Abstract
Background
A major hepatic resection for malignancies requires an adequate post-operative liver reserve. Portal vein embolization (PVE) with intra-arterial therapy (IAT) may increase future liver remnant (FLR) hypertrophy. As such, the feasibility, safety and efficacy of IAT+PVE were investigated.
Methods
Between 2000 to 2011, 86 patients with malignancy of the liver were identified from a multi-institutional database. Twenty-nine patients underwent sequential IAT+PVE, 25 had PVE alone and 32 had IAT alone. Clinicopathological data were evaluated.
Results
Most patients had hepatocellular carcinoma (HCC) (65.1%) and 31.4% had secondary metastatic disease. A complete or partial response using European Association for the Study of the Liver (EASLD) criteria was seen in 48.3% of patients undergoing IAT+PVE vs. 56.6% among patients undergoing IAT (P = 0.601). The median increase in percentage FLR volume was comparable in IAT+PVE (7.4%) vs. PVE only (7.9%) (P = 0.203). There were no IAT+PVE-associated deaths and only one complication. Among patients treated with IAT+PVE (n = 29), 27 underwent a subsequent hepatic resection. Peri-operative morbidity and mortality was 29.6% and 7.4%, respectively. Among the patients with HCC who underwent curative intent surgery after IAT+PVE, the median survival was 59.0 months.
Conclusions
Sequential IAT and PVE are feasible and safe. Utilization of IAT+PVE before a resection can lead to long-term survival and should be considered in the treatment of patients with advanced hepatic malignancies.
Keywords: chemoembolization, portal vein embolization, liver, malignancy, safety, outcome
Introduction
A major hepatic resection is the only curative option for many patients with advanced malignancies of the liver. In recent decades, improvements in surgical technique, increased understanding of hepatic anatomy and advances in peri-operative care have resulted in improvements in peri-operative mortality associated with a liver resection. Mortality is now reported to be less than 5% at most high-volume centres.1–5 However, post-operative liver failure remains a serious concern associated with high mortality.6 A number of studies have identified the risk factors associated with post-operative liver failure including cirrhosis, chemotherapy induced steatohepatitis, sepsis and cholestasis.6,7 Most efforts to reduce the risk of liver failure after resection have focused on insuring an adequate post-operative hepatic reserve.4,6–8
Characterization of an adequate hepatic reserve has resulted in the development of scoring systems and biochemical tests.9,10 However, in most centres pre-operative measurement of the future liver remnant (FLR) is utilized to ensure that an adequate minimal functional liver volume will be left after resection to avoid liver insufficiency. In general, patients with normal underlying hepatic parenchyma require an FLR of 20% to 30%, whereas patients with underlying liver disease may require an FLR of 30% to 50%.11 Pre-operative portal vein embolization (PVE) has been utilized as a strategy to induce hypertrophy of the FLR to mitigate the risk of liver failure among patients undergoing a major hepatic resection. However, there have been some concerns over tumour progression after portal vein occlusion. Several previous studies have demonstrated either primary or metastatic tumour progression after pre-operative PVE.12–15 Preclinical evidence in mouse models has also suggested that portal vein occlusion may increase the growth rate of liver metastasis through growth factor secretion.16 Other studies have suggested that PVE may be associated with tumour progression through various potential routes including a combination of growth factor secretion and increased hepatic artery flow.17
Because of the risk of tumour progression and the desire to treat the intrahepatic disease around the time of PVE, there has been interest in combining PVE with intra-arterial therapy (IAT). Sequential IAT and PVE may allow for the treatment of intrahepatic disease, while also increasing the hypertrophy of the FLR owing to occlusion of both the portal and arterial flow to the tumour-bearing liver. However, combined IAT and PVE may also result in more morbidity as a result of double occlusion of hepatic vascular in-flow. Data on sequential IAT and PVE prior to major hepatic resection remain poorly defined. Most reports on the topic have been single institution series and therefore may have limited generalizability.18–20 The current series utilized an international multi-centre database derived from six major hepatobiliary centres. The short- and long-term outcomes of patients who were managed with sequential IAT and PVE prior to hepatic resection were examined. Specifically, the aim was to determine the feasibility, safety and efficacy of combined sequential IAT and PVE.
