Abstract
We demonstrate that membranes consisting of certain triblock-copolymers were tight for CO2. Using a novel approach, we provide evidence for aquaporin facilitated CO2 diffusion. Plant aquaporins obtained from heterologous expression were inserted into triblock copolymer membranes. These were employed to separate a chamber with a solution maintaining high CO2 concentrations from one with depleted CO2 concentrations. CO2 diffusion was detected by measuring the pH change resulting from membrane CO2 diffusion from one chamber to the other. An up to 21 fold increase in diffusion rate was determined. Besides the supply of this proof of principle, we could provide additional arguments in favour of protein facilitated CO2 diffusion to the vivid on-going debate about the principles of membrane gas diffusion in living cells.
Aquaporins are membrane-spanning pore-forming proteins, facilitating the transport of water and certain uncharged solutes across biological membranes in almost all living organisms. Aquaporin proteins exhibit a characteristic conserved structure. Hydropathy plot analyses of the primary sequence predicted a topology of six transmembrane helices (I–VI) connected by five loops (loops A–E). The highly conserved loops B and E dipping into the membrane include a conserved signature motif, asparagine-proline-alanine (NPA motif), which is directly involved in the water transport mechanism1. Inserted into a membrane, aquaporins arrange in tetramers, in which four monomers giving four individual water conducting pores form a putative fifth pore in the centre of the protein complex. In plants, aquaporins were subdivided into distinct groups related to their localisation in the cell. The largest group is called PIP for plasma membrane intrinsic protein. The PIP group was further divided into PIP2, which are highly water selective, and PIP1, which are almost impermeable to water.
Aquaporin function was determined mainly in living cells, and it is generally accepted that aquaporins facilitate membrane water diffusion. A possible function concerning facilitating CO2 membrane diffusion has caused an on-going scientific debate. This debate accounts for publications concerning significant differences of CO2 diffusion rates in animals and plants with or without specific aquaporins. Conversely, theoretical considerations, and experimental data obtained from lipid bilayer CO2 diffusion as well as CO2 diffusion rate comparisons in animals and plants in the presence or absence of aquaporins in some cases showed no, or just slight, differences. Most profoundly, a protein facilitated CO2 diffusion seems to be a violation of the so-called Meyer-Overton rule, which implies that lipid bilayer membranes such as biomembranes do not impose resistance to diffusion of small hydrophobic molecules like CO2. A consequence of this solubility-diffusion model is that any protein, even if it is highly permeable for CO2, would reduce the rate of CO2 diffusion. In fact, Gutknecht and co-workers, for example, could show that an artificial bilayer consisting of egg lecithin and cholesterol does not constitute a substantial barrier to diffusion of CO2 (permeability coefficient PCO2 ≈ 0,35 cm/s)2. Combining CO2 diffusion studies and an analytical model Missner et al. predicted a PCO2 in lipid bilayers of 3,2 cm/s3. However, studies with biomembranes report 10 to 1000 times reduced CO2 diffusion rates4,5,6,7. Under these conditions, the Meyer-Overton rule is not applicable and protein facilitated CO2 diffusion would make sense if high CO2 transport rates are required.
One of the major pitfalls of the debate from both sides is that these could only rely on data from CO2 permeable membranes. In this case the membranes, such as artificial bilayers, were highly permeable to CO2, the figures were close to the theoretical considerations confirming Meyer-Overton. In the case of distinct cell membranes, the figures were orders of magnitude below the theoretical value possibly due to intrusion of biological components or coverage with proteins and other substances. These may cause a decrease in overall CO2 diffusion rates and aquaporin facilitated CO2 diffusion becomes necessary. To obtain data about aquaporin CO2 conductivity, besides circumventing the background and disadvantage of a CO2 permeable membrane, we decided to analyse a possible aquaporin dependent CO2 flux in a CO2 tight membrane. These conditions were provided by an artificial membrane consisting of block-copolymers. Thus, we are using a highly sensitive approach to analyse if specific biological components, the aquaporins, have the capacity to increase CO2 diffusion rates.
