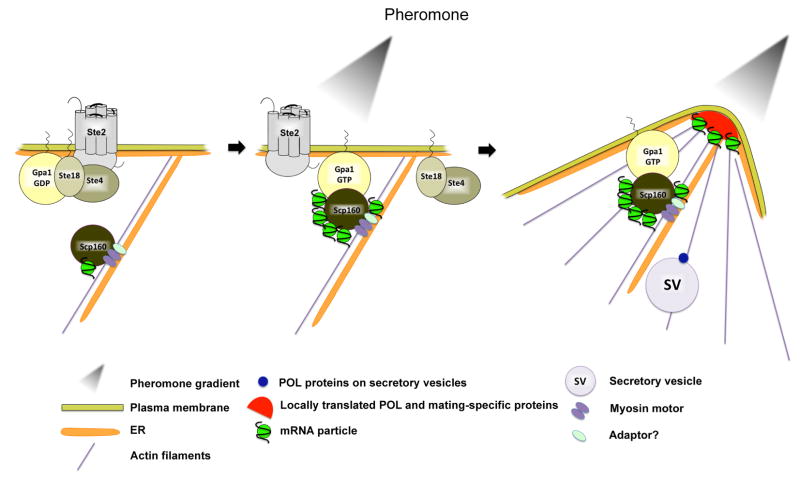
Figure 7. A model for the role of Scp160 in pheromone-mediated chemotropic growth.
Left panel - Untreated budding yeast cells have the inactive α-factor pheromone receptor (Ste2) present at the cell surface in a complex with the inactive G protein α, β, γ subunits (e.g. Gpa1-GDP, Ste18, Ste4, respectively). Scp160 binds to mRNAs, interacts either directly or indirectly with a myosin motor protein (i.e. Myo4), and resides on cortical ER (cER) membranes. The existence of a putative adaptor required for Scp160 to bind to ER membranes and/or the myosin motor is illustrated. Center panel – Upon pheromone (α-factor) binding to the receptor, GDP-GTP catalyzed exchange on Gpa1 results in the release of Ste18/Ste4 to activate MAP kinase cascade involved in actin polarization, cell wall integrity, and cell cycle control (not shown). In addition, Gpa1-GTP binds to Scp160 and increases the amount of associated mRNAs (i.e. SRO7, SEC3, FUS3, STE7, etc.). Right panel – Scp160 and cER are targeted to the shmoo tip, perhaps via Myo4, along actin filaments oriented along the polarization axis via Ste18/Ste4 signaling. mRNA and cER delivery allows for the localized translation and enrichment of polarity/secretion and mating pathway components at the site of polarization (to facilitate shmoo growth).
EXPERIMENTAL PROCEDURES
Yeast growth and manipulation
Standard methods for the growth of yeast were used (Rose et al., 1990). See Extended Experimental Procedures for details on plasmid construction. For α-factor-treatment, cells were grown to early log phase on either rich (YPD) or synthetic selective medium, harvested, and treated with α-factor (5μM; Sigma, St. Louis, MO, USA) for 2 hrs at 26°C, with the exception of the microfluidic assays. The quantitative mating assay, based on the complementation of auxotrophic markers present in MATa and MATα cells after crossing, was performed as described previously (Grote, 2008), and is described in the Extended Experimental Procedures. Time-lapse microscopy methods are also detailed in the Extended Experimental Procedures. Yeast strains are listed in Table S2.
Microfluidics and gradient sensing
Gradient sensing experiments were performed on yeast [e.g. wild-type (BY4741) or JFy4493 (W303 background), scp160Δ, and SCP160KH14Δ-expressing cells] as described (Segall, 1993; Paliwal et al., 2007). Briefly, microfluidic chips were filled with YPD containing casein (100 ug/ml) for 1hr to coat internal surfaces in order to reduce non-specific pheromone adsorption. All subsequent media contained casein (20μg/ml). Cells were introduced from the cell inlet and lined up against one edge of the test chamber by inducing a cross-flow of media from the inlet to the cell outlet. The pressure in the chip was increased briefly to allow cells to transit the chamber and then decreased to trap the cells therein. Next, media containing pheromone and a red fluorescent marker dye (see below) was flowed along the side channels and into the media outlet at the bottom. Fluid pressure on both sides of the test chambers was equal, hence, no cross-flow was present. Gradient creation occurs as a result of pheromone diffusion across the chamber and was visualized with Alexa Fluor 555 hydrazide (Invitrogen). The “high” pheromone concentration used in the experiments was 750nM and the “low” pheromone concentration was set at 100nM. Statistics regarding the mean angle to the gradient normal were compiled at 2, 4, and 6hrs after exposure to pheromone at a concentration range of 350–500nM. Polar plots for the data compiled at 2hrs are shown in Figure S5 for wild-type, scp160Δ, and SCP160KH14Δ cells. To counter secreted Bar1 activity, the pH of media was 3.5. In addition, a cross-flow from the low pheromone side-channel to the high pheromone side-channel was imposed for 1min after every 30min.