Methods
Patients and data collection
Utilizing an international multi-institutional database, 86 patients with an advanced malignancy of the liver diagnosed between January 2000 and June 2011 were identified from one of six institutions (Johns Hopkins University School of Medicine, Baltimore, MD, USA; Ohio State University, Columbus, Ohio, USA; University of Calgary Hospital, Calgary, Canada; Curry Cabral Hospital, Lisbon, Portugal; Eastern Hepatobiliary Surgery Hospital, Shanghai, China; and Ospedale Mauriziano Umberto I, Turin, Italy). The Institutional Review Boards of each respective institution approved the study. The study cohort consisted of 29 patients who had undergone a prospectively planned sequential IAT+PVE, compared with patients who had undergone either PVE (n = 25) or IAT (n = 32) alone.
Standard demographical and clinicopathological data were collected including patient and primary tumour characteristics. Specifically, as previously described,21 data collected included patient demographics; primary tumour size, number; serum laboratory exams [e.g. international normalized ratio (INR), bilirubin, creatinine etc.]; and model of end-stage liver disease (MELD) score. Detailed data on imaging characteristics were also collected. Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) were assessed using standard European Association for the Study of the Liver (EASL) criteria,22 as previously described.23 For those patients who underwent a resection, a pathological response was also assessed according to established criteria.24 Data on the type of IAT and PVE treatment, as well as peri-procedural and long-term outcomes were also collected. IAT was performed using conventional trans-arterial chemoembolization (cTACE) with either cisplatin+doxorubicin+mitomycin C or doxorubicin drug-eluting beads (DEB). PVE was performed using either cyanoacrylate, microspheres or coils as described previously.25–27 In general, the doses of chemotherapy for conventional TACE were 100 mg of cisplatin, 50 mg of doxorubicin and 10 mg of mitomycin C mixed with lipiodol 1–2 to 1 depending on arterial flow. The volume used was usually 10 cc of chemo + 5–15 cc of lipiodol. The size of the microspheres used to embolize as part of conventional TACE was 100 to 300 microns. The size of the DEB-TACE particles was 100 to 300 microns loaded with 1 vial or 50 mg of doxorubicin. In general, 1 or 2 vials were used per case. For PVE, the particles were usually 100–500 microns, whereas for the N-Butyl Cyanoacrylate or glue typically two tubes or 2 ml of total mixed with lipiodol at four times the volume of glue, therefore approximately 8 ml was used.
Cross-sectional volumetry was performed after PVE to assess contralateral liver hypertrophy.28,29 Survival status was determined using both hospital records as well as the Social Security Death Index.
Data analysis
Summary statistics were obtained using established methods and presented as percentages or median values. The chi-square test was used to compare categorical data, whereas the Mann-Whitney U-test was used for continuous data. Factors including procedure-related complications, change in FLR, tumour response on imaging and pathology, as well as overall survival were compared among patients undergoing sequential IAT+PVE versus either IAT or PVE alone. Survival times were estimated using the Kaplan–Meier method and differences were examined using the log-rank test. A P-value of less than 0.05 was considered significant. All tests were performed using the SPSS version 18.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics and demographics
The patient and tumour characteristics of the 86 patients who were diagnosed with a primary or secondary hepatic tumour and who were included in the current study are detailed in Table 1. Fifty-six patients had a primary hepatic cancer and 27 had secondary metastatic disease. Overall, 59 patients had a solitary liver lesion and 21 had bilobar hepatic metastasis. The median size of the largest lesion was 6.2 cm (range, 1.5–15.0 cm). Ten patients had concurrent extrahepatic metastatic disease.
Table 1.
Baseline characteristics of patients according to treatment received
| IAT (n = 32) | PVE (n = 25) | IAT & PVE (n = 29) | P-value | |
|---|---|---|---|---|
| Age at diagnosis (years); median (range) | 61.1 (13.4–74.0) | 62.4 (42.4–71.3) – | 60.1 (48.2–69.8) | 0.316 |
| Male gender; n | 22 | 17 | 24 | 0.300 |
| Race | ||||
| White; n | 19 | 23 | 23 | <0.001 |
| Black; n | 13 | 1 | 1 | |
| Other; n | 0 | 1 | 5 | |
| Cirrhotic liver; n | 22 | 1 | 11 | <0.001 |
| Chemistries | ||||
| Serum bilirubin (mg/dl); median (range) | 1.0 (0.2–3.8) | 0.7 (0.3–4.3) | 0.9 (0.3–1.7) | 0.078 |
| INR; median (range) | 1.1 (1.1–1.4) | 1.0 (0.9–1.4) | 1.1 (1.0–1.2) | 0.141 |
| Serum creatinine (mg/dl); median (range) | 1.0 (0.4–1.4) | 0.9 (0.7–1.2) | 0.9 (0.6–1.2) | 0.481 |
| Tumour histology | ||||
| HCC; n | 27 | 5 | 24 | <0.001 |
| CRLM; n | 2 | 16 | 1 | |
| Neuroendocrine; n | 3 | 2 | 3 | |
| Cholangiocarcinoma; n | 0 | 2 | 1 | |
| Tumour size (cm); median (range) | 6.0 (1.5–9.4) | 5.5 (1.8–12.1) | 10.2 (2.9–15.0) | 0.006 |
| Lymph node metastatsis; n | 4 | 10 | 0 | <0.001 |
| Extrahepatic disease; n | 4 | 3 | 5 | 0.851 |
| Estimated hepatic involvementa | ||||
| 0–25%; n | 13 | 6 | 5 | 0.032 |
| 26–50%; n | 10 | 11 | 7 | |
| 51–75%; n | 9 | 3 | 14 | |
| >75%; n | 0 | 1 (4.8) | 0 | |
| MELD score; median (range) | 8.4 (4.4–15) | 7.6 (2.5–11) | 7.6 (3.0–8.2) | 0.043 |
Data were missing for seven patients.