Results
Measurement of CO2 transport
In the range of maximal theoretical values for CO2 diffusion, the so-called unstirred water layers (USL) on both sides of the membrane will restrict CO2 diffusion rates. Therefore, it is required to analyse membrane transport of CO2 with a tool capable of considering USL effects. The scanning pH electrode as described by Missner et al.3,8,9 is an appropriate device to analyse such high diffusion rates. The approach was adapted to determine CO2 diffusion over artificial planar membranes. Block-copolymer membranes were introduced into a modified two-chamber system (Fig. 1). In this two-chamber system, a gradient driven flux of CO2 across a membrane from one compartment with a high CO2 concentration to another with a lower concentration was measured. A defined CO2 concentration was maintained by the addition of carbonic anhydrase. The enzyme was added to both chambers in a concentration ensuring that the conversion reaction to/from bicarbonate was not rate limiting. Increase or decrease of bicarbonate as a result of CO2 concentration changes caused changes in pH and this was recorded by the scanning pH electrode. Both compartments have a volume of approximately 2 ml. Under the conditions applied, the CO2 gradient across the membrane remained stable for more than an hour, admitting measurements comparatively independent of time.
Figure 1. Modification of the two chamber system.
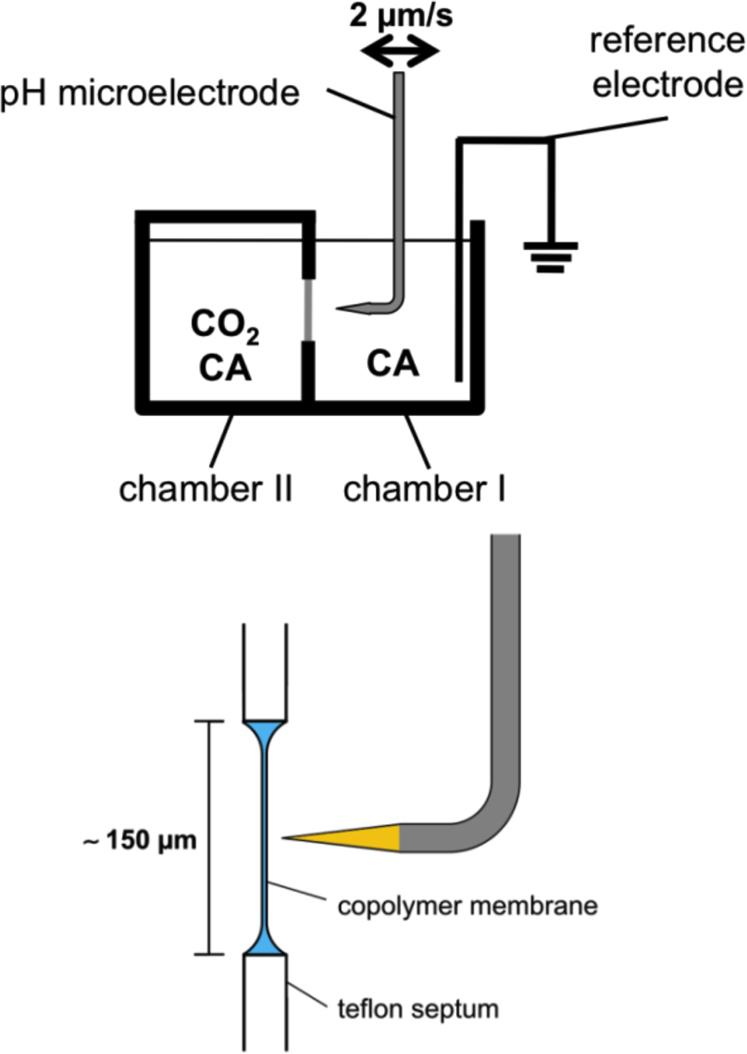
A PTFE septum with small hole (∼150 µm diameter) has been clamped between two half chambers. A copolymer membrane has been spread across the hole. Chamber II contains a CO2 and carbonic anhydrase containing buffer solution. Both compartments contained carbonic anhydrase. To minimize loss of CO2 from the reservoir chamber II has been closed with a glass slide. Diffusion of CO2 from chamber II across the membrane into chamber I is measured as a function of pH.
