Abstract
The maximal force a muscle produces depends among others on the length of the muscle and therefore on the positions of the joints the muscle crosses. Long and short toe flexor muscles (TFM) cross the ankle joints and metatarsal phalangeal joints (MPJ) and work against gravity during human locomotion. The purpose of this study was to describe the maximal moments around the MPJ during maximal voluntary isometric contractions (MVIC) of the TFM as a function of ankle joint and MPJ position. Twenty men performed MVIC of the TFM in a custom-made dynamometer. Ankle and MPJ angles were modified after each contraction. External moments of force around the MPJ were determined. Moments ranged between 6.3 ± 2.6 Nm and 14.2 ± 5.8 Nm. Highest moments were produced at 0°–10° ankle joint dorsal flexion and 25°–45° MPJ dorsal flexion. Lowest moments were generated at 35° ankle joint plantar flexion and 0° MPJ dorsal flexion. In conclusion, if the ankle is plantar-flexed, dorsal flexion of the MPJ avoids a disadvantage of the force–length relationship of TFM. Therefore, MPJ dorsal flexion is a necessary function in the push-off phase of human locomotion to work against the loss of the mechanical output at the forefoot caused by plantar flexion of the ankle.
Keywords: force–length relationship, metatarsal phalangeal joint, moment–angle relationship, muscle strength
Introduction
The propulsive forces in human locomotion mainly result from a push on the ground by the ankle plantar flexor muscles in late stance (Winter, 1980, 1983; Winter & Sienko, 1988; Pandy & Andriacchi, 2010). Beside the large triceps surae, the long toe flexor muscles (TFM) flexor hallucis longus and flexor digitorum longus generate an internal plantar flexion moment around the ankle and lift the heel from the ground. The long TFM are active in the mid-stance phase of walking (Fujita, 1985; Arndt et al. 2002) and are also responsible for load transmission from the shank to the ground (Ferris et al. 1995). Jacob (2001) estimated that the long TFM exert about 61% body weight during the second peak of the ground reaction force in the gait cycle. The long TFM originate in the calf, proceed over the ankle and metatarsal phalangeal joints (MPJ), insert on the distal phalanges and functionally achieve plantar flexion of the foot and/or the toes. The long TFM constitute nearly 10% of the physiological cross-sectional area of all shank muscles (Wickiewicz et al. 1983). The flexor hallucis longus muscle has the largest physiological cross-sectional area (muscle volume divided by fiber length) of long and short TFM (Friederich & Brand, 1990; Kura et al. 1997) and therefore might produce the largest forces.
In the push-off phase, the heel leaves the ground and MPJ dorsal flexion increases (Bojsen-Møller & Lamoreux, 1979; Leardini et al. 1999; Oleson et al. 2005). In this phase the resultant ground reaction force acts distal to the MPJ and causes an external dorsal flexion moment around the MPJ (Miyazaki & Yamamoto, 1993). In interaction with the long TFM, the major short TFM abductor hallucis, abductor digiti minimi, flexor digitorum brevis and quadratus plantae work against the external dorsal flexion moment by producing an internal plantar flexion moment around the MPJ. The short TFM are active when the heel leaves the ground and MPJ dorsal flexion increases (Mann & Inman, 1964). The short TFM have a stabilizing effect on the medial longitudinal arch. Fiolkowski et al. (2003) blocked the tibial nerve, which innervates the short TFM, and found an increase of longitudinal arch flattening when the activity of the intrinsic muscles decreased. The major short TFM originate at the calcaneus and insert on the proximal phalanges supporting the long TFM in plantar flexion of the toes. They provide nearly 80% of the physiological cross-sectional area of the foot muscles (Kura et al. 1997).
The maximal force a muscle can exert depends among others on the length of the muscle and therefore on the angles of the joints the muscle crosses (Blix, 1894; Gordon et al. 1966, Grieve et al. 1978). To estimate the net resultant mechanical output at a joint, many studies since the early 1950s describe moment–angle relationships around the hip, knee and ankle joint (Clarke et al. 1950; Elkins et al. 1951; Houtz et al. 1957; Williams & Stutzman, 1959; Campney & Wehr, 1965; Jensen et al. 1971; Murray et al. 1977; Fugl-Meyer et al. 1980). These moment–angle relationships are important to estimate the mechanical output at a joint, to design variable strength training, and to construct resistance-exercising machines. Moment–angle relationships (i) contain internal muscle forces of all synergists and antagonists as well as external resultant forces and moments, (ii) are regarded as a plot that describes the maximal muscular force capability at a joint as a function of joint angles in maximal voluntary isometric contractions (MVIC), and (iii) are best achieved by maximal isometric contractions, when the joint angle and therefore the length of the muscle are more or less constant.