IAT, intra-arterial therapy; PVE, portal vein embolization; CRLM, colorectal liver metastatsis; HCC, hepatocellular carcinoma, INR, international normalized ratio; MELD, model for end-stage liver disease.
Although the distributions of certain factors (e.g. age, gender, INR, creatinine and the presence of extra-hepatic disease) were similar among the three study groups, univariate analyses revealed several differences (Table 1). While most patients who underwent sequential IAT+PVE (n = 24) and those who underwent IAT alone (n = 27) had HCC, patients who underwent PVE alone more often had colorectal liver metastasis as a diagnosis (n = 16) (P < 0.001). Patients who underwent IAT+PVE had the largest burden of intrahepatic disease. Specifically, the median size of the largest intrahepatic lesion was 10.2 cm among patients treated with IAT+PVE vs. 6.0 cm and 5.5 cm, respectively, for patients undergoing IAT or PVE alone (P = 0.006). There was also a corresponding difference in the extent of hepatic involvement (>50% intrahepatic tumour burden: IAT+PVE, 53.9% vs. IAT alone, 28.1% vs. PVE alone, 29.1%; P = 0.032).
IAT and PVE procedural details and associated morbidity
Details regarding the IAT and PVE procedures are outlined in Table 2. Overall, among patients undergoing IAT, patients were treated with either cTACE with cisplatin+doxorubicin+mitomycin C (n = 29) or DEB (n = 32). The median number of IAT treatments was 1 (range, 1 to 4). All patients who were treated with sequential IAT+PVE (n = 29) had IAT therapy as the initial liver-directed therapy. Compared with patients who underwent IAT alone (n = 10), patients who underwent sequential IAT+PVE (n = 19) were more commonly treated with doxorubicin DEB (P = 0.010) (Table 2). Overall, repeat IAT treatment was performed among 44.8% of IAT+PVE patients vs. 71.0% of the IAT patients. Repeat IAT therapy most often consisted of DEB (84.6%) among IAT+PVE patients, whereas 18 of the 22 IAT-only patients who underwent a repeat IAT received cTACE (P < 0.001).
Table 2.
Details of procedures undergone by the patients
| IAT (n = 32) | PVE (n = 25) | IAT & PVE (n = 29) | P-value | |
|---|---|---|---|---|
| Portal vein embolization | ||||
| Vein(s) occluded | ||||
| Right portal vein; n | – | 20 | 26 | 0.350 |
| Left portal vein; n | – | 2 | 0 | |
| Right portal vein+left segment 4; n | – | 3 | 3 | |
| Embolization material | – | |||
| N-butyl cyanoacrylate; n | – | 22 | 12 | 0.014 |
| Microspheres; n | – | 1 | 9 | |
| Coils+polyvinyl alcohol; n | – | 2 | 6 | |
| Procedure-related complications; n | – | 3 | 0 | 0.093 |
| Intra-arterial therapy regimen 1 | ||||
| Extent of tumour | ||||
| Unilobar; n | 20 | – | 18 | 0.941 |
| Bilobar; n | 12 | – | 11 | |
| Chemotherapy regimen | – | |||
| Cisplatin+doxorubicin+mitomycin C; n | 22 | – | 10 | 0.010 |
| Doxorubicin DEB; n(%) | 10 | – | 19 | |
| Procedure-related complications; n | 1 | – | 1 | 0.902 |
| Accompanied systemic chemotherapy; n | 1 | – | 4 | 0.181 |
| Intra-arterial therapy regimen 2 | ||||
| Patients undergoing second IAT; n | 22a | – | 13 | 0.402 |
| Chemotherapy regimen; n | – | |||
| Cisplatin+doxorubicin+mitomycin C; n | 18 | – | 2 | <0.001 |
| Doxorubicin DEB; n | 4 | – | 11 | |
| Procedure-related complications; n | 0 | – | 1 | |
| Accompanied systemic chemotherapy; n | 0 | – | 3 | 0.040 |
| Surgical resection | ||||
| Patients undergoing surgery; n | 0 | 19 | 27 | <0.001 |
| Type of operation | ||||
| Hemi-hepatectomy | – | 7 | 15 | 0.171 |
| Extended resection | – | 12 | 12 | |
| Margin status | – | |||
| R0 | – | 19 | 26 | 0.594 |
| R1 | – | 0 | 1 | |
| Procedure-related complications; n | – | 6 | 8 | 0.891 |
| Operative mortality, n | – | 1 | 2 | 0.190 |
From a total of 31 patients. One patient had complications arising from the first IAT.