Triblock-copolymer membranes as a biomimetic model system to study membrane transport
Triblock-copolymer membranes exhibiting defined permeability properties became an attractive biomimetic system with properties relevant for nanotechnological applications. Immobilisation of functional biological molecules such as membrane proteins is conceivable10. Depending on the nature of the employed copolymer, these artificial membranes can exhibit characteristics comparable to that of lipid bilayer membranes. According to the copolymer's attributes the resulting membrane has distinct intrinsic permeability properties11. As mentioned above, lipid bilayers or cell membranes, exhibit a substantially high background CO2 permeability representing an error source. For discovery of a suitable copolymer as source for the construction of a membrane with a low CO2 conductivity, a set of different triblock-copolymers based on poly-2-methyloxazoline (PMOXA) and poly-dimethylsiloxane (PDMS) with different A/B ratios were tested. Triblock-copolymers used in preliminary experiments for this study are listed in Table 1. The substances were dissolved in n-decane and were spread across the aperture of the two chamber PTFE septum. The scanning pH microelectrode was advanced towards the resulting copolymer membrane patch and the pH decrease was documented. pH decrease was related to the rate of CO2 flux (JM,CO2) from the chamber with a higher apparent CO2 concentration to that with the lower one. The CO2 concentration in the vicinity of the membrane increased and consequently, a gradual decrease in pH was detected for ABA3 copolymer membranes. JM,CO2 was determined as described by Missner et al. (2008). For ABA1 and ABA2 membranes no change of pH has been observed (Fig. 2a) indicating CO2 impermeability (Fig. 2b). ABA3 membranes showed a substantial CO2 permeability. Accordingly, ABA1 was chosen for further experiments.
Table 1. Configuration of ABA block copolymers used for the present study.
| A (PMOXA) | B (PDMS) | A (PMOXA) | |
|---|---|---|---|
| ABA1 | 20 | 41 | 20 |
| ABA2 | 12 | 55 | 12 |
| ABA3 | 15 | 110 | 15 |
Figure 2. CO2 flux across block-copolymer membranes.
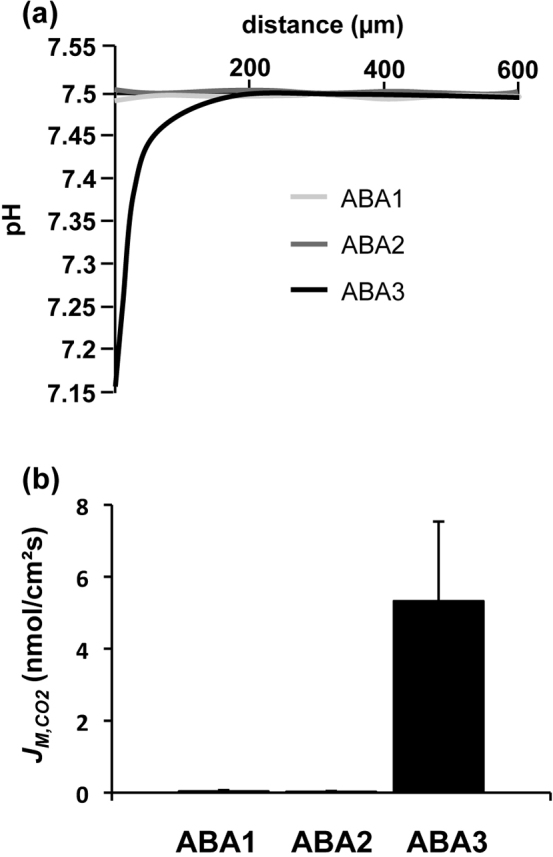
(a) Experimental pH profiles in response to CO2 diffusion across three types of poly-methyloxazoline-poly-dimethylsiloxane-poly-methyloxazoline based triblock copolymer membranes. (b) Average membrane flux of CO2 (± S.E.; n = 10 each) calculated from the slope of the pH traces within 50 µm from the membrane.
Reconstitution of aquaporin proteins into planar polymeric membranes
Although block-copolymer membranes are two- to three-fold thicker than conventional lipid bilayers, they can be used as a matrix for membrane-spanning proteins. The proteins often remain functional, despite the thickness of the membranes in comparison to lipid bilayers and the polymerisation reaction of the reactive block-copolymers12. As an example, functional protein integration was successfully performed with the E. coli aquaporin AQPZ. In this case, water transport rates were found to be increased after integration of the aquaporin11. For the analysis of protein facilitated CO2 transport, the tobacco PIP1 aquaporin NtAQP1 was employed. From data obtained in the heterologous expression/analysis system yeast and from physiological observations in plants concerning photosynthesis, NtAQP1 was considered to increase CO2 transport rates. For comparison, NtPIP2;1, a PIP2 aquaporin from tobacco, which was found to be permeable for water and to a much lesser degree for CO2 was also subjected to the analysis. Nontheless, all these conclusions came from data relying on membranes with a high internal CO2 permeability. Prior to integration, the protein has to be synthesized to a considerable concentration. For this purpose, it was expressed as a 6xhis tagged fusion protein in S. cerevisiae. Via His-tag affinity to Ni2+, NtAQP1 and NtPIP2;1 were purified according to Otto et al.5. The purified protein was inserted into copolymer membranes (80 µg protein per 6 mg triblock-copolymer) as described in the methods section. Both pH and reference electrodes were inserted into chamber I in order to detect a decrease of pH due to membrane CO2 flux. The pH electrode was moved perpendicularly towards the copolymer membrane and the pH signal was continuously recorded. The CO2 concentration in the vicinity of the membrane increased and consequently, a gradual decrease in pH was detected for copolymer membranes containing NtAQP1 and NtPIP2;1, but not for membranes treated with control protein fractions containing neither of the proteins (Fig. 3a). When the PIP1 aquaporin NtAQP1 was inserted into the copolymer membrane, average membrane flux of CO2 was increased 21-fold compared to control membranes (4.18 ± 0.66 nmol CO2/m2s compared to 0.2 ± 0.08 nmol CO2/m2s for control measurements). Insertion of the NtPIP2;1 increased the CO2 permeability of the membrane 12-fold (2.39 ± 0.17 nmol CO2/m2s; Fig. 3b).
