Abstract
Background
Spontaneous regression of hepatocellular carcinoma (HCC) is well documented, although the aetiology of this phenomenon remains unknown.
Methods
A review of the English literature was performed for reports of spontaneous regression of HCC. Reports were classified by mechanism based on the available information.
Results
Spontaenous regression of HCC has been identified in 75 patients. The most common mechanisms of regression identified were tumour hypoxia (n= 21, 28.0%), a systemic inflammatory response (n= 25, 33.3%) and unknown (n= 29, 38.7%). In patients where tumour hypoxia was described as the aetiology, mechanisms included spontaneous hepatic artery thrombosis and sustained systemic hypotension. In patients where a systemic inflammatory response was the aetiology, mechanisms included cholangitis, trauma and elevated cytokine levels.
Discussion
Spontaneous regression of HCC is most commonly associated with tumour hypoxia or a systemic inflammatory response. Determining the aetiology of spontaneous regression may identify potential therapeutic pathways. Tumour hypoxia is already the basis of treatment modalities such as hepatic artery embolization and the anti-angiogenic agent sorafenib. However, treatment modalities for HCC do not currently include immune-directed therapies; this may prove to be a worthy target for future research.
Keywords: hepatocellular carcinoma, hepatoma, regression, tumour hypoxia, systemic inflammatory response
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and results in over half a million deaths annually.1 Unfortunately, most patients present with advanced disease, at which point therapeutic options are limited. Even with current treatments, the mortality of this disease is high with a median survival shown in some studies to be as little as 20 months after the onset of symptoms.2
Spontaneous regression of cancer was originally defined by Everson and Cole3 in 1959 as being ‘the partial or complete disappearance of a malignant tumour in the absence of all treatment, or in the presence of therapy which is considered to be inadequate to exert a significant influence on neoplastic disease’. Spontaneous regression is a well-established phenomenon in certain malignancies including renal cell carcinoma, neuroblastoma and choriocarcinoma.4 However, regression of HCC appears to be a rare event, but has also been described.
In 1972, Johnson and colleagues5 first described spontaneous regression of HCC in a 3-year-old girl who had developed biopsy-proven HCC while on chronic androgen-anabolic steroid treatment for aplastic anaemia. One year after her diagnosis, she was admitted to the hospital for treatment of Staphylococcus aureus septicaemia at which time her imaging demonstrated near complete resolution of her HCC. Other than her bacteraemia, the only reported event in the patient's condition was the cessation of her steroid treatment.5 Since this initial report, within the English literature, over 80 additional patients have been reported to have undergone spontaneous regression of their HCC.
The true incidence of spontaneous regression of HCC is difficult to determine accurately, however, Oquinena and colleagues6 estimated this value by collating data from 10 randomized controlled trials involving 1640 patients with HCC. Based on the incidence of regression in the control groups, it was calculated to be 0.4%.6
Several mechanisms have been suggested to explain the aetiology of spontaneous regression of HCC, including the administration of herbal remedies,7–10 or the withdrawal of a possible causative agent such as alcohol,11,12 tobacco,9 or exogenous androgens.5,13 Two additional theories cited in the literature as possible mechanisms of regression are (i) tumour hypoxia and (ii) systemic inflammatory activation. These pathways have been explored for their therapeutic effect in other malignancies as well. One example of this is the use of interleukin-2 (IL-2) for the treatment of melanoma, an agent which is known to be a potent inducer of pro-inflammatory cytokines.14,15
The present study reviews the published literature on spontaneous HCC regression and attempts to classify these reports by mechanism. Determining of the aetiology of spontaneous regression may help identify future therapeutic pathways for HCC treatment.
Methods
A PubMed search of the English literature was performed using the key words ‘regression’ and ‘hepatocellular carcinoma’. All resulting articles were reviewed and assessed for inclusion. A manual search was then performed on all references cited in these publications. Spontaneous regression was defined as the reduction in size or disappearance of a HCC, as determined radiologically or pathologically in the absence of any possible direct effects from standard treatments. Any reports that did not meet this definition were excluded from further classification and analysis. A patient from the local institution who underwent spontaneous regression of their HCC and has not been previously reported was added to the analysis (Fig. 1).