Immunoprecipitation of protein and RNA
Standard procedures were used for the precipitation and detection of proteins from lysed yeast, and are detailed in the Extended Experimental Procedures. RNA extraction from the eluates of IPs and subsequent RT-PCR and real-time PCR procedures are detailed in the Extended Experimental Procedures.
Supplementary Material
Highlights.
mRNAs encoding polarity and mating pathway components localize to the yeast mating projection (shmoo) tip
RNA delivery depends upon Myosin 4 and the transport of cortical endoplasmic reticulum
Scp160 is a pheromone-activated RNA-binding protein that binds and delivers mRNAs
Loss of RNA-binding to Scp160 blocks chemotropism and inhibits mating
Acknowledgments
We thank H. Dohlman, J. Fridovich-Keil, M. Schuldiner, and P. Takizawa for strains and other reagents; and L. Haim-Vilmovsky, R. Herbst, and G. Zipor for plasmid construction. This work was supported by grants to J.E.G. from the Minerva Foundation, Germany and Y. Leon Benoziyo Foundation for Molecular Medicine, Weizmann Institute of Science; and by NIH grants GM084332 and RR020839 to A.L. J.E.G. holds the Besen-Brender Chair in Microbiology and Parasitology, Weizmann Institute of Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Aronov S, Gelin-Licht R, Zipor G, Haim L, Safran E, Gerst JE. mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol Cell Biol. 2007;27:3441–3455. doi: 10.1128/MCB.01643-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Taneja KL, Kislauskis EH, Sundell CL, Powers CM, Ross A, Singer RH. Actin filaments and the spatial positioning of mRNAs. Adv Exp Med Biol. 1994;358:183–189. doi: 10.1007/978-1-4615-2578-3_17. [DOI] [PubMed] [Google Scholar]
- Baum S, Bittins M, Frey S, Seedorf M. Asc1p, a WD40-domain containing adaptor protein, is required for the interaction of the RNA-binding protein Scp160p with polysomes. Biochem J. 2004;380:823–830. doi: 10.1042/BJ20031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Blumer KJ, Thorner J. Receptor-G protein signaling in yeast. Annu Rev Physiol. 1991;53:37–57. doi: 10.1146/annurev.ph.53.030191.000345. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J Cell Biol. 2003;160:811–816. doi: 10.1083/jcb.200301035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brykailo MA, Corbett AH, Fridovich-Keil JL. Functional overlap between conserved and diverged KH domains in Saccharomyces cerevisiae SCP160. Nucleic Acids Res. 2007;35:1108–1118. doi: 10.1093/nar/gkl1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor A, Snyder M. Bud-site selection and cell polarity in budding yeast. Curr Opin Microbiol. 2002;5:179–186. doi: 10.1016/s1369-5274(02)00300-4. [DOI] [PubMed] [Google Scholar]
- Dodson RE, Shapiro DJ. An estrogen-inducible protein binds specifically to a sequence in the 3′ untranslated region of estrogen-stabilized vitellogenin mRNA. Mol Cell Biol. 1994;14:3130–3138. doi: 10.1128/mcb.14.5.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman HG. G proteins and pheromone signaling. Annu Rev Physiol. 2002;64:129–152. doi: 10.1146/annurev.physiol.64.081701.133448. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Pool M, Seedorf M. Scp160p, an RNA-binding, polysome-associated protein, localizes to the endoplasmic reticulum of Saccharomyces cerevisiae in a microtubule-dependent manner. J Biol Chem. 2001;276:15905–15912. doi: 10.1074/jbc.M009430200. [DOI] [PubMed] [Google Scholar]
- Gerst JE. Message on the web: mRNA and ER co-trafficking. Trends Cell Biol. 2008;18:68–76. doi: 10.1016/j.tcb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Guo M, Aston C, Burchett SA, Dyke C, Fields S, Rajarao SJ, Uetz P, Wang Y, Young K, Dohlman HG. The yeast G protein alpha subunit Gpa1 transmits a signal through an RNA binding effector protein Scp160. Mol Cell. 2003;12:517–524. doi: 10.1016/s1097-2765(03)00307-1. [DOI] [PubMed] [Google Scholar]