IAT, intra-arterial therapy; PVE, portal vein embolization; DEB, drug-eluting beads.
Among the 54 patients who underwent PVE, 46 patients had the right portal vein embolized (IAT+PVE, 89.7% vs. PVE alone, 80.0%) (P = 0.351). Six patients underwent PVE of the right hemi-liver with concurrent embolization of segment 4 (IAT+PVE, 10.3% vs. PVE alone, 12.0%). While most PVE-alone patients were treated with N-butyl cyanoacrylate (n = 18), IAT+PVE patients were treated using several different approaches (N-butyl cyanoacrylate in 12; microspheres in 9; and coils+polyvinyl alcohol in 6) (P = 0.014). Of the 29 patients who underwent sequential liver-directed therapy, the median time between the initial IAT and subsequent PVE was 60 days (range, 28 to 102).
Morbidity and mortality after IAT and PVE were uncommon. Five patients experienced a peri-procedural complication; there was only one death. The death occurred in a patient who had undergone IAT alone. This patient was 71 years old and had HCC with a pre-IAT bilirubin of 3.8 and MELD score of 15. After IAT therapy with cTACE, the patient developed worsening liver insufficiency and died post-procedure day 26. Among those patients who underwent PVE alone, there were no peri-procedural deaths but there were three patients who had complications. Complications included a hepatic abscess, haemolytic anaemia and sepsis. Similarly, there were no procedural-related deaths after sequential IAT+PVE. One patient did experience a complication of a hepatic abscess related to IAT therapy, but this resolved with conservative management and antibiotics.
IAT and PVE: the impact on FLR, tumour response and surgical therapy
The efficacy of IAT was assessed comparing patients who underwent sequential IAT+PVE vs. patients who underwent IAT only (Table 3). Targeted lesions were evaluated for treatment response by assessing tumour necrosis on cross-sectional imaging using established EASLD criteria.22,23 Among patients treated with IAT alone, 16 and 1 patients had a PR or CR, respectively. Thirteen patients had SD. Among the patients who underwent sequential IAT+PVE, 12 and 2 patients, respectively, had a PR or CR whereas 15 patients had SD on cross-sectional imaging using EASLD criteria. No patient in either group had PD. Overall there was no difference in the response rate (PR+CR) using EASLD criteria when comparing IAT alone (56.6%) vs. sequential IAT+PVE (48.3%) (P = 0.601).
Table 3.
Response to procedures by type of procedure undergone by patients
| IAT (n = 32) | PVE (n = 25) | IAT & PVE (n = 29) | P-value | |
|---|---|---|---|---|
| Percent increase in FLR volume; median (range) | 0.94 (0–1.4) | 7.9 (4.1–14.7) | 7.4 (2.2–11.6) | 0.017 |
| EASL responsea | ||||
| Complete response, n | 1 | – | 2 | 0.601 |
| Partial response, n | 16 | – | 12 | |
| Stable disease, n | 13 | – | 15 | |
| Pathological response (% necrosis)b | ||||
| 0–25; n | – | 7 | 4 | 0.843 |
| 26–50%; n | – | 14 | 4 | |
| 51–75%; n | – | 0 | 2 | |
| 75–100%; n | – | 1 | 9 | |
| Recurrence; n | N/A | 7 | 9 | 0.809 |
| Condition at final follow-up | ||||
| Alive without evidence of disease; n | 0 | 8 | 18 | <0.001 |
| Alive with disease; n | 25 | 13 | 5 | |
| Died of disease; n | 3 | 3 | 5 | |
| Died of other causes; n | 4 | 1 | 1 | |
One patient in the IAT group did not return for response evaluation. Data missing for another patient. Total in group is 30 patients.