Figure 1.
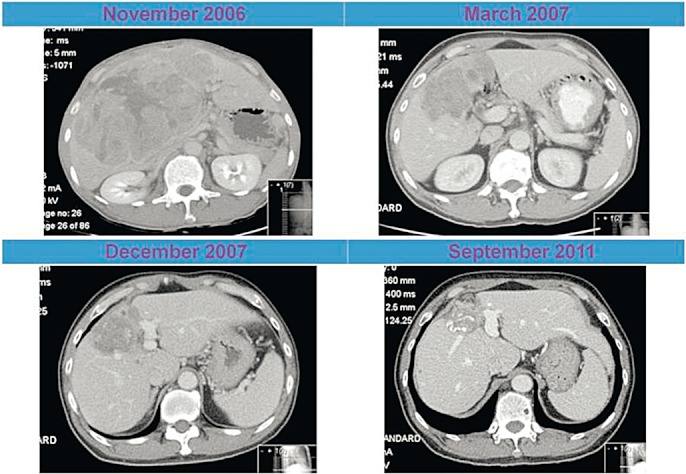
Computed tomography (CT) images of a 59-year-old male with spontaneous regression of hepatocellular carcinoma (HCC)
Once patients were assessed, they were categorized by mechanism of regression into one of three categories: tumour hypoxia, systemic inflammatory response and unknown or other. In some instances the mechanism was proposed by the authors whereas in others the report described a situation in which the aetiology could clearly be categorized as either tumour hypoxia or activation of the inflammatory system.
Using the data available in the published reports, demographic and clinical features were recorded, including age, gender, presence of cirrhosis, type of underlying liver disease (hepatitis B, hepatitis C, alcoholic cirrhosis or other) and degree of tumour response. When provided, survival time in months since the original diagnosis was also recorded.
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The two cohorts of tumour hypoxia and systemic inflammatory response were compared. For nominal data where the denominator was incomplete the reduced figure is provided. Univariate analysis was conducted in the standard fashion using a Mann–Whitney U-test to compare continuous non-parametric variables, and a chi-square test to compare categorical variables.
Results
The PubMed search of the English literature identified 84 patients with spontaneous regression of HCC. Of these, 75 matched the definition of ‘spontaneous’ as defined above. Nine patients were excluded because regression occurred after the use of a standard of care treatment directed at the primary tumour, including transhepatic arterial embolization (n= 4), systemic chemotherapy (n= 2), radiofrequency ablation (n= 1) and the administration of multiple accepted treatment modalities (n= 2).
The mean age of patients with regression was 64 years [range 5–89, standard deviation (SD) ± 14]. Of the 75 patients, 73% (50/70) were male. Seventy-five percent (43/57) had cirrhosis. Information on underlying liver disease was present in 70 of the patients, demonstrating 12 patients (17%) with hepatitis B, 32 patients (46%) with hepatitis C, 9 patients (14%) with alcoholic cirrhosis and 17 patients (24%) with no known underlying hepatic pathology. Information on the degree of tumour regression was available in 67 cases, of which 32 patients (48%) had a partial regression, and the remaining 35 patients (52%) had a complete regression. Median survival time from diagnosis of HCC was 29 months (range 4–240).
Of the 75 patients with true spontaneous regression included in this review, 21 (28.0%) could be attributed to tumour hypoxia and 25 (33.3%) to a systemic inflammatory response. Spontaneous regression in the remaining 29 (38.7%) patients was as a result of an unknown mechanism. Other than these categories, no other mechanism was consistently suggested by more than two case reports.