The moment–angle relationship of a muscle about a joint is related to the force–length relationship of the contractile element of the muscle and the variable moment arm. The force–length relationship describes the maximal, active, isometric force a muscle can exert as a function of the length of the contractile elements of the muscle. Based on the cross-bridge theory (Huxley, 1957), Gordon et al. (1966) suggested experimentally that the length-dependence of muscle force is related to the amount of overlap between the actin and myosin filaments in the sarcomere. If muscles cross one joint at which they produce movements, length changes of a muscle are a function of the appropriate joint angle (Grieve et al. 1978). Among all the joints of the foot, the ankle and MPJ undergo the largest ranges of motion in the sagittal plane (Leardini et al. 1999). In the push-off phase of walking, running, sprinting and jumping, the ankle achieves plantar flexion while the MPJ effects dorsal flexion (Bobbert & van Ingen Schenau, 1988; Stefanyshyn & Nigg, 1998; Leardini et al. 1999). While moving in opposite directions, these joints may change length and therefore force potential of TFM. To the authors’ best knowledge, the dependence of muscle strength to joint angle position is a well known effect, but was not described for long and short TFM crossing the MPJ and ankle so far. However, the potential of TFM to produce force as a function of ankle and MPJ angle is unclear. Therefore, the purpose of the study was to analyse the moments around the MPJ during MVIC as a function of (i) ankle angle and (ii) MPJ angle. The moment–angle relationship in this article will be regarded as a plot that describes the maximal muscular force capability at a joint as a function of joint angles in maximal isometric contractions.
Materials and methods
Participants
For analysing the moment–angle relationship of long and short TFM, 20 male sport students (27 ± 3 years; 74 ± 6 kg) participated in this cross-sectional study. All participants had no medical afflictions and were given precise information about the content and order of the study. The study was conducted with the approval of the institution’s ethics committee.
Measurement of MPJ plantar flexion moments
In a custom-made dynamometer (Fig. 1), angles of the right ankle (20°, 35° plantar flexion; 0°, 10°, 20° dorsal flexion) and MPJ (0°, 25°, 35°, 45° dorsal flexion) were modified randomly and external moments of force around the MPJ during MVIC of TFM were determined. More precisely, the external moments of force around the transverse axis of the dynamometer represent the moments of force produced by the TFM. Subjects were seated upright on a chair without a backrest. The upper extremities were maintained in the crossed position in front of the chest during all MVIC. The left foot was positioned beside the testing apparatus. The right forefoot, midfoot and rearfoot were fixed in the dynamometer with Velcro® straps. The toes were placed on an angle adjustable plate, which was connected to a 1 kN strain-gauge load cell (DMS, HBM, Darmstadt; accuracy 99%) by a pulley (Ø 5.7 cm) and a teflon-coated wire. Raw data were amplified and digitized using an analog-to-digital card (6024E; National Instruments Corp., TX, USA). The digitized data were sampled by a personal computer using DasyLab (National Instruments Corp., TX, USA). The position of the first MPJ in the anterior-posterior direction was controlled with an adjustable heel cap at the back and a pin, which was directed to the first MPJ joint space at the height of the transverse axis of the dynamometer. Before starting the measurements, subjects conducted a warm-up period including three to five submaximal isometric contractions on the testing machine.
Fig. 1.
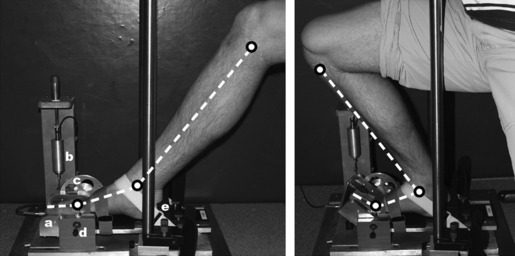
Dynamometer to determine external moments around the MPJ. Toes were positioned on an angle adjustable plate (a) connected to a load cell (b) by a pulley (c). First MPJ joint space was aligned with the transverse axis of the dynamometer by an external pin (d). The foot was fixed with a heel cap (e) and Velcro® straps. Left: Maximal MPJ and ankle plantar flexion angles due to short contractile elements. Right: Maximal MPJ and ankle dorsal flexion angles due to long contractile elements.
