Abstract
Objectives
In patients diagnosed with incidental gallbladder cancer (GC), the benefit and optimal extent of further surgery remain unclear. The aims of this study were to analyse outcomes in patients who underwent liver resection following a diagnosis of incidental GC and to determine factors associated with longterm survival.
Methods
A retrospective analysis of patients diagnosed with incidental GC between June 1999 and June 2010 was performed. Data covering demographics, clinical and surgical characteristics and local pathological stage were analysed.
Results
A total of 24 patients were identified. All patients underwent a resection of segments IVb and V and lymphadenectomy. Histological examination revealed residual disease in 10 patients, all of whom presented with recurrent disease at 3–12 months. Overall 5-year survival was 53%. Increasing T-stage (P < 0.001), tumour–node–metastasis (TNM) stage (P= 0.003), and the presence of residual tumour in the resected liver (P < 0.001) were all associated with worse survival.
Conclusions
Aggressive re-resection of incidental GC offers the only chance for cure, but its efficacy depends on the extent of disease found at the time of repeat surgery. The presence of residual disease correlated strongly with T-stage and was the most relevant prognostic factor for survival in patients treated with curative resection.
Introduction
Gallbladder cancer (GC) is the sixth most common gastrointestinal malignancy worldwide.1 In Argentina it predominates in the Province of Salta with an incidence of 6.7/100 000 population.2 The prognosis is dismal as the majority of patients are diagnosed in advanced stages of malignancy. It is estimated that only 15% of GC patients will survive for >5 years after diagnosis.3 Longterm survival has been reported mostly in patients with early tumours that are diagnosed incidentally on pathological examination of cholecystectomy specimens.4 Radical surgery for GC has been associated with the likelihood of longterm survival.5,6 This applies not only in cases of per primum surgery, but also in the revision of radical surgery for incidental GC.6,7 The rationale for performing revision surgery in patients who have undergone simple cholecystectomy for invasive GC is based on the premise that as the tumour stage (T-stage) increases, so does the chance that residual disease will be found in the gallbladder fossa, as well as in the regional lymph nodes.6,7 In the era of laparoscopic cholecystectomy, the identification of incidental GC has dramatically increased and it now constitutes the most common presentation of this disease.8,9 Several aspects of the surgical approach are still under debate, such as the extent of liver resection and lymphadenectomy, the need for common bile duct resection and the concomitant performance of a pancreatoduodenectomy.10,11 The therapeutic efficacy and appropriate extent of the resection remain unclear.12 No difference in longterm survival between patients undergoing one and those undergoing more than one operation has been reported.13
The objectives of this study were to analyse the results of liver resection in patients with incidental GC and to determine factors associated with longterm survival.
Materials and methods
A retrospective analysis was performed of data for patients referred to the Hospital Dr C Argerich with a diagnosis of GC between June 1999 and June 2010. Patients were identified from the Liver and Transplant Division's prospective computerized database. Preoperative assessment included clinical history, routine blood tests, carbohydrate antigen 19-9 (CA 19-9), transabdominal ultrasound and computed tomography (CT) of the chest, abdomen and pelvis with enhanced triphasic contrast of the liver.
A total of 60 patients with GC were identified; 40 (67%) patients had presented with incidental tumours diagnosed by subsequent pathological examination. The surgical approach involved resection of liver segments IVb and V with supraduodenal lymphadenectomy. Operative mortality included any death attributed to liver resection and all deaths occurring within 30 days of partial hepatectomy. Explanted specimens were reviewed by a single experienced liver pathologist. Involvement of liver, lymph nodes and port sites, surgical margins, and vascular and perineural invasion were assessed. A curative resection (negative margin, R0) was defined as the complete removal of any clinically evident tumour lesion(s) with negative pathological margins. Any infiltration of the resection margin by tumour cells in the histological specimen was defined as an R1 resection. Residual disease was defined by findings of microscopic liver involvement, positive nodes or positive port sites in the pathological examination after radical surgery. Clinical staging of disease was performed according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system.14 Chemotherapy was given in patients with residual disease. Postoperative follow-up was performed by physical examination and CT at 6-month intervals.