Figure 3. Aquaporin facilitated CO2 diffusion across ABA1 block copolymer membranes.
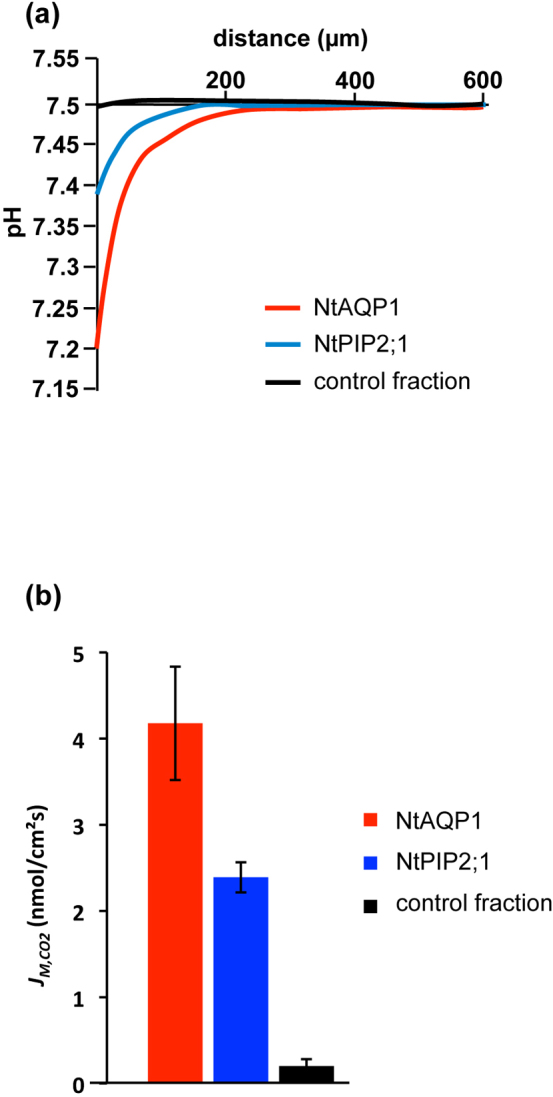
(a) Experimental pH profiles in response to CO2 diffusion across ABA1 membranes containing NtAQP1 or NtPIP2;1 as well as control membranes. Insertion of NtAQP1 protein reduces the membranes resistance to CO2 diffusion dramatically, NtPIP2;1 to a minor extent. (b) Average membrane flux of CO2 (± S.E.) calculated from the slope of the pH traces within 50 µm from the membrane (n = 6 for NtAQP1; n = 14 for NtPIP2;1; n = 10 for control fraction).
Discussion
The present work describes an apparent discrepancy of membrane diffusion studies in an artificial system with measurements in biological systems. The latter revealed in general no, or just a small, increase in CO2 diffusion by PIP2 aquaporins, compared to NtAQP1 or human aquaporin 14,5,13,14,15,16. It might, however, indicate the different sensitivity of the deployed systems. NtAQP1 facilitated CO2 transport has been studied in yeast cells or Xenopus oocytes as well as plant cells and the overall CO2 transport was much lower in these studies4,5,13 than presented here. These are biological membranes and, as mentioned above, have a certain level of background CO2 permeability. In contrast, the planar block-copolymer system used for the present study has no detectable background CO2 permeability and therefore is able to unravel the full CO2 transport capacity of NtAQP1. The effect of NtPIP2;1 on membrane CO2 permeability as measured in block-copolymer membranes could be covered by the intrinsic CO2 membrane permeability of biological membranes. Additionally, the present CO2 transport studies were done at room temperature whereas studies employing yeast cells are generally performed at 10°C. This may additionally mask the lower CO2 transport activity of NtPIP2;1 in yeast cells due to thermal effects on the protein flexibility.