Before testing, subjects were asked to relax their foot muscles and the force signal caused by passive structures and fixation in the dynamometer was reset by the software. During MVIC, the subjects tried to pull down the plate with their toes against resistance within 3 s and to hold the peak plateau for 2–3 s. Maximal force was calculated as the mean value of a 2-s time window of the plateau region. Trials were repeated if a maximal effort was not sustained for the named period or when subjects judged the attempt to be less than maximal. Ankle angles in the sagittal plane were controlled with a motion analyses system (VICON Motion Systems, Nexus, Oxford, UK). Three retro-reflective, spherical markers (Ø 14 mm) were attached to the tibial epicondyle medialis, malleolus medialis and metatarsal I of the subject’s right leg (Fig. 1). The ankle angle was defined as the interior angle between the shank and the foot. The shank segment was defined as a line connecting the tibial epicondyle medialis and the malleolus medialis; the foot segment was defined as the line connecting the malleolus medialis and the metatarsal I. Trials were repeated if the ankle position did not remain at the predefined angle (± 2°), a maximal effort was not sustained for the named period or participants assessed the attempt to be less than maximal. The front plate angles of the dynamometer representing the MPJ angles were adjusted with the help of a measuring device on the dynamometer. Neutral position of both joints was achieved by adjusting the shank perpendicular to the longitudinal axis of the flattened foot. This position was set as the reference position (0°). All MVIC were separated by a rest period of at least 3 min. The order of testing MVIC in different joint angles was random to avoid effects of fatigue. The examiner instructed each subject to pull down only with the toes during MVIC and not to lift the heel of the foot being tested.
Reproducibility of MVIC of toe flexor muscles
In a pilot study the reproducibility of the external moments of force acting around the transverse axis of the dynamometer during MVIC of TFM was assessed with a group of 10 men performing two sets of three repeated MVIC a day. The best trial of each set was used for analysis.
Statistics
A paired t-test and the Pearson correlation coefficient were chosen to determine the differences between the trials. A General Linear Model for repeated measures was used to compare the main effects of ankle joint and MPJ position on the external moments around the MPJ. Main effects across the conditions were identified with the Bonferroni confidence interval. The level of significance was defined as α = 0.05.
Results
The external moments of force acting around the transverse axis of the dynamometer during MVIC of long and short TFM during two measurements a day showed no significant (P ≥ 0.05) differences between the trials. Correlation between trials within one day was indicated with r = 0.91 (P < 0.001) in 95% of the data (Fig. 2).
Fig. 2.
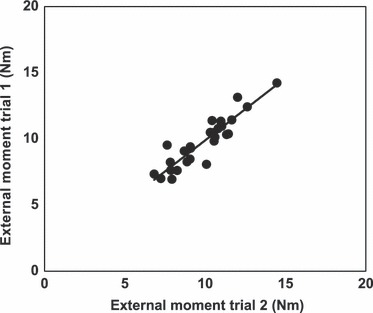
Reproducibility of the moments acting around the MPJ during two MVIC at neutral (0°) joint angles (r = 0.91).
Maximal MPJ plantar flexion moments produced by TFM as a function of ankle joint and MPJ position are shown in Fig. 3. Highest moments (14.2 ± 5.8 Nm and 14.2 ± 4.9 Nm) were measured at 0°–10° ankle dorsal flexion and 45° MPJ dorsal flexion. Lowest moments (6.3 ± 2.6 Nm) were measured at 35° ankle plantar flexion and 0° MPJ position. The difference between the highest and lowest moments produced by TFM was 7.9 Nm (P = 0.008). In 0° MPJ dorsal flexion, moments significantly decreased from 12.5 (± 4.7) Nm to 6.3 (± 2.6) Nm with increasing ankle plantar flexion angle (Fig. 3).
Fig. 3.
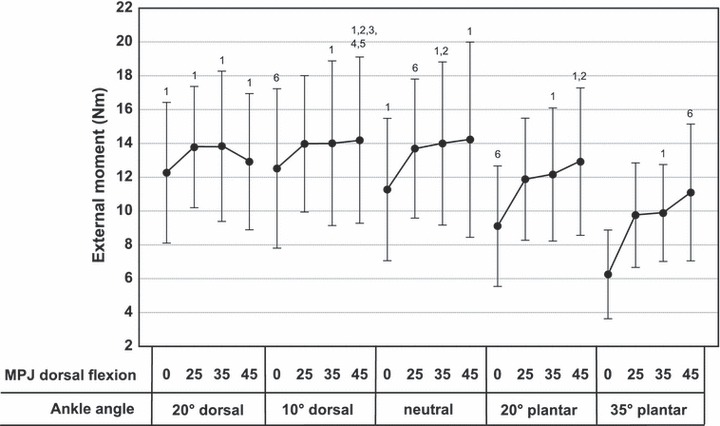
Mean external moments (± standard deviation) around the MPJ as a function of ankle joint and MPJ position. 1 – significant difference (P < 0.01) to 0° MPJ dorsal flexion and 35° ankle plantar flexion; 2 – significant difference (P < 0.01) to 25° MPJ dorsal flexion and 35° ankle plantar flexion; 3 – significant difference (P < 0.01) to 35° MPJ dorsal flexion and 35° ankle plantar flexion; 4 – significant difference (P < 0.05) to 45° MPJ dorsal flexion and 35° ankle plantar flexion; 5 – significant difference (P < 0.01) to 0° MPJ dorsal flexion and 20° ankle plantar flexion; 6 – significant difference (P < 0.05) to 0° MPJ dorsal flexion and 35° ankle plantar flexion.