Data on demographic features, clinical characteristics, local pathological stages, surgical procedures, morbidity, pathological features and factors for longterm survival were analysed.
All statistical analyses were performed using spss Version 15 (SPSS, Inc., Chicago, IL, USA). Significance was calculated using the chi-squared test and correlations were measured using cross tabs. Survival curves were estimated with the Kaplan–Meier method and compared using the log-rank test in statistical analysis. P-values of < 0.05 were considered to indicate statistical significance.
Results
Of the 40 patients with incidental GC, 24 were candidates for curative treatment. Twenty of these 24 patients were female. The mean age of the patients was 57.4 years (range: 38–78 years). Twenty-two patients had been referred from other institutions.
Laparoscopic cholecystectomy had been performed in four patients and open cholecystectomy in 20. Gallbladder cancer was diagnosed at the time of cholecystectomy or after the procedure on analysis of the pathological specimen in two and 22 patients, respectively.
Pathological analysis of the gallbladder confirmed the T-stage of the disease as T-stage Ib, II and III in one, 12 and 11 patients, respectively.
At the time of referral (median 28 days, range: 0–150 days), 15 patients were found to be inoperable as a result of disease progression (n= 13) or refusal of surgical treatment (n= 2). Only one patient was found to have disseminated peritoneal disease at laparotomy. All non-resected patients subsequently presented with disease progression in the liver parenchyma and died at 3–6 months.
All resected patients in this study were treated by laparotomy and resection of liver segments IVb and V with an N1 lymphadenectomy. In the four patients who had undergone previous laparoscopic cholecystectomy, port site resection was performed at the time of laparotomy. No mortality was observed. Five patients suffered perioperative morbidity. Median postoperative stay was 5.5 days (range: 4–10 days).
Pathology
In 23 patients, the IVb–V liver resections were R0. Histological examination revealed adenocarcinoma in all patients. Histopathological staging is shown in Table 1. Seventeen tumours were reported as moderately differentiated. Positive lymph nodes were identified in three patients. The median number of lymph nodes dissected was three (range: 1–10 lymph nodes). Microscopic vascular invasion and perineural invasion were each found in two patients. Microscopic tumour involvement of the liver was present in seven patients, all of whom had stage III disease. One of the four patients who underwent port site resection was found to have metastatic disease in the resected port site. Residual disease was found in 10 of 24 patients; one of these patients had stage II disease and nine had stage III disease (P < 0.001).
Table 1.
Histopathological staging in 24 patients with incidental gallbladder cancer who underwent resection of liver segments IVb–V according to the American Joint Committee on Cancer Staging Manual, 7th edition
| Stage | Patients, n |
|---|---|
| I | 1 |
| II | 10 |
| IIIa | 9 |
| IIIb | 2 |
| IVb | 2 |
Survival
The median length of follow-up was 25 months (range: 6–132 months). Figure 1 summarizes overall survival in the 24 resected patients. Five-year overall survival by tumour–node–metastasis (TNM) stage was 100% in stage II and 22.5% in stage III disease (Fig. 2). Variables studied by statistical analysis to determine their relationship to survival are shown in Table 2. Four patients presented with local recurrence (n= 3 in the liver, n= 1 in lymph nodes; median time to recurrence: 7.5 months; range: 4–12 months) and six patients relapsed with metastatic disease (median time to relapse: 6 months; range: 3–9 months), most commonly in the peritoneum. Recurrence was found to increase significantly according to T-stage. The only patient with a T-stage Ib tumour died 6 months after surgery from gastrointestinal bleeding without tumour recurrence. All patients with positive lymph nodes, port site implants and microscopic involvement of the gallbladder fossa died within 15 months. A significant difference in survival emerged in patients with non-residual disease according to whether they had T-stage II (P = 0.002) or III (P = 0.01) disease.
Figure 1.