No pathology specimens were available for patients undergoing IAT as none underwent surgery. Data were available for a limited number of patients in the other two groups.
IAT, intra-arterial therapy; PVE, portal vein embolization; FLR, future liver remnant; EASL, European Association for the Study of the Liver.
The degree of FLR hypertrophy was also assessed comparing patients who underwent sequential IAT+PVE vs. patients who underwent PVE alone. The median time to FLR assessment from time of PVE was 47.8 days (range, 15 to 95). The percent increase in FLR was comparable after PVE alone vs. sequential IAT+PVE (PVE alone, 7.9% vs. sequential IAT+PVE, 7.4%; P = 0.203) (Fig. 1).
Figure 1.
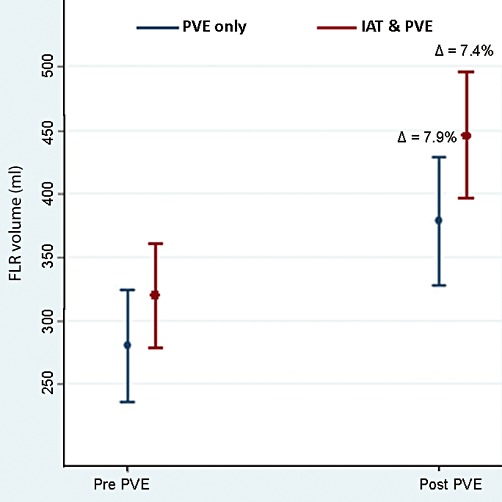
The percent increase in future liver remnant (FLR) was comparable after portal vein embolization (PVE) alone vs. sequential intra-arterial therapy (IAT)+PVE (PVE alone, 7.9% vs. sequential IAT+PVE, 7.4%; P = 0.203)
Among patients who underwent PVE alone (n = 25), 19 underwent a resection. In contrast among patients treated with IAT+PVE (n = 29), 27 patients underwent subsequent hepatic resection. In this latter group, a hepatic resection consisted of hemi-hepatectomy (n = 15) or extended hepatic resection (n = 12). The surgical margin was microscopically negative in the majority of patients (n = 26). On final pathological analysis, greater than 50% tumour necrosis was noted among most patients (n = 11), with many patients having 75% or more tumour necrosis (n = 9) (Fig. 2).
Figure 2.
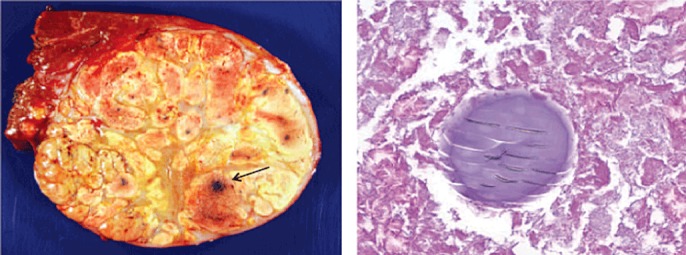
Histological findings showing tumour response. Presence of extensive necrosis with no viable tumour. Note the embedded drug-eluting beads (DEB) post-intra-arterial therapy (IAT) (arrow)
Morbidity and mortality after a hepatic resection among those patients who had pre-operative sequential IAT+PVE was noted in 8 and 2 patients, respectively. The two post-operative deaths were related to liver insufficiency/failure in cirrhotic patients who had pre-operative FLR volumes of 598 ml (27%) and 530 ml (34%). Among all patients who underwent resection after sequential IAT+PVE, the median and 3-year survival was 58 months and 87.4%, respectively. Among patients with HCC (n = 24) who underwent surgery after IAT+PVE, the median survival was 59.0 months and 1-, and 3-year survival was 85.7% and 66.7%, respectively. At a median follow-up of 16.7 months, 18 patients with HCC who underwent resection after IAT+PVE were alive and disease-free.