In maximal ankle (20°) and MPJ (45°) dorsal flexion, moments declined to about 90% of the maximum (P > 0.05). In maximal ankle (35°) and MPJ (0°) plantar flexion, TFM produced 44% of the maximal voluntary moment (P < 0.001).
Based on the results of the study by Refshauge et al. (1995), external moments around the MPJ as a function of estimated fascicle length changes of flexor hallucis longus muscle are shown in Fig. 4. The rest length of flexor hallucis longus muscle was estimated with 19–25 cm (Wickiewicz et al. 1983; Friederich & Brand, 1990; Fukunaga et al. 1992). External moments around the MPJ increased with fascicle lengthening up to 15 mm and then decreased.
Fig. 4.
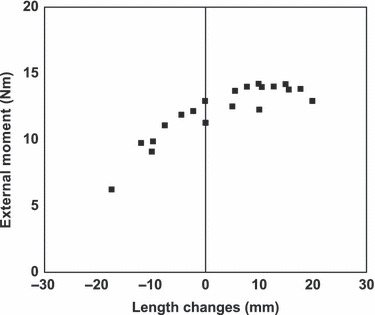
Estimation of fascicle rest length changes of flexor hallucis longus muscle in different joint angle positions based on the data by the study of Refshauge et al. (1995) as a function of external MPJ moments. Positive values indicate a lengthened muscle, negative values indicate a shortened muscle. Length changes of 0 mm indicate neutral ankle joint and 0° MPJ position.
Discussion
This study examined for the first time the moments of force around the MPJ caused by TFM as a function of joint angles. The purpose of this work was to investigate the change of the voluntary mechanical output of TFM at the MPJ as a function of ankle and MPJ angles. It was found that the highest moments (14.2 ± 5.8 Nm) were achieved in ankle and MPJ dorsal flexion and the lowest moments (6.3 ± 2.6 Nm) in ankle plantar flexion and MPJ neutral position. Previous studies (Senda et al. 1999; Nihal et al. 2002; Allen & Gross, 2003) determined forces produced by TFM at strain gauges or pressure sensors but the moments of force around the MPJ were not described, which makes a comparison with the results of our study difficult. A study by Endo et al. (2002) calculated TFM strength as the maximal voluntary moment developed in the sagittal plane by TFM about a reference axis through the first MPJ, while subjects stood on a force plate trying to reach as far forward as possible. The results described MPJ moments between 12 and 20 Nm, which conform to the voluntary produced maximal moments (14.2 ± 5.8 Nm) measured in our study. One of the first dynamic measurements of MPJ moments showed maximal moments in fast walking of 20 to 25 Nm (Miyazaki & Yamamoto, 1993).
The moment–angle relationship of muscles about joints is determined by the force–length properties of the muscles. Altering joint angles is directly linked to changing muscle length (Hawkins & Hull, 1990). Grieve et al. (1978) already investigated the changes in the length of muscles as a function of the angles of the joints they cross. For the flexor hallucis longus muscle, changes in fascicle length per degree MPJ rotation of 0.22 mm and per degree ankle rotation of 0.50 mm were calculated (Refshauge et al. 1995). According to this, in our study a range of MPJ dorsal flexion of 45° could correspond to a total change in flexor hallucis longus fascicle length of approximately 10 mm. A range of ankle rotation of 55° could correspond to a total change in flexor hallucis longus fascicle length of 27.5 mm. Approximately, total length changes of the flexor hallucis longus fascicles of nearly 40 mm could occur in maximal range of ankle and MPJ rotation (Fig. 4). Rest length changes of 27 mm resulted in moment changes of 8 Nm, or 44% of the maximum (0° MPJ dorsal flexion/35° ankle plantar flexion to 45° MPJ dorsal flexion/neutral ankle position). Arampatzis et al. (2006) showed that rest length changes of gastrocnemius medialis muscle of 25 mm resulted in ankle moment changes during MVIC of 70 Nm (63% of maximum). However, one should consider that the moments around the MPJ are produced by the flexor hallucis longus muscle as well as the flexor digitorum longus muscle and the short TFM.