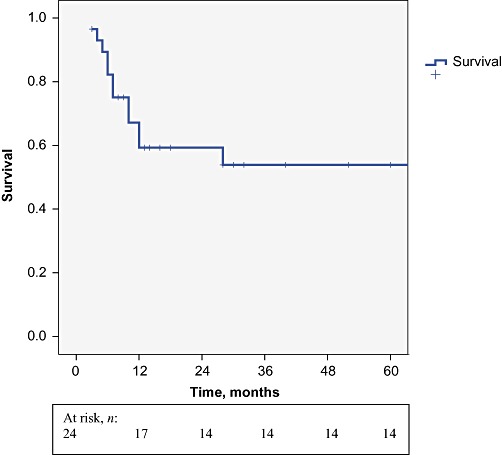
Five-year overall survival in 24 patients who underwent resection for incidental gallbladder cancer
Figure 2.
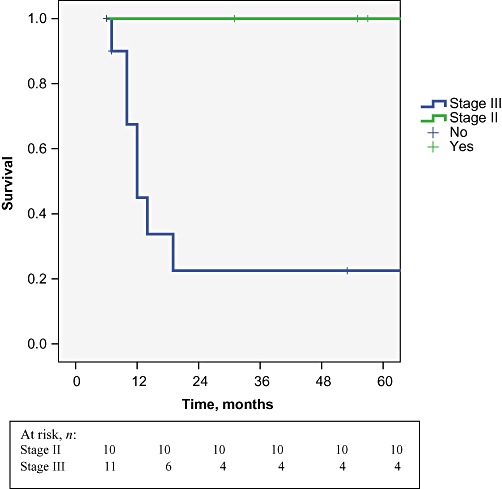
Five-year overall survival in patients with tumour–node–metastasis stage II and III disease
Table 2.
Analysis of variables related to recurrence and survival in 24 patients with incidental gallbladder cancer who underwent resection of liver segments IVb–V
| Variable | Patients, n | Recurrence, n (%) | P-value | Survival, months, median (range) | P-value |
|---|---|---|---|---|---|
| Age >65 years | 5 | 3 (60%) | 0.2 | 10 (6–55) | 0.8 |
| Age ≤65 years | 19 | 6 (31%) | 42 (6–132) | ||
| Positive lymph nodes | 3 | 3 (100%) | 0.01 | 10 (10–15) | 0.2 |
| Negatives lymph nodes | 21 | 6 (28%) | 42 (6–132) | ||
| Vascular invasion present | 2 | 2 (100%) | 0.05 | 11 (10–12) | 0.05 |
| Vascular invasion absent | 22 | 7 (32%) | 31 (6–132) | ||
| R0 resection | 23 | 8 (35%) | 0.1 | 31 (6–132) | 0.4 |
| R1 resection | 1 | 1 (100%) | 12 | ||
| T-stage | |||||
| Ib | 1 | 0 | 0.01 | 6 | 0.001 |
| II | 10 | 1 (10%) | 65 (15–132) | ||
| III | 12 | 8 (66%) | 10 (6–84) | ||
| Tumour–node–metastasis (TNM) stage | |||||
| I | 1 | 0 | 0.006 | 6 | 0.003 |
| II | 10 | 0 | 65 (31–132) | ||
| IIIa | 9 | 5 (55%) | 12 (6–84) | ||
| IIIb | 2 | 2 (100%) | 14.5 (10–15) | ||
| IVb | 2 | 2 (100%) | 8 (6–10) | ||
| Residual disease present | 10 | 10 (100%) | 0.001 | 10 (6–15) | <0.001 |
| Residual disease absent | 14 | 0 | 65 (6–132) | ||
Discussion
Gallbladder cancer is the most common biliary tract tumour, but remains a relatively infrequent gastrointestinal malignancy. The rate of incidental presentation has increased in recent years and this presentation predominates in Western countries.15 The 67% rate of incidental presentation reported in the present study is similar to those reported by institutions in the USA and Chile.16 Shih et al. reported a series of 53 patients with incidental GC which demonstrated that a laparoscopic approach increased the rate of early detection and longterm survival if patients were treated in centres that regularly perform radical surgery for this type of tumour.9 In the current study, the most common T-stage was T-stage II, but only five patients had previously undergone laparoscopic cholecystectomy. The low incidence of laparoscopy is explained by the inclusion in the present study of a high percentage of patients referred from regional centres in Argentina that continue to undertake a high proportion of conventional cholecystectomies.