Discussion
Surgery is being increasingly considered for patients with a large intrahepatic burden of disease that requires a major hepatic resection. Determination of resectability is based on preserving blood flow and biliary drainage to two contiguous liver segments while also preserving enough remnant liver to avoid liver failure.30,31 To mitigate the risk of post-operative liver insufficiency, PVE has been employed to increase the FLR. However, there is some concern that while PVE may induce hypertrophy of the FLR, there may also be concomitant tumour growth during this period of time.12–17 Some investigators have suggested that IAT combined with PVE may have an increased therapeutic effect on the intrahepatic tumour owing to obstruction of the tumour-feeding vessels and suppression of intrahepatic spread by portal vein invasion.25,32–34 However, double occlusion of the blood supply to the tumour-bearing liver may result in an increased risk of ischaemia, liver damage and complications. Less than a handful of studies have reported on the use of sequential IAT+PVE for patients with advanced liver malignancies.18–20 Two of the three previous reports included fewer than 20 patients19,20 and all previous studies were single institution experiences.18–20 The current study is important because it examined an international experience with sequential IAT+PVE. This enabled a wide range of IAT+PVE techniques in a more ‘real-world’ setting to be reported, making these findings more broadly applicable. In the present study, IAT+PVE was noted to be feasible and safe, with no related deaths and only one patient complication. Sequential IAT+PVE did not result in additional FLR hypertrophy, but a subset of patients with extensive intrahepatic disease who did undergo a resection after IAT+PVE were noted to have significant tumour necrosis on final pathology and experienced long-term survival.
One of the main concerns with sequential IAT+PVE regards the double occlusion of vascular inflow to the tumour-bearing hemi-liver. Parenchymal damage can occur after IAT alone as evidenced by elevation in liver enzymes including aspartate aminotransferase (AST) and alanine aminotransferase (ALT).18,35,36 Most often these elevations are transient; however, the concern with IAT+PVE is that any ischaemic effect may be more severe and long standing. In the study by Yoo et al., the authors reported that nearly all patients treated with IAT+PVE had only a mild elevation in AST and ALT within 3 days after IAT that quickly normalized and no patient had abnormal elevation in enzymes after subsequent PVE.18 In this study, only one patient who underwent IAT+PVE had markedly and persistently elevated liver enzymes that that did not normalize 3 months after PVE. Of note, Yoo et al. noted that no patient experienced a significant complication after IAT+PVE. In the present study, we similarly found that IAT+PVE was well tolerated with no peri-procedural deaths and only one serious complication. While liver function enzymes after IAT+PVE were not formally assessed, the one specific complication – hepatic abscess – did appear to be related to relative ischaemia and super-infection of the treated hemi-liver. In aggregate, data from the current study as well as data published by others,18–20 demonstrate that pre-operative sequential IAT+PVE can be performed safely.
In the present study, the median increase in percentage FLR volume was not different in the IAT+PVE group compared with the PVE only group. In fact, the percent increase in FLR was virtually identical after sequential IAT+PVE (7.4%) vs. PVE alone (7.9%) (Fig. 1). In contrast, Yoo et al. had reported that sequential IAT+PVE was an effective method to increase the rate of FLR hypertrophy and was associated with a significantly higher percent change in FLR.18 Specifically, in the study by Yoo et al., the mean increase in percentage FLR volume was 7.3% in the IAT+PVE group compared with 5.8% in the PVE-only group.18 The reasons for the difference in the present findings compared with the study by Yoo et al. are probably multi-factorial. Different techniques were employed for the IAT and PVE procedures in each study. Specifically, gelatin sponge embolization was the main method of PVE in the Yoo et al. study, whereas a variety of approaches were used in the present study (Table 2). In addition, Yoo and colleagues routinely performed IAT using a cisplatin-lipiodol emulsion, which tends to be more ‘embolic’ than the DEB IAT therapy that was delivered to half (52.5%) of the patients in the present study. These differences may explain, in part, the lower than expected change in FLR in the ‘control’ PVE alone group of only 5.8% in the Yoo et al. study.18 It is interesting to note that the change in FLR for the IAT+PVE (7.4%) and PVE only (7.9%) groups in the present study were nearly identical to the IAT+PVE group (7.3%) in the Yoo et al. study. Furthermore, the reported FLR changes in the Yoo et al. study had wide standard errors that overlapped considerably between the two groups (IAT+PVE: 7.3% ± 3.6% vs. PVE only group: 5.8% ± 4.5%) – again calling into question any significant difference between the groups. In turn, taken together, IAT+PVE does not appear to be associated with a marked difference in FLR hypertrophy compared with PVE alone.