When the long TFM were shortened in maximal ankle (35°) and MPJ (0°) plantar flexion, lowest moments (6.3 ± 2.6 Nm) around the MPJ were generated. Particularly with regard to the shortened flexor hallucis longus muscle and flexor digitorum longus muscle, we conclude that the moments around the MPJ of 6.3 (± 2.6) Nm were mainly produced by the short TFM. Therefore, in plantar flexion positions of the ankle and MPJ, the short TFM generated nearly 40% of the maximal voluntary moment at the MPJ. Then, if the ankle angle changed to 10° dorsal flexion and the long TFM were lengthened successively, the moments increased to 12.5 (± 4.7) Nm, or 88% of the maximal voluntary moment. If the MPJ angle additionally changed to 45° dorsal flexion, the highest moments (14.2 ± 4.9 Nm) were produced between 0° and 10° ankle dorsal flexion, and 25° and 45° MPJ dorsal flexion. We expect that in this joint angle configuration the myofilaments actin and myosin were optimally overlapped and could generate the highest forces. The pennation of the muscle plays an important part in transmitting forces from the contractile element to the tendon. Since TFM are pinnate muscles (Friederich & Brand, 1990; Fukunaga et al. 1992; Ledoux et al. 2001), another possible explanation for the different measured moments are angle changes of muscle pennation. The degree of pennation depends on muscle length (Woittiez et al. 1984) and influences the moment produced by a muscle at a joint. However, the pennation angles of long and short TFM are relatively small and the effect of the pennation angle on the force production does not seem to be great (Wickiewicz et al. 1983; Friederich & Brand, 1990; Ledoux et al. 2001). Therefore, one should consider that shortening or lengthening of TFM by changing joint angles could alter the pennation angle but would change the produced joint moments around the MPJ marginally.
For multiarticular muscles, it is suggested that shortening at one joint could be offset by lengthening at another (Motoshi et al. 2005). Because ankle and MPJ move in opposite directions during stance (Leardini et al. 1999), it is supposed that the flexor hallucis longus muscle enables energy transfer from the proximal leg extensor muscles into the distal foot segments (Kirane et al. 2008). If multiarticular muscles transfer the energy produced by uniarticular muscles to the ground (Zajac, 1993; Prilutsky & Zatsiorsky, 1994; Jacobs et al. 1996), one can assume that stronger TFM may increase the mechanical output at the forefoot and ankle and therefore enhance performance.
The maximal voluntary isometric force of TFM can be as large as 10–17% of the MVIC of ankle plantar flexor muscles (Fugl-Meyer et al. 1980; Gravel et al. 1990; Maganaris et al. 1998b; Harbo et al. 2012) and as large as 50–66% of ankle dorsal flexor muscles (Maganaris, 2001; Dragert & Zehr, 2011). In maximal ankle (20°) and MPJ (45°) dorsal flexion, the long and short TFM generated 90% of the maximal voluntary produced moment. We assume that in this joint angle configuration the contractile elements of TFM were lengthened marginally and produced slightly smaller forces than in moderate dorsal flexed ankle positions. It is possible that moments produced in these joint angle positions reflect a point on the descending limb of the force–length relationship of TFM. One can assume that altered ankle and MPJ angles mainly changed the length of the contractile elements of the long and short TFM and therefore the voluntary mechanical output at the MPJ.
If we assume that moment–angle relationships are representative of the force–length relationships, we should consider two things: 1) the moment at a given joint angle represents the net resultant mechanical output of all synergists and antagonists crossing the joint and 2) the muscle length and tendon moment arm length are non-linear functions of joint angle (Kawakami et al. 1998; Maganaris et al. 1998a, 1999). Considering the moment arm length, Rassier et al. (1999) concluded that the joint angle of maximal muscle force might not correspond to the joint angle of maximal moment. Here, the distance from the MPJ centre of rotation to the acting force exerted by the TFM was defined as the internal TFM moment arm at the MPJ. Although the excursion of the tendon was highly limited by the hallux sesamoids, the metatarsals and the plantar surface, tendon excursions of only 1 mm resulted in changes of the joint moment of 10% (presuming an internal moment arm length in rest of 1 cm).
The measured joint moments were the sum of the moments produced voluntarily by the activated agonist muscles minus the moments of the co-activated antagonist muscles. Since the maximal produced moment is a function of the given joint angle, the level of muscle activation and antagonist co-activation may change with joint angle. As a result, the agonist and antagonist muscle activation level could alter the shape of the moment–angle relationship and the angle at which maximal joint moments occurred. For the gastrocnemius muscle, it was found that muscle activity during MVIC decreased at a shortened muscle length (Cresswell et al. 1995; Arampatzis et al. 2006). This must be due not only to an impaired neuromuscular transmission but also to a decrease in the number of fibres within the EMG electrode recording volume. For the knee extensor muscles it has been reported that agonist activation (Newman et al. 2003) and antagonist co-activation (Kellis & Baltzopoulos, 1997) levels are more or less consistent across joint angles (O’Brien et al. 2009). Furthermore, during MVIC of the triceps surae muscle, the antagonist muscle was submaximally activated at all joint positions and did not influence the exerted moment (Arampatzis et al. 2006). However, no information about the activation level of toe flexor and extensor muscles during MVIC in different joint positions is currently available in the literature. Since it is not possible to measure muscle activity of all toe flexor and extensor muscles with surface electromyography, this study could provide no information about the muscle activity of agonist and antagonist muscles.