The results from this investigation provide clear experimental evidence that aquaporins have the capability to increase CO2 transport, although to different extents. Unstirred water layers close to the membrane may affect the overall CO2 transport to some extent, but were not limiting in our experiments as it was the case in the study by Missner et al.3. Thus, not only the theoretical considerations about the gas permeability of aquaporins could be confirmed, but also a missing link in the chain of evidence in favour of the CO2 facilitating function is provided. Now, the observations supporting protein facilitated CO2 diffusion start from theory, extend to molecular evidence, and reach into physiological changes. Considering that biological membranes in some cases show very low permeability for CO217 and that relatively high CO2 transport rates must be assured for a cell to survive, our studies indicate for the requirement of these proteins for the exchange of CO2. Concerning biophysical characteristics, other gasses are similar to CO2 and it is possible that also for these, a protein facilitated membrane transport has to be considered.
Uncovering the molecular basics of membrane gas transport and understanding how gasses move into and within cells, tissues and whole living organisms bears potential scientific and technical implications for environmental engineering and sensor technology. The novel findings and biomimetic membrane systems described above, in future may be used to improve separation processes important for technical and medical applications like sensing and purification of technical gasses. The presented system allows studies on gas conductivity properties of candidate membrane proteins in a system with minimal background permeability.
Methods
Microelectrode measurements
The pH measurements were performed as described by Missner et al.3. A scanning pH-sensitive microelectrode was moved by a motorized hydraulic micromanipulator (MHW-103, Narishige, Tokyo, Japan) within the stagnant water layer with a velocity of 2 µm s−1 towards or away from the cells8. The travel speed of the micromanipulator was regularly checked using a digital sliding caliper. The electrodes had a sensitivity of 56±0.3 mV/pH, which was determined by making a three-point calibration in buffer solutions with a defined pH before and after each experiment. The addition of carbonic anhydrase (CA) did not affect the sensitivity of the electrode. Voltage recordings were performed each second using an electrometer (Duo 773, World Precision Instruments, Berlin, Germany). The filtered signal (LHBF-48x, npi Electronic GmbH, Tamm, Germany) was recorded on a personal computer via an A/D converter box (USB-6008, National Instruments, Austin, Texas, USA). The electrodes were manufactured from borosilicate glass (GB 150F-10, Science Products GmbH, Hofheim, Germany) pulled to a tip size of 2–4 µm, silanized (Dichloro-dimethylsilane, VWR International GmbH, Darmstadt, Germany), and then filled with a proton-sensitive mixture (Hydrogen Ionophore II - Cocktail A, Selectophore, Fluka). A leaf without a lower epidermis was connected to the chambers (Fig. 1). The chambers were filled with a CO2 and carbonic anhydrase (Sigma; 1 mg ml-1)-containing buffer solution (0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 30 mM NaHCO3, 30 mM HEPES pH 7.5). The liquid in both chambers was continuously agitated by magnetic stirring bars. JM,CO2 was calculated as described by Missner et al.3.
Production of NtAQP1 and NtPIP2;1 protein
Recombinant NtAQP1 and NtPIP2;1 protein was heterologously expressed in yeast a 6xhis tagged fusion protein and was purified essentially as described by Otto et al.5. In brief, isolation of plasma membrane fractions from yeast cells was performed according to Panaretou and Piper18. Total membranes from glass bead lysed yeast cells were collected by centrifugation at 22,000 x g for 30 minutes and subsequently fractionated on a sucrose-step gradient (1.1, 1.65, and 2.25 M sucrose in 2 mM EDTA, 25 mM imidazole/HCl, pH 7; 9 ml each) by centrifugation for 15 h at 80,000 × g. Plasma membranes were obtained from the 2.25 M/1.65 M interface. The combined plasma membrane fractions were washed with 20 mm Tris/HCl, pH 7.5, 150 mm NaCl, 10% glycerol. Aquaporins were solubilized with 2% dodecylmaltoside (DDM). Via His-tag affinity to Ni2+ NtAQP1 and NtPIP2;1 were purified using an Äkta prime chromatography system (GE Healthcare). Identity of the aquaporin containing fractions and purity of the proteins was confirmed by Western blot analysis with an NtAQP1 or NtPIP2;1 specific antibody raised in chicken or rabbit, respectively. Chromatography fractions not containing aquaporin protein were used as control fractions for CO2 transport studies (see below).