Rather than reducing the tumour size, the goal of IAT treatment is to induce tumour necrosis. IAT alone has been associated with a mean tumour necrosis of 50% to 60% and the effect of IAT has been shown to be related to the size of the targeted lesion.37 In the present study, the median tumour size among patients undergoing IAT+PVE was almost 10 cm and over one-half of patients had greater than 50% estimated tumour involvement of the liver. In spite of this high burden of disease, IAT+PVE was associated with a good therapeutic response. Specifically, 48.3% of patients had either a partial or complete response based on EASLD criteria on cross-sectional imaging. In addition, among those patients who underwent a resection, 47.4% of patients were noted to have significant tumour necrosis of 75% to 100%. Ogata et al. had similarly noted a higher response for IAT among patient treated with combined IAT+PVE.19 IAT+PVE resulted not only in high pathological response rates, but also long-term survival in a subset of patients. Among patients who underwent a resection after sequential IAT+PVE the median survival was 58 months. In examining only HCC patients treated with IAT+PVE and resection, the median survival was 59.0 months and, perhaps more interestingly, three-quarters of these patients were alive and disease free. Other investigators have suggested that combined IAT+PVE may decrease the rate of intrahepatic recurrence by pre-operatively suppressing potential intrahepatic spread via the portal vein as well as occluding arterioportal shunts.18,32–34 Future studies will be needed to evaluate more fully the impact of IAT+PVE on overall and disease-free survival.
The present study has a number of limitations that need to be considered. In spite of assembling the experience of some of the largest hepato-biliary centres in the world, only a small number of patients who underwent sequential IAT+PVE could be identified. Because of the small number of patients who underwent IAT+PVE identified for this study, there were limitations with regard to statistical modelling and power. In addition, like all retrospective studies, the data may be subject to selection bias. Given the relative heterogeneity among patient and tumour characteristics, as well as the types of therapy administered, comparisons of patients treated with IAT+PVE vs. IAT or PVE alone may be limited. While direct comparisons may be problematic, the present study achieved its main objective of defining the safety and feasibility of IAT+PVE as well as providing general information regarding the effect of IAT+PVE on FLR and tumour necrosis.
In conclusion, sequential IAT+PVE prior to a hepatic resection is both feasible and safe. Combined therapy did not result in an appreciable increase in percent FLR compared with PVE alone. However, sequential IAT and PVE was associated with a significant tumour response both by EASLD criteria and on final pathology among those patients who underwent a resection. Furthermore, patients treated with IAT+PVE prior to a resection had a median survival approaching 5 years and many were disease-free at the time of last follow-up. As such, utilization of IAT+PVE prior to resection should be considered with advanced hepatic malignancies. Sequential IAT+PVE should especially be considered in those patients with a small FLR who have a large burden of intra-hepatic disease that would benefit from treatment while awaiting hypertrophy. Combined IAT+PVE in this setting may provide for hypertrophy of the FLR while also treating the disease and allowing for assessment of the treatment response.
Conflicts of interest
None declared.
References
- 1.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. Epub 2000/07/18. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion -7. Epub 2002/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. Epub 1999/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute's experience with 625 patients. J Am Coll Surg. 2007;204:580–587. doi: 10.1016/j.jamcollsurg.2007.01.035. Epub 2007/03/27. [DOI] [PubMed] [Google Scholar]
- 5.Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 6.Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145–155. doi: 10.1007/s00534-008-0017-y. Epub 2008/12/27. [DOI] [PubMed] [Google Scholar]
- 7.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. Epub 2008/12/19. [DOI] [PubMed] [Google Scholar]
- 8.Tamandl D, Gruenberger B, Klinger M, Herberger B, Kaczirek K, Fleischmann E, et al. Liver resection remains a safe procedure after neoadjuvant chemotherapy including bevacizumab: a case-controlled study. Ann Surg. 2010;252:124–130. doi: 10.1097/SLA.0b013e3181deb67f. Epub 2010/06/22. [DOI] [PubMed] [Google Scholar]
- 9.Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. Epub 2004/04/06. [DOI] [PubMed] [Google Scholar]