In human locomotion, the body is moved from one place to another. Caused by gravity, the external forces acting on the body are the reaction forces between the feet and the ground. During propulsion, the heel leaves the ground, the ankle plantar flexes and the MPJ achieves dorsal flexion. In fast running, the ankle starts plantar flexion after approximately 60% of stance (Stefanyshyn & Nigg, 1998; Kuitunen et al. 2002) and the MPJ becomes more and more dorsal flexed. In this phase, the point of force application moves distally to the MPJ axis and causes an external dorsal flexion moment around the joint. This dorsal flexion moment is actively balanced by a plantar flexion moment resulting from contractions of the TFM to regulate the angular momentum of the whole body (Miyazaki & Yamamoto, 1993). Especially when the body position and therefore the ground reaction force vector clearly points forward, e.g. in the early acceleration phase of fast running (Kugler & Janshen, 2010), a stable body posture must be maintained by regulating the angular momentum of the whole body (Fig. 5). As higher acceleration during running is mainly attained by applying a greater forward lean (Kugler & Janshen, 2010), MPJ dorsal flexion and TFM strength have the potential to regulate postural stability. The length of long and short TFM acting about the MPJ could be affected by the foot-to-ground interface. Modern footwear influences the maximal dorsal flexion of the MPJ in daily activities as well as in sport (Bojsen-Møller & Lamoreux, 1979; Stefanyshyn & Nigg, 2000). Bojsen-Møller & Lamoreux (1979) demonstrated that soft midsoles caused a greater amount of dorsal flexion of the toes at the MPJ in walking than conventional stiff midsoles did. Stiff shoe midsoles which reduce MPJ dorsal flexion, could affect TFM capacity. As stiff shoe midsole constructions and insoles decrease MPJ dorsal flexion (Bojsen-Møller & Lamoreux, 1979; Stefanyshyn & Nigg, 2000), the TFM are unable to work in their optimal muscle length to produce force, therefore impairing their ability to induce internal MPJ plantar flexion moments to control the angular momentum of the whole body. Considering this, the stiffening of shoe midsoles or insoles to decrease energy dissipation at the MPJ seems to be controversial (Stefanyshyn & Nigg, 2000).
Fig. 5.
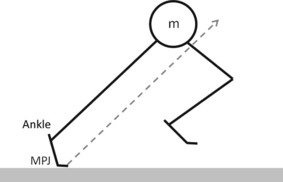
Schematic diagram of ankle plantar flexion and MPJ dorsal flexion of the support leg in high accelerating movements due to a forward lean of the body. The ground reaction force (dashed arrow) causes external moments around the MPJ and ankle and induces a moment acting about the centre of mass (m), generating an angular momentum of the whole body.
After discussing the results, one should consider that this study is not without limitations. First, not every participant could place the fifth toe on the front plate of the dynamometer. The reason is that toes and metatarsals are different in length between participants and the metatarsal phalangeal level has an oblique axis passing through the heads of the second and fifth metatarsal bones (Bojsen-Møller & Lamoreux, 1979), which is not conformable to the transverse axis of the dynamometer. Secondly, during MVIC in dynamometers, the measured moment–angle relationship can be affected by the shift in the ‘internal’ angle from the dynamometer’s ‘external’ angle (Arampatzis et al. 2004, 2005). These shifts are the result of deformation of the fixation on the dynamometer, deformation of the dynamometer itself, the participant’s soft tissue, and motions between forefoot and rearfoot. Thirdly, it is probable that the maximal moments are affected by the ability of the participants to recruit their TFM. The maximal voluntary push of the toes against resistance was a very unusual task for the subjects. Participants need some test trials before the measurements to familiarize with the apparatus and TFM activation.
Concluding remarks
In conclusion, the main results of the present work showed for the first time that TFM produce maximal voluntary moments around the MPJ in 0°–10° ankle dorsal flexion and 25°–45° MPJ dorsal flexion. If the ankle is plantar flexed, dorsal flexion of the MPJ avoids the disadvantage of the force–length relationship of TFM. Therefore, MPJ dorsal flexion is a necessary function in the push-off phase of human locomotion to work against the loss of the mechanical output at the forefoot caused by plantar flexion of the ankle.
Acknowledgments
The authors have no conflict of interest to declare.
Author contributions
Jan-Peter Goldmann was responsible for the conception and design of the study and acquisition, analysis and interpretation of data. Gert-Peter Brüggemann is Head of the Institute of Biomechanics and Orthopaedics and was included in the conception and design of the study and analysis and interpretation of data. All authors revised the article critically for important intellectual content and final approval.