The definition of the optimal treatment for incidental GC is still controversial. When it is feasible, radical re-resection offers the only potential for cure, but there is debate over the selection of patients for a second operation according to T-stage and the extent of resection. In a recent US study in 4631 patients with GC, only 2% were treated with the recommended surgery and only 5% of patients with T-stage I–II disease underwent further surgery.5 Dismal results were related to the aggressive biology of this cancer and advanced disease at presentation. Recently, 5-year survival rates as high as 38% have been reported in patients undergoing radical resection,17,18 which indicates that loco-regional recurrence may be reduced with optimal surgical management. In the present series, R0 resection was achieved in 96% of patients and the 5-year survival rate was 53%, which is comparable with rates reported in other series.18 However, a high proportion of patients with incidental GC are not eligible for potentially curative re-resection, as evidenced by the 35% of patients with disseminated disease who did not undergo re-resection in the current study. Debate on the appropriate extent of resection is ongoing.8 A Japanese study found no statistical differences in survival or recurrence rates in patients with T-stage II–III disease who underwent gallbladder bed resection compared with segmentectomy IVb–V.19 Goetze and Paolucci analysed data from the German Registry and found that for T-stage II tumours, resection of liver segments IVb–V seems to be the minimum volume of resection required.20 It is the present authors' belief that segmentectomy IVb–V should be performed because the procedure is associated with low morbidity and has the advantage of removing micro-metastases in the drainage area of the cystic vein.9,19,21,22 Resection of the laparoscopic port sites has a staging purpose, but offers no advantage in patients with confirmed implants that usually progress with peritoneal recurrence.8,13,23,24 A recent study found that port site resection was not associated with improved survival or recurrence and concluded that it should not be considered mandatory during definitive surgical treatment.25 A role for laparoscopy in re-exploration and resection for incidental GC has been advocated, but more definitive prospective studies are needed.26
Previous studies have shown the presence of parenchymal involvement in liver resections for incidental GC at rates of 20% and 0%, respectively.21,27 In the present series, seven patients who underwent re-resection were found to have residual disease in the liver bed. In a multicentre study involving 115 patients who underwent repeat surgery for incidental GC, residual tumour in the liver bed was found in only 15% of patients, but this series included a predominance (69%) of T-stage II patients.25 In a recent report analysing the impact of accurate staging in GC, 38% of residual disease in the gallbladder fossa was shown to occur in incidental tumours.28 Tumour stage correlates with the likelihood of residual disease in the liver and lymph nodes.28 Whether there is any prognostic benefit from re-resection in patients with residual disease is currently undetermined. In the present study, all patients with residual disease died in <15 months. Other authors have reported low survival rates, especially when residual disease was located in the liver.15,25 Prognosis is also correlated with nodal status and lymph node metastases have been reported as the most frequent site of additional disease.25 Although a median of three nodes per patient were dissected in the current study, only two patients were found to have lymph node metastases. The findings of a recent study by Ito et al.,28 showing that the number of lymph nodes evaluated is critical for stratifying recurrence risk and cancer-specific survival, are supported elsewhere.29–32 Radical surgery of GC with positive lymph nodes can lead to longterm survival in a subset of patients.25,33 The identification of patients with potential residual disease will be crucial to improve survival in incidental GC. It might be useful to use 18FDG-positron emission tomography (PET)-CT in the selection of patients.34,35 At present, adjuvant chemotherapy has not been demonstrated to provide any benefit in patients with residual disease.36
In the present series, patients with stage II disease appeared to benefit most, achieving a 5-year survival rate of 100%, whereas those reported elsewhere ranged from 61% to 91%.8,18 In a previous study of 443 radical operations for GC, the prognostic factors of age, T-stage, lymph node involvement (N-stage), gender and adjuvant radiotherapy were found to be associated with better survival.3 The present analysis, which was limited to incidental GC, showed that vascular invasion, tumour extension, TNM stage and the presence of residual disease in resected specimens were prognostic factors for survival. Similarly, in a study by Zaydfudim et al. of 4048 US patients, tumour extension and nodal invasion were identified as factors associated with longterm survival.37
In conclusion, the present study confirms that preoperative T-stage and TNM-stage are important factors for determining survival. Aggressive re-resection of incidental GC offers the only chance for cure, but its efficacy depends on the extent of disease found at repeat surgery. Residual disease was predominant in the liver (29%), correlated strongly with T-stage and was the most relevant prognostic factor for survival in patients treated with curative resection. Future adjuvant therapies in this setting will be crucial to improve the results of radical surgery in incidental GC.