The ‘tumour hypoxia’ category16–36 included patients with an occlusive portal vein thrombosis, spontaneous hepatic artery thrombosis, angiospasm during angiography, development of a large arterioportal shunt and a sustained period of hypotension because of a massive gastrointestinal bleed (Table 1).
Table 1.
Reports of spontaneous HCC regression with description of inciting event
| Systemic inflammatory response | |
| Heianna et al.45 | Regression of lung metastases after TACE of a primary lesion |
| Ohta et al.42 | Regression after cholangitis, complete necrosis on biopsy |
| Randolph et al.43 | Regression after febrile illness with rigors |
| Blondon et al.38 | Regression after capsular rupture |
| Takeura et al.51 | Regression after trauma with multiple bone fractures |
| Huz et al.50 | Biopsy of regressed tumour site demonstrating chronic inflammatory infiltrate |
| Tumour hypoxia | |
| Alqutub et al.16 | Regression after occlusive thrombus of the right portal vein |
| Kondo et al.20 | Variceal bleeding with prolonged systemic hypotension |
| Morimoto et al.22 | Biopsy of the regressed tumour site demonstrating organized hepatic arterial thrombi |
| Suzuki et al.35 | Regression after angiospasm observed during angiography |
The ‘systemic inflammatory response’ category37–52 included patients with cholangitis, a persistent febrile illness, bacteraemia, the detection of elevated levels of cytokines and trauma leading to bone fractures. Also included were patients in whom pulmonary metastases regressed after embolization of the primary lesion, or where the primary hepatic tumour regressed after radiation of bony metastases (Table 1).
The remaining patients were labelled as having an ‘other or unknown’ mechanism.5,7–13,20,53–70 Some possibilities suggested by the authors of reports in the unknown category included the use of herbal medications, smoking cessation, alcohol cessation, withdrawal of androgen therapy and administration of Vitamin K. However, most of the reports in this group did not have any mechanism of regression suggested.
The two cohorts of tumour hypoxia and systemic inflammatory response were then compared. Univariate analysis did not show any significant association of mechanism of regression with age, gender, liver disease, cirrhosis, completeness of response or survival time.
Discussion
Based on the current review of the literature, two common mechanisms of spontaneous HCC regression were identified: tumour hypoxia and systemic inflammatory activation.
Tumour hypoxia
The current analysis revealed multiple patients in which regression appeared to be associated with tumour hypoxia. Several occurrences were related to occlusion of either the hepatic artery or portal vein, which probably led to a direct ischaemic insult. Other patients experienced profound systemic hypoperfusion, such as sustained hypotension associated with a massive variceal bleed. Tumour hypoxia as a mechanism is intuitively appealing in that it mirrors established treatment modalities for HCC. For example, both hepatic artery embolization and the agent sorafenib can be considered to rely upon the induction of tumour hypoxia for their effect.71
Transhepatic arterial chemoembolization occludes the arterial supply to the tumour72 and artificially produces a similar effect to the reports of regression of HCC after spontaneous arterial thrombosis. Sorafenib's efficacy is based on the principle of ‘anti-angiogenesis’ as described by Folkman in 1971.73 This small molecule is an inhibitor of several tyrosine kinases, including several of the vascular endothelial growth factor receptors whose downstream pathways have been shown to be overactive in human HCC.74 This drug is thought to work by inhibiting neovascularization of the malignancy and as a result appears to function in a manner that mimics the tumour hypoxia mechanism of spontaneous regression.71
Systemic inflammatory response
However, tumour hypoxia does not explain all of the reported patients with HCC, in particular those involving the regression of metastatic disease. In these instances, a systemic process must be the inciting factor underlying the spontaneous regression. Interestingly, several reports documented the presence of elevated cytokine levels, suggesting a systemic inflammatory response. Abiru et al.37 noted elevated IL-18 in three patients with regression of HCC. IL-18 has been shown to induce interferon (IFN)-gamma production by T-cells and natural killer (NK) cells, thus potentially producing enhanced cytotoxic activity targeted at cancer cells. Jozuka et al.40 proposed a similar mechanism after they detected elevated levels of NK cell activity, IL-2, IL-6, IL-12 and IFN-gamma throughout the course of the patient's spontaneous regression.