References
- Allen RH, Gross MT. Toe flexors strength and passive extension range of motion of the first metatarsophalangeal joint in individuals with plantar fasciitis. J Orthop Sports Phys Ther. 2003;33:468–478. doi: 10.2519/jospt.2003.33.8.468. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, De Monte G, et al. Differences between measured and resultant joint moments during voluntary and artificially elicited isometric knee extension contractions. Clin Biomech (Bristol, Avon) 2004;19:277–283. doi: 10.1016/j.clinbiomech.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Morey-Klapsing G, Karamanidis K, et al. Differences between measured and resultant joint moments during isometric contractions at the ankle joint. J Biomech. 2005;38:885–892. doi: 10.1016/j.jbiomech.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Stafilidis S, et al. Effect of different ankle- and knee-joint positions on gastrocnemius medialis fascicle length and EMG activity during isometric plantar flexion. J Biomech. 2006;39:1891–1902. doi: 10.1016/j.jbiomech.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Arndt A, Ekenman I, Westblad P, et al. Effects of fatigue and load variation on metatarsal deformation measured in vivo during barefoot walking. J Biomech. 2002;35:621–628. doi: 10.1016/s0021-9290(01)00241-x. [DOI] [PubMed] [Google Scholar]
- Blix M. Die Lange und die Spannung des Muskels. Scand Arch Physiol. 1894;5:149–206. [Google Scholar]
- Bobbert MF, van Ingen Schenau GJ. Coordination in vertical jumping. J Biomech. 1988;21:249–262. doi: 10.1016/0021-9290(88)90175-3. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller F, Lamoreux L. Significance of free-dorsiflexion of the toes in walking. Acta Orthop Scand. 1979;50:471–479. doi: 10.3109/17453677908989792. [DOI] [PubMed] [Google Scholar]
- Campney HK, Wehr RW. Significance of strength variation through a range of joint motion. Phys Ther. 1965;45:773–779. doi: 10.1093/ptj/45.8.773. [DOI] [PubMed] [Google Scholar]
- Clarke HH, Elkins EC, Martin GM, et al. Relationship between body position and the application of muscle power to movements of the joints. Arch Phys Med Rehabil. 1950;31:81–89. [PubMed] [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Dragert K, Zehr EP. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res. 2011;208:217–227. doi: 10.1007/s00221-010-2472-3. [DOI] [PubMed] [Google Scholar]
- Elkins EC, Leden UM, Wakim KG. Objective recording of the strength of normal muscles. Arch Phys Med Rehabil. 1951;32:639–647. [PubMed] [Google Scholar]
- Endo M, Ashton-Miller JA, Alexander NB. Effects of age and gender on toe flexor muscle strength. J Gerontol A Biol Sci Med Sci. 2002;57:M392–M397. doi: 10.1093/gerona/57.6.m392. [DOI] [PubMed] [Google Scholar]
- Ferris L, Sharkey NA, Smith TS, et al. Influence of extrinsic plantar flexors on forefoot loading during heel rise. Foot Ankle Int. 1995;16:464–473. doi: 10.1177/107110079501600802. [DOI] [PubMed] [Google Scholar]
- Fiolkowski P, Brunt D, Bishop M, et al. Intrinsic pedal musculature support of the medial longitudinal arch: an electromyography study. J Foot Ankle Surg. 2003;42:327–333. doi: 10.1053/j.jfas.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. J Biomech. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Gustafsson L, Burstedt Y. Isokinetic and static plantar flexion characteristics. Eur J Appl Physiol. 1980;45:221–234. doi: 10.1007/BF00421330. [DOI] [PubMed] [Google Scholar]
- Fujita M. Role of the metatarsophalangeal (MTP) joints of the foot in level walking. Nippon Seikeigeka Gakkai Zasshi. 1985;59:985–997. [PubMed] [Google Scholar]
- Fukunaga T, Roy RR, Shellock FG, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;10:928–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel D, Richards CL, Filion M. Angle dependency in strength measurements of the ankle plantar flexors. Eur J Appl Physiol. 1990;61:182–187. doi: 10.1007/BF00357596. [DOI] [PubMed] [Google Scholar]
- Grieve DW, Cavanagh PR, Pheasant S. Prediction of gastrocnemius length from knee and ankle joint posture. In: Asmussen E, Jorgensen K, editors. Biomechanics. Baltimore: University Park Press; 1978. pp. 405–412. [Google Scholar]
- Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112:267–275. doi: 10.1007/s00421-011-1975-3. [DOI] [PubMed] [Google Scholar]
- Hawkins D, Hull ML. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J Biomech. 1990;23:487–494. doi: 10.1016/0021-9290(90)90304-l. [DOI] [PubMed] [Google Scholar]