Conflicts of interest
None declared.
References
- 1.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB. 2008;10:327–331. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo GF. Cáncer de vesícula biliar. Oncol Clin. 2002;8:831–836. [Google Scholar]
- 3.Jensen EH, Abraham A, Habermann EB, Al-Refaie WB, Vickers SM, Virnig BA, et al. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2009;13:722–727. doi: 10.1007/s11605-008-0772-8. [DOI] [PubMed] [Google Scholar]
- 4.Dong XD, DeMatos P, Prieto VG, Seigler HF. Melanoma of the gallbladder: a review of cases seen at Duke University Medical Center. Cancer. 1999;85:32–39. [PubMed] [Google Scholar]
- 5.Batra Y, Pal S, Dutta U, Gark PK, Makharia G. Gallbladder cancer in India: a dismal picture. J Gastrointest Hepatol. 2005;20:309–314. doi: 10.1111/j.1440-1746.2005.03576.x. [DOI] [PubMed] [Google Scholar]
- 6.Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu O. Gallbladder cancer: defining indications of primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833–840. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 7.Wakai T, Shirai Y, Hatakeyama K. Radical second resection provides survival benefit with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg. 2002;26:867–871. doi: 10.1007/s00268-002-6274-z. [DOI] [PubMed] [Google Scholar]
- 8.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101–2115. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 9.Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoist S, Panis Y, Fagniez PL. Longterm results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118–122. doi: 10.1016/s0002-9610(97)00269-9. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor VK, Benjamin IS. Resectional surgery for gallbladder cancer. Br J Surg. 1998;85:145–146. doi: 10.1046/j.1365-2168.1998.00715.x. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki R, Takeda Y, Hoshikawa K, Takahashi M, Funato O, Nitta H, et al. Longterm results of central inferior (S4a+S5) hepatic subsegmentectomy and pancreatoduodenectomy combined with extended lymphadenectomy for gallbladder carcinoma with subserous or mild liver invasion (pT2–3) and nodal involvement: a preliminary report. Hepatogastroenterology. 2004;51:215–218. [PubMed] [Google Scholar]
- 13.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior non-curative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green F, Page D, Fleming I. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 15.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102:620–625. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 16.Butte JM, Matsuo K, Gonen M, D'Angelica MI, Waugh E, Allen PJ, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centres in three countries. J Am Coll Surg. 2012;212:50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Fong Y, Wagman L, Gonen M, Crawford J, Reed W, Swanson R, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006;243:767–771. doi: 10.1097/01.sla.0000219737.81943.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong SL, Garcea G, Thomasset SC, Neal CP, Lloyd DM, Berry DP, et al. Ten-year experience in the management of gallbladder cancer from a single hepatobiliary and pancreatic centre with review of the literature. HPB. 2008;10:446–458. doi: 10.1080/13651820802392346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, et al. Hepatic resection in 485 R0 pT2 and pT3 cases of advanced carcinoma of the gallbladder: results of a Japanese Society of Biliary Surgery survey – a multicentre study. J Hepatobiliary Pancreat Surg. 2009;16:204–215. doi: 10.1007/s00534-009-0044-3. [DOI] [PubMed] [Google Scholar]