While many patients with cytokine elevation were associated with infections, high levels were also seen in non-infectious patients. The report by Blondon et al.38 describes a patient in whom tumour rupture and the peritoneal dissemination of cancer cells led to the activation of an immune response against the tumour antigens. In a report where regression of the primary HCC tumour after radiation therapy delivered to metastases,49,52 the authors noted that tumour necrosis factor (TNF)-alpha was elevated after radiotherapy and proposed that tumour lysis may have led to activation of a host immune response directed at tumour antigens.
Pathological evidence of an inflammatory mechanism for regression was demonstrated in one patient where a biopsy of the previous tumour site revealed the presence of a chronic inflammatory infiltrate.50 Wada et al.75 reported that patients with HCC whose resected tumours demonstrated a lymphocytic infiltration had a better prognosis than those without the infiltrate; the authors attributed the improved survival to the anti-tumour effect induced by cellular and humoral immunity. Together, these reports provide reasonable support to the theory that a systemic inflammatory response is associated with spontaneous regression.
In conclusion, of the 75 patients who underwent true spontaneous regression of HCC in the literature, 28.0% can be attributed to tumour hypoxia and 33.3% to activation of a systemic inflammatory response. Although the current findings suggest that tumour hypoxia and the inflammatory response are important components of spontaneous regression, the true mechanism of regression is certainly multifactorial and complex. There are likely interactive effects between these two phenomena and other causative elements which together lead to regression. These possible aetiologies of tumour regression hint at intriguing methods of treating HCC. Tumour hypoxia is already the basis of treatment modalities such as hepatic artery embolization and the anti-angiogenic agent sorafenib. Treatment modalities for HCC do not currently include immune-directed therapies but this is an active area of research. Future publications of spontaneous regression of HCC should include consideration of the underlying mechanism.
Conflicts of interest
None declared.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.The Cancer of the Liver Italian Program (Clip) Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1999;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 3.Everson T, Cole W. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366–380. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole WH. Spontaneous regression of cancer and the importance of finding its cause. Natl Cancer Inst Monogr. 1976;44:5–9. [PubMed] [Google Scholar]
- 5.Johnson FL, Lerner KG, Siegel M, Feagler JR, Majerus PW, Hartmann JR, et al. Association of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet. 1972;2:1273–1276. doi: 10.1016/s0140-6736(72)92649-9. [DOI] [PubMed] [Google Scholar]
- 6.Oquiñena S, Guillen-Grima F, Iñarrairaegui M, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:254–257. doi: 10.1097/MEG.0b013e328324b6a2. [DOI] [PubMed] [Google Scholar]
- 7.Cheng HM, Tsai MC. Regression of hepatocellular carcinoma spontaneous or herbal medicine related? Am J Chin Med. 2004;32:579–585. doi: 10.1142/S0192415X04002211. [DOI] [PubMed] [Google Scholar]