- 10.Mitsumori A, Nagaya I, Kimoto S, Akaki S, Togami I, Takeda Y, et al. Preoperative evaluation of hepatic functional reserve following hepatectomy by technetium-99m galactosyl human serum albumin liver scintigraphy and computed tomography. Eur J Nucl Med. 1998;25:1377–1382. doi: 10.1007/s002590050311. Epub 1998/11/18. [DOI] [PubMed] [Google Scholar]
- 11.Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB. 2010;12:289–299. doi: 10.1111/j.1477-2574.2010.00181.x. Epub 2010/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias D, De Baere T, Roche A, Ducreux RM, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 13.Barbaro B, Di Stasi C, Nuzzo G, Vellone M, Giuliante F, Marano P. Preoperative right portal vein embolization in patients with metastatic liver disease. Metastatic liver volumes after RPVE. Acta Radiol. 2003;44:98–102. Epub 2003/03/13. [PubMed] [Google Scholar]
- 14.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. Epub 2001/08/02. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–727. doi: 10.1080/02841850701424514. Epub 2007/08/31. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol. 2006;45:35–42. doi: 10.1016/j.jhep.2006.02.020. Epub 2006/05/16. [DOI] [PubMed] [Google Scholar]
- 17.de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423–430. doi: 10.1245/s10434-008-0222-6. Epub 2008/12/04. [DOI] [PubMed] [Google Scholar]
- 18.Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251–1257. doi: 10.1245/s10434-010-1423-3. Epub 2010/11/12. [DOI] [PubMed] [Google Scholar]
- 19.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. Epub 2006/06/17. [DOI] [PubMed] [Google Scholar]
- 20.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. Epub 2004/07/14. [DOI] [PubMed] [Google Scholar]
- 21.Demirjian A, Peng P, Geschwind JF, Cosgrove D, Schutz J, Kamel IR, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011;15:2089–2097. doi: 10.1007/s11605-011-1614-7. Epub 2011/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. Epub 2005/10/27. [DOI] [PubMed] [Google Scholar]
- 23.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466–473. doi: 10.1148/radiol.2502072222. Epub 2009/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. Epub 2006/10/25. [DOI] [PubMed] [Google Scholar]
- 25.Yoo H, Ko GY, Gwon DI, Kim JH, Yoon HK, Sung KB, et al. Preoperative portal vein embolization using an amplatzer vascular plug. Eur Radiol. 2009;19:1054–1061. doi: 10.1007/s00330-008-1240-2. Epub 2008/12/06. [DOI] [PubMed] [Google Scholar]
- 26.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness–study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. Epub 2003/03/05. [DOI] [PubMed] [Google Scholar]
- 27.Ko GY, Sung KB, Yoon HK, Kim JH, Weon YC, Song HY. Preoperative portal vein embolization with a new liquid embolic agent. Radiology. 2003;227:407–413. doi: 10.1148/radiol.2272011702. Epub 2003/03/15. [DOI] [PubMed] [Google Scholar]
- 28.Zacharia TT. Assessment of future remnant liver regeneration after portal vein embolization using three-dimensional CT and MR volumetric analyses. Australas Radiol. 2006;50:543–548. doi: 10.1111/j.1440-1673.2006.01625.x. Epub 2006/11/17. [DOI] [PubMed] [Google Scholar]
- 29.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. Epub 2009/09/05. [DOI] [PubMed] [Google Scholar]
- 30.Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol. 2009;3:131–144. doi: 10.1586/egh.09.8. Epub 2009/04/09. [DOI] [PubMed] [Google Scholar]
- 31.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. Epub 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 32.Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, et al. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: perioperative outcome and survival. Surgery. 2009;145:399–405. doi: 10.1016/j.surg.2008.10.009. Epub 2009/03/24. [DOI] [PubMed] [Google Scholar]
- 33.Murata S, Tajima H, Nakazawa K, Onozawa S, Kumita S, Nomura K. Initial experience of transcatheter arterial chemoembolization during portal vein occlusion for unresectable hepatocellular carcinoma with marked arterioportal shunts. Eur Radiol. 2009;19:2016–2023. doi: 10.1007/s00330-009-1349-y. Epub 2009/02/25. [DOI] [PubMed] [Google Scholar]
- 34.Kang BK, Kim JH, Kim KM, Ko GY, Yoon HK, Gwon DI, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma after attempted portal vein embolization in 25 patients. AJR Am J Roentgenol. 2009;193:W446–W451. doi: 10.2214/AJR.09.2479. Epub 2009/10/22. [DOI] [PubMed] [Google Scholar]
- 35.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007;25:978–986. doi: 10.1200/JCO.2006.09.8657. Epub 2007/03/14. [DOI] [PubMed] [Google Scholar]
- 37.Herber S, Biesterfeld S, Franz U, Schneider J, Thies J, Schuchmann M, et al. Correlation of multislice CT and histomorphology in HCC following TACE: predictors of outcome. Cardiovasc Intervent Radiol. 2008;31:768–777. doi: 10.1007/s00270-007-9270-8. Epub 2008/01/16. [DOI] [PubMed] [Google Scholar]