Synthesis of ABA Polymer
Symmetric poly-(2-methyloxazoline)-block-poly-(dimethylsiloxane)-block-poly-(2-methyloxazoline) (PMOXAn-PDMSm-PMOXAn) polymers of different block lengths were synthesized by the approach described by Nardin et al.19, except for ABA1, for which the PDMS (Mw = 3000 g/mol, PDI = 1.12) was obtained from ABCR GmbH, Germany. Polymers were verified by 1H NMR. Molecular weights and polydispersity indices (PDI) were determined by gel permeation chromatography (GPC) and were 6550 g/mol for ABA1 (PDI = 1.61), 6325 g/mol for ABA2 (PDI = 1.64) and 10700 g/mol for ABA3 (PDI = 1.62).
Production of aquaporin protein containing triblock-copolymer membranes
6 mg ABA1 copolymer were dissolved in 100 µl n-decane and mixed with aquaporin fraction (80 µg aquaporin protein) at 4°C for 2 h. After phase separation by centrifugation the decane phase was collected and membranes were spread across the Teflon septum using the painting technique. Presence of a membrane was verified by microscopic inspection and by resistance measurements. For control experiments ABA1/decane was treated with fractions from the His-tag affinity chromatography not containing aquaporin protein.
Author Contributions
N.U. and R.K. designed the study. B.O., A.E. and N.U. performed the experiments. W.M. and F.I. provided materials and know-how. R.K. and N.U. drafted and edited the final manuscript.
Acknowledgments
We thank J. Zilles, Department of Civil and Environmental Engineering, University of Illinois at Urbana-Champaign, for providing copolymers.
References
- Sui H., Han B. G., Lee J. K., Walian P. & Jap B. K. Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878 (2001). [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A. & Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes. Effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol 69, 779–794 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner A. et al. Carbon dioxide transport through membranes. J Biol Chem 283, 25340–25347 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N. et al. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20, 648–657 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B. et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J Biol Chem 285, 31253–31260 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V., Cartron J. P., Ripoche P. & Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 22, 64–73 (2008). [DOI] [PubMed] [Google Scholar]
- Endeward V. & Gros G. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. J Physiol 567, 253–265 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko Y. N., Denisov G. A. & Pohl P. Weak acid transport across bilayer lipid membrane in the presence of buffers. Theoretical and experimental pH profiles in the unstirred layers. Biophys J 64, 1701–1710 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner A., Kugler P., Antonenko Y. N. & Pohl P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc Natl Acad Sci USA 105, E123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belegrinou S. et al. Biomimetic supported membranes from amphiphilic block copolymers. Soft Matter 6, 179–186 (2010). [Google Scholar]
- Kumar M., Grzelakowski M., Zilles J., Clark M. & Meier W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. .Proc Natl Acad Sci USA 104, 20719–20724 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin C. & Meier W. Hybrid materials from amphiphilic block copolymers and membrane proteins. Rev Mol Biotechnol 90, 17–26 (2002). [DOI] [PubMed] [Google Scholar]
- Uehlein N., Lovisolo C., Siefritz F. & Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425, 734–737 (2003). [DOI] [PubMed] [Google Scholar]
- Uehlein N., Sperling H., Heckwolf M. & Kaldenhoff R. The Arabidopsis aquaporin PIP1;2 rules cellular CO(2) uptake. Plant Cell Environ 35, 1077–1083 (2012). [DOI] [PubMed] [Google Scholar]
- Endeward V., Cartron J. P., Ripoche P. & Gros G. Red cell membrane CO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus Clin Biol 13, 123–127 (2006). [DOI] [PubMed] [Google Scholar]
- Musa-Aziz R., Chen L. M., Pelletier M. F. & Boron W. F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., Kaldenhoff R., Genty B. & Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60, 2235–2248 (2009). [DOI] [PubMed] [Google Scholar]
- Panaretou B. & Piper P. Isolation of yeast plasma membranes. Meth Mol Biol 313, 27–32 (2006). [DOI] [PubMed] [Google Scholar]
- Nardin C., Thoeni S., Widmer J., Winterhalter M. & Meier W. Nanoreactors based on (polymerized) ABA-triblock copolymer vesicles. Chem Commun 1433–1434 (2000). [Google Scholar]