- 8.Chien RN, Chen TJ, Liaw YF. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1992;87:903–905. [PubMed] [Google Scholar]
- 9.Kato H, Nakamura M, Muramatsu M, Orito E, Ueda R, Mizokami M. Spontaneous regression of hepatocellular carcinoma: two case reports and a literature review. Hepatol Res. 2004;29:180–190. doi: 10.1016/j.hepres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Takeda Y, Togashi H, Shinzawa H, Miyano S, Ishii R, Karasawa T, et al. Spontaneous regression of hepatocellular carcinoma and review of literature. J Gastroenterol Hepatol. 2000;15:1079–1086. doi: 10.1046/j.1440-1746.2000.02202.x. [DOI] [PubMed] [Google Scholar]
- 11.Storey RE, Huerta AL, Khan A, Laber DA. Spontaneous complete regression of hepatocellular carcinoma. Med Oncol. 2010;28:948–950. doi: 10.1007/s12032-010-9562-8. [DOI] [PubMed] [Google Scholar]
- 12.Gottfried EB, Steller R, Paronetto F, Lieber CS. Spontaneous regression of hepatocellular carcinoma. Gastroenterology. 1982;82:770–774. [PubMed] [Google Scholar]
- 13.McCaughan GW, Bilous MJ, Gallagher ND. Longterm survival with tumour regression in androgen induced liver tumours. Cancer. 1985;56:2622–2626. doi: 10.1002/1097-0142(19851201)56:11<2622::aid-cncr2820561115>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 15.Mier JW, Vachino G, van der Meer JW, Numerof RP, Adams S, Cannon JG, et al. Induction of circulating tumor necrosis factor as the mechanism for the febrile response to interleukin-2 in cancer patients. J Clin Immunol. 1988;8:426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- 16.Alqutub A, Peck D, Marotta P. Spontaneous regression of a large hepatocellular carcinoma: case report. Ger Med Sci. 2011;22:9. doi: 10.3205/000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feo CF, Marrosu A, Scanu AM, Ginesu GC, Fancellu A, Migaleddu V, et al. Spontaneous regression of hepatocellular carcinoma: report of a case. Eur J Gastroenterol Hepatol. 2004;16:933–936. doi: 10.1097/00042737-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Gaffey MJ, Joyce JP, Carlson GS, Esteban JM. Spontaneous regression of hepatocellular carcinoma. Cancer. 1990;65:2779–2783. doi: 10.1002/1097-0142(19900615)65:12<2779::aid-cncr2820651228>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY, Sun PL, Chang HC, Perng DS, Chen YS. Spontaneous regression of advanced hepatocellular carcinoma: a case report. Cases J. 2009;2:6251. doi: 10.4076/1757-1626-2-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo S, Okusaka T, Ueno H, Ikeda M, Morizane C. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol. 2006;11:407–411. doi: 10.1007/s10147-006-0591-4. [DOI] [PubMed] [Google Scholar]
- 21.Misawa K, Hata Y, Manabe K, Matsuoka S, Saito M, Takada J, et al. Spontaneous regression of hepatocellular carcinoma. J Gastroenterol. 1999;34:410–414. doi: 10.1007/s005350050285. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Mizuno H, Fushimi H. Spontaneous necrosis of hepatocellular carcinoma: a case report. Dig Surg. 2002;19:413–418. doi: 10.1159/000065822. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani H, Yamazaki O, Matsuyama M, Horii K, Shimizu S, Oka H, et al. Spontaneous regression of hepatocellular carcinoma: report of a case. Surg Today. 2005;35:1081–1086. doi: 10.1007/s00595-005-3066-8. [DOI] [PubMed] [Google Scholar]