- Haim L, Zipor G, Aronov S, Gerst JE. A genomic integration method to visualize localization of endogenous mRNAs in living yeast. Nat Methods. 2007;4:409–412. doi: 10.1038/nmeth1040. [DOI] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Irie K, Tadauchi T, Takizawa PA, Vale RD, Matsumoto K, Herskowitz I. The Khd1 protein, which has three KH RNA-binding motifs, is required for proper localization of ASH1 mRNA in yeast. Embo J. 2002;21:1158–1167. doi: 10.1093/emboj/21.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Lang BD, Fridovich-Keil JL. Scp160p, a multiple KH-domain protein, is a component of mRNP complexes in yeast. Nucleic Acids Res. 2000;28:1576–1584. doi: 10.1093/nar/28.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Katayama Y, Schmid M, Burkacky O, Brauchle C, Lamb DC, Jansen RP. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic. 2008;9:1256–1267. doi: 10.1111/j.1600-0854.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- Li AM, Watson A, Fridovich-Keil JL. Scp160p associates with specific mRNAs in yeast. Nucleic Acids Res. 2003;31:1830–1837. doi: 10.1093/nar/gkg284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277:383–387. doi: 10.1126/science.277.5324.383. [DOI] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy R, Moradi EK, Niu W, Hart GT, Davis M, McGary KL, Ellington AD, Marcotte EM. Systematic definition of protein constituents along the major polarization axis reveals an adaptive reuse of the polarization machinery in pheromone-treated budding yeast. J Proteome Res. 2009;8:6–19. doi: 10.1021/pr800524g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S, Iglesias PA, Campbell K, Hilioti Z, Groisman A, Levchenko A. MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature. 2007;446:46–51. doi: 10.1038/nature05561. [DOI] [PubMed] [Google Scholar]
- Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. I Establishment and maintenance of polarity states. J Cell Sci. 2000;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Reinke CA, Kozik P, Glick BS. Golgi inheritance in small buds of Saccharomyces cerevisiae is linked to endoplasmic reticulum inheritance. Proc Natl Acad Sci USA. 2004;101:18018–18023. doi: 10.1073/pnas.0408256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics. New York: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Rosoff WJ, Urbach JS, Esrick MA, McAllister RG, Richards LJ, Goodhill GJ. A new chemotaxis assay shows the extreme sensitivity of axons to molecular gradients. Nat Neurosci. 2004;7:678–682. doi: 10.1038/nn1259. [DOI] [PubMed] [Google Scholar]
- Segall JE. Polarization of yeast cells in spatial gradients of alpha mating factor. Proc Natl Acad Sci USA. 1993;90:8332–8336. doi: 10.1073/pnas.90.18.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard KA, Gerber AP, Jambhekar A, Takizawa PA, Brown PO, Herschlag D, DeRisi JL, Vale RD. Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc Natl Acad Sci U S A. 2003;100:11429–11434. doi: 10.1073/pnas.2033246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shimoi H, Ito K. Identification of genes required for growth under ethanol stress using transposon mutagenesis in Saccharomyces cerevisiae. Mol Genet Genomics. 2001;265:1112–1119. doi: 10.1007/s004380100510. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, Sil A, Swedlow JR, Herskowitz I, Vale RD. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 1997;389:90–93. doi: 10.1038/38015. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, Vale RD. The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc Natl Acad Sci USA. 2000;97:5273–5278. doi: 10.1073/pnas.080585897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber V, Wernitznig A, Hager G, Harata M, Frank P, Wintersberger U. Purification and nucleic-acid-binding properties of a Saccharomyces cerevisiae protein involved in the control of ploidy. Eur J Biochem. 1997;249:309–317. doi: 10.1111/j.1432-1033.1997.00309.x. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A. Establishing cell polarity in development. Nat Cell Biol. 2002;4:E39–44. doi: 10.1038/ncb0202-e39. [DOI] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- Yoon BC, Zivraj KH, Holt CE. Local Translation and mRNA Trafficking in Axon Pathfinding. Results Probl Cell Differ. 2009;48:269–288. doi: 10.1007/400_2009_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NN, Dudgeon DD, Paliwal S, Levchenko A, Grote E, Cunningham KW. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol Biol Cell. 2006;17:3409–3422. doi: 10.1091/mbc.E06-03-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.