- 20.Goetze TO, Paolucci V. Benefit of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg. 2008;247:104–108. doi: 10.1097/SLA.0b013e318154bf5d. [DOI] [PubMed] [Google Scholar]
- 21.Scheingraber S, Justinger C, Stremovskaia T, Weinrich M, Igna D, Schilling MK. The standardized surgical approach improves outcome of gallbladder cancer. World J Surg Oncol. 2007;5:55–60. doi: 10.1186/1477-7819-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Araida T, Azuma T, Takasaki K. Bisubsegmental liver resection for gallbladder cancer. Hepatogastroenterology. 1998;45:14–19. [PubMed] [Google Scholar]
- 23.Marker AV, Butte JM, Oxemberg J, Kuk D, Gonen M, Fong Y, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 2012;19:409–417. doi: 10.1245/s10434-011-1850-9. [DOI] [PubMed] [Google Scholar]
- 24.Giuliante F, Ardito F, Vellone M, Clemente G, Nuzzo G. Port-sites excision for gallbladder cancer incidentally found after laparoscopic cholecystectomy. Am J Surg. 2006;191:114–116. doi: 10.1016/j.amjsurg.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478–1486. doi: 10.1007/s11605-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 26.De Arexabala X, Leon J, Hepp J, Maluenda F, Roa I. Gallbladder cancer: role of laparoscopy in the management of potentially resectable tumours. Surg Endosc. 2010;24:2192–2196. doi: 10.1007/s00464-010-0925-1. [DOI] [PubMed] [Google Scholar]
- 27.Smith G, Parks R, Madhavan K, Garden J. A 10-year experience in the management of gallbladder cancer. HPB. 2003;5:159–166. doi: 10.1080/13651820310000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–325. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 29.Shirai Y, Yoshida K, Tsukada K. Radical surgery for gallbladder carcinoma: longterm results. Ann Surg. 1992;216:565–568. doi: 10.1097/00000658-199211000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Longterm results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1992;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki M, Itoh H, Ambiru S. Radical surgery for advanced gallbladder carcinoma. Br J Surg. 1996;83:478–481. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- 32.Steinert R, Nestler G, Sagynaliev E. Laparoscopic cholecystectomy and gallbladder cancer. J Surg Oncol. 2006;26:682–689. doi: 10.1002/jso.20536. [DOI] [PubMed] [Google Scholar]
- 33.Shibata K, Uchida H, Iwaki K, Kai S, Otha M, Kitano S. Lymphatic invasion: an important factor for stage T1b–T3 gallbladder cancer and an indication for additional radical resection of incidental gallbladder cancer. World J Surg. 2009;33:1035–1041. doi: 10.1007/s00268-009-9950-4. [DOI] [PubMed] [Google Scholar]
- 34.Butte JM, Redondo F, Waugh E, Meneses M, Pruzzo R, Parada H, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB. 2009;11:585–591. doi: 10.1111/j.1477-2574.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla PJ, Barreto SG, Arya S, Shikhande SV, Hawaldar R, Purandare N, et al. Does PET-CT scan have a role prior to radical re-resection for incidental gallbladder cancer? HPB. 2008;10:439–445. doi: 10.1080/13651820802286910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GC): 10-year experience at Memorial Sloan–Kettering Cancer Center (MSKCC) J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 37.Zaydfudim V, Feurer ID, Kelly WJ, Wright PC. The impact of tumour extent (T stage) and lymph node involvement (N stage) on survival after surgical resection for gallbladder adenocarcinoma. HPB. 2008;10:420–427. doi: 10.1080/13651820802320057. [DOI] [PMC free article] [PubMed] [Google Scholar]