- 24.Sibartie V, Moriarty J, Crowe J. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 2008;103:1050–1051. doi: 10.1111/j.1572-0241.2007.01772_14.x. [DOI] [PubMed] [Google Scholar]
- 25.Tocci G, Conte A, Guarascio P, Visco G. Spontaneous remission of hepatocellular carcinoma after massive gastrointestinal haemorrhage. Br Med J. 1999;300:641–642. doi: 10.1136/bmj.300.6725.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uenishi T, Hirohashi K, Tanaka H, Ikebe T, Kinoshita H. Spontaneous regression of a large hepatocellular carcinoma with portal vein tumor thrombi: report of a case. Surg Today. 2000;30:82–85. doi: 10.1007/PL00010054. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Fujiwara K, Nakagawa S, Kanishima S, Ohta Y, Oka Y, et al. A case of spontaneous regression of hepatocellular carcinoma with bone metastasis. Cancer. 1985;56:667–671. doi: 10.1002/1097-0142(19850801)56:3<667::aid-cncr2820560339>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Imaoka S, Sasaki Y, Masutani S, Ishikawa O, Furukawa H, Kabuto T, et al. Necrosis of hepatocellular carcinoma caused by spontaneously arising arterial thrombus. Hepatogastroenterology. 1994;41:359–362. [PubMed] [Google Scholar]
- 29.Iiai T, Sato Y, Nabatame N, Yamamoto S, Makino S, Hatakeyama K. Spontaneous complete regression of hepatocellular carcinoma with portal vein tumor thrombus. Hepatogastroenterology. 2003;50:1628–1630. [PubMed] [Google Scholar]
- 30.Peddu P, Huang D, Kane PA, Karani JB, Knisely AS. Vanishing liver tumours. Clin Radiol. 2008;63:329–339. doi: 10.1016/j.crad.2007.08.009. Epub 2007 Nov 26. [DOI] [PubMed] [Google Scholar]
- 31.van Halteren HK, Salemans JM, Peters H, Vreugdenhil G, Driessen WM. Spontaneous regression of hepatocellular carcinoma. J Hepatol. 1997;27:211–215. doi: 10.1016/s0168-8278(97)80304-2. [DOI] [PubMed] [Google Scholar]
- 32.Yano Y, Yamashita F, Kuwaki K, Fukumori K, Kato O, Kiyomatsu K, et al. Partial spontaneous regression of hepatocellular carcinoma: a case with high concentrations of serum lens culinaris agglutinin-reactive alpha fetoprotein. Kurume Med J. 2005;52:97–103. doi: 10.2739/kurumemedj.52.97. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki M, Furuse J, Yoshino M, Moriyama N, Kanemoto H, Okumura H. Spontaneous regression of hepatocellular carcinoma: a case report. Jpn J Clin Oncol. 1997;27:278–281. doi: 10.1093/jjco/27.4.278. [DOI] [PubMed] [Google Scholar]
- 34.Meza-Junco J, Montaño-Loza AJ, Martinez-Benítez B, Cabrera-Aleksandrova T. Spontaneous partial regression of hepatocellular carcinoma in a cirrhotic patient. Ann Hepatol. 2007;6:66–69. [PubMed] [Google Scholar]
- 35.Suzuki M, Okazaki N, Yoshino M, Yoshida T. Spontaneous regression of a hepatocellular carcinoma – a case report. Hepatogastroenterology. 1989;36:160–163. [PubMed] [Google Scholar]
- 36.Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Yoshida T, et al. Necrosis of hepatocellular carcinoma as a result of subintimal injury incurred by hepatic angiography: report of two cases. Am J Gastroenterol. 1986;81:979–983. [PubMed] [Google Scholar]
- 37.Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of interleukin 18. Am J Gastroenterol. 2002;97:774–775. doi: 10.1111/j.1572-0241.2002.05580.x. [DOI] [PubMed] [Google Scholar]
- 38.Blondon H, Fritsch L, Cherqui D. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: possible role of immune mechanisms. Eur J Gastroenterol Hepatol. 2004;16:1355–1359. doi: 10.1097/00042737-200412000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Gomez Sanz R, Moreno Gonzalez E, Colina Ruiz-Delgado F, Garcia-Munoz H, Ochando Cerdan F, Gonzalez-Pinto I. Spontaneous regression of a recurrent hepatocellular carcinoma. Dig Dis Sci. 1998;43:323–328. doi: 10.1023/a:1018802321581. [DOI] [PubMed] [Google Scholar]
- 40.Jozuka H, Jozuka E, Suzuki M, Takeuchi S, Takatsu Y. Psycho-neuro-immunological treatment of hepatocellular carcinoma with major depression – a single case report. Curr Med Res Opin. 2003;19:59–63. doi: 10.1185/030079902125001362. [DOI] [PubMed] [Google Scholar]
- 41.Markovic S, Ferlan-Marolt V, Hlebanja Z. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:392–393. [PubMed] [Google Scholar]
- 42.Ohta H, Sakamoto Y, Ojima H, Yamada Y, Hibi T, Takahashi Y, et al. Spontaneous regression of hepatocellular carcinoma with complete necrosis: case report. Abdom Imaging. 2005;30:734–737. doi: 10.1007/s00261-005-0313-9. [DOI] [PubMed] [Google Scholar]
- 43.Randolph AC, Tharalson EM, Gilani N. Spontaneous regression of hepatocellular carcinoma is possible and might have implications for future therapies. Eur J Gastroenterol Hepatol. 2008;20:804–809. doi: 10.1097/MEG.0b013e3282f2bbcc. [DOI] [PubMed] [Google Scholar]
- 44.Stoelben E, Koch M, Hanke S, Lossnitzer A, Gaertner HJ, Schentke KU, et al. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg. 1998;383:447–452. doi: 10.1007/s004230050158. [DOI] [PubMed] [Google Scholar]
- 45.Heianna J, Miyauchi T, Suzuki T, Ishida H, Hashimoto M, Watarai J. Spontaneous regression of multiple lung metastases following regression of hepatocellular carcinoma after transcatheter arterial embolization. A case report. Hepatogastroenterology. 2007;54:1560–1562. [PubMed] [Google Scholar]
- 46.Matsuo R, Ogata H, Tsuji H, Kitazono T, Shimada M, Taguchi K, et al. Spontaneous regression of hepatocellular carcinoma – a case report. Hepatogastroenterology. 2001;48:1740–1742. [PubMed] [Google Scholar]
- 47.Lam KC, Ho JC, Yeung RT. Spontaneous regression of hepatocellular carcinoma: a case study. Cancer. 1982;50:332–336. doi: 10.1002/1097-0142(19820715)50:2<332::aid-cncr2820500228>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 48.Li AJ, Wu MC, Cong WM, Shen F, Yi B. Spontaneous complete necrosis of hepatocellular carcinoma: a case report. Hepatobiliary Pancreat Dis Int. 2003;2:152–154. [PubMed] [Google Scholar]
- 49.Mochizuki T, Takehara Y, Nishimura T, Takahashi M, Kaneko M. Regression of hepatocellular carcinoma. Am J Roentgenol. 1991;156:868–869. doi: 10.2214/ajr.156.4.1848389. [DOI] [PubMed] [Google Scholar]
- 50.Huz JI, Melis M, Sarpel U. Spontaneous regression of HCC is most often associated with tumor hypoxia or systemic inflammatory response. 2012. Poster session at the 2012 American HepatoPancreatoBiliary Association Annual Meeting, Miami, FL. [DOI] [PMC free article] [PubMed]
- 51.Takeura C, Tokoro T, Tanahashi Y, Yoshida J, Kagawa T, Kato Y, et al. Two cases of spontaneous regression of hepatocellular carcinoma with extrahepatic metastasis. 2012. Poster session at the 2012 American HepatoPancreatoBiliary Association Annual Meeting, Miami, FL.
- 52.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayres RC, Robertson DA, Dewbury KC, Millward-Sadler GH, Smith CL. Spontaneous regression of hepatocellular carcinoma. Gut. 1990;31:722–724. doi: 10.1136/gut.31.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Poggio P, Mattiello M, Gilardoni L, Jamoletti C, Colombo S, Zabbialini G. The mysterious case of spontaneous disappearance of hepatocellular carcinoma. Dig Liver Dis. 2009;41:e21–e25. doi: 10.1016/j.dld.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Grossmann M, Hoermann R, Weiss M, Jauch KW, Oertel H, Staebler A, et al. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1995;90:1500–1503. [PubMed] [Google Scholar]
- 56.Kaczynski J, Hansson G, Remotti H, Wallerstedt S. Spontaneous regression of hepatocellular carcinoma. Histopathology. 1998;32:147–150. doi: 10.1046/j.1365-2559.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee HS, Lee JS, Woo GW, Yoon JH, Kim CY. Recurrent hepatocellular carcinoma after spontaneous regression. J Gastroenterol. 2000;35:552–556. doi: 10.1007/s005350070080. [DOI] [PubMed] [Google Scholar]
- 58.Nam SW, Han JY, Kim JI, Park SH, Cho SH, Han NI, et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol. 2005;20:488–492. doi: 10.1111/j.1440-1746.2005.03243.x. [DOI] [PubMed] [Google Scholar]
- 59.Nishijima N, Marusawa H, Kita R, Osaki Y, Chiba T. Education and Imaging. Hepatobiliary and pancreatic: spontaneous regression of hepatocellular cancer demonstrated by contrast-enhanced ultrasonography. J Gastroenterol Hepatol. 2009;24:1153. doi: 10.1111/j.1440-1746.2009.05884.x. [DOI] [PubMed] [Google Scholar]
- 60.Nouso K, Uematsu S, Shiraga K, Okamoto R, Harada R, Takayama S, et al. Regression of hepatocellular carcinoma during vitamin K administration. World J Gastroenterol. 2005;11:6722–6724. doi: 10.3748/wjg.v11.i42.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oquiñena S, Iñarrairaegui M, Vila JJ, Alegre F, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci. 2009;54:1147–1153. doi: 10.1007/s10620-008-0447-z. Epub 2008 Aug 21. [DOI] [PubMed] [Google Scholar]
- 62.Ozeki Y, Matsubara N, Tateyama K, Kokubo M, Shimoji H, Katayama M. Spontaneous complete necrosis of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:391–392. [PubMed] [Google Scholar]
- 63.Terasaki T, Hanazaki K, Shiohara E, Matsunaga Y, Koide N, Amano J. Complete disappearance of recurrent hepatocellular carcinoma with peritoneal dissemination and splenic metastasis: a unique clinical course after surgery. J Gastroenterol Hepatol. 2000;15:327–330. doi: 10.1046/j.1440-1746.2000.02092.x. [DOI] [PubMed] [Google Scholar]
- 64.Toyoda H, Sugimura S, Fukuda K, Mabuchi T. Hepatocellular carcinoma with spontaneous regression of multiple lung metastases. Pathol Int. 1999;49:893–897. doi: 10.1046/j.1440-1827.1999.00956.x. [DOI] [PubMed] [Google Scholar]
- 65.Vardhana HG, Panda M. Spontaneous regression of hepatocellular carcinoma: potential promise for the future. South Med J. 2007;100:223–224. doi: 10.1097/SIH.0b013e3180322322. [DOI] [PubMed] [Google Scholar]
- 66.Lin TJ, Liao LY, Lin CL, Shih LS, Chang TA, Tu HY, et al. Spontaneous regression of hepatocellular carcinoma: a case report and literature review. Hepatogastroenterology. 2004;51:579–582. [PubMed] [Google Scholar]
- 67.McDermott WV, Khettry U. Clear cell carcinoma of the liver with spontaneous regression of metastases. J Surg Oncol. 1999;57:206–209. doi: 10.1002/jso.2930570315. [DOI] [PubMed] [Google Scholar]
- 68.Herrera A, Erdozain JC, Molina E, Conde P, Palomo V. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:1288–1289. [PubMed] [Google Scholar]
- 69.Izuishi K, Ryu M, Hasebe T, Kinoshita T, Konishi M, Inoue K. Spontaneous total necrosis of hepatocellular carcinoma: report of a case. Hepatogastroenterology. 2000;47:1122–1124. [PubMed] [Google Scholar]
- 70.Jang TJ, Lee JI, Kim DH, Kim JR, Lee HK. Spontaneous regression of hepatocellular carcinoma – a case report. Korean J Intern Med. 2000;15:147–150. doi: 10.3904/kjim.2000.15.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57:1122–1129. doi: 10.1007/s10620-012-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res. 2010;40:943–953. doi: 10.1111/j.1872-034X.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 73.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 74.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 75.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]