- Houtz SJ, Lebow MJ, Beyer FR. Effect of posture on strength of the knee flexor and extensor muscles. J Appl Physiol. 1957;11:475–480. doi: 10.1152/jappl.1957.11.3.475. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Jacob HA. Forces acting in the forefoot during normal gait – an estimate. Clin Biomech (Bristol, Avon) 2001;16:783–792. doi: 10.1016/s0268-0033(01)00070-5. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Bobbert MF, van Ingen Schenau GJ. Mechanical output from individual muscles during explosive leg extensions: the role of biarticular muscles. J Biomech. 1996;29:513–523. doi: 10.1016/0021-9290(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Jensen RH, Smidt GL, Johnston RC. A technique for obtaining measurements of force generated by hip muscles. Arch Phys Med Rehabil. 1971;52:207–215. [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. The effects of antagonist moment on the resultant knee joint moment during isokinetic testing of the knee extensors. Eur J Appl Physiol Occup Physiol. 1997;76:253–259. doi: 10.1007/s004210050244. [DOI] [PubMed] [Google Scholar]
- Kirane YM, Michelson JD, Sharkey NA. Evidence of isometric function of the flexor hallucis longus muscle in normal gait. J Biomech. 2008;41:1919–1928. doi: 10.1016/j.jbiomech.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Kugler F, Janshen L. Body position determines propulsive forces in accelerated running. J Biomech. 2010;43:343–348. doi: 10.1016/j.jbiomech.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Kuitunen S, Komi PV, Kyrolainen H. Knee and ankle joint stiffness in sprint running. Med Sci Sports Exerc. 2002;34:166–173. doi: 10.1097/00005768-200201000-00025. [DOI] [PubMed] [Google Scholar]
- Kura H, Luo ZP, Kitaoka HB, et al. Quantitative analysis of the intrinsic muscles of the foot. Anat Rec. 1997;249:143–151. doi: 10.1002/(SICI)1097-0185(199709)249:1<143::AID-AR17>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Leardini A, Benedetti MG, Catani F, et al. An anatomically based protocol for the description of foot segment kinematics during gait. Clin Biomech (Bristol, Avon) 1999;14:528–536. doi: 10.1016/s0268-0033(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Ledoux WR, Hirsch BE, Church T, et al. Pennation angles of the intrinsic muscles of the foot. J Biomech. 2001;34:399–403. doi: 10.1016/s0021-9290(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Maganaris CN. Force-length characteristics of in vivo human skeletal muscle. Acta Physiol Scand. 2001;172:279–285. doi: 10.1046/j.1365-201x.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. J Physiol. 1998a;510(Pt 3):977–985. doi: 10.1111/j.1469-7793.1998.977bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Differences in human antagonistic ankle dorsiflexor coactivation between legs; can they explain the moment deficit in the weaker plantarflexor leg? Exp Physiol. 1998b;83:843–855. doi: 10.1113/expphysiol.1998.sp004164. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in the tibialis anterior tendon moment arm from rest to maximum isometric dorsiflexion: in vivo observations in man. Clin Biomech (Bristol, Avon) 1999;14:661–666. doi: 10.1016/s0268-0033(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Mann R, Inman VT. Phasic activity of intrinsic muscles of the foot. J Bone Joint Surg Am. 1964;46:469–481. [PubMed] [Google Scholar]
- Miyazaki S, Yamamoto S. Moment acting at the metatarsophalangeal joints during normal barefoot level walking. Gait & Posture. 1993;1:133–140. [Google Scholar]
- Motoshi K, Azim J, Leonard TR, et al. Multifunctionality of the cat medial gastrocnemius during locomotion. J Biomech. 2005;38:1291–1301. doi: 10.1016/j.jbiomech.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Murray MP, Baldwin JM, Gardner GM, et al. Maximum isometric knee flexor and extensor muscle contractions: normal patterns of torque versus time. Phys Ther. 1977;57:637–643. doi: 10.1093/ptj/57.6.637. [DOI] [PubMed] [Google Scholar]
- Newman SA, Jones G, Newham DJ. Quadriceps voluntary activation at different joint angles measured by two stimulation techniques. Eur J Appl Physiol. 2003;89:496–499. doi: 10.1007/s00421-003-0836-0. [DOI] [PubMed] [Google Scholar]
- Nihal A, Goldstein J, Haas J, et al. Toe flexor forces in dancers and non-dancers. Foot Ankle Int. 2002;23:1119–1123. doi: 10.1177/107110070202301207. [DOI] [PubMed] [Google Scholar]
- O’Brien TD, Reeves ND, Baltzopoulos V, et al. The effects of agonist and antagonist muscle activation on the knee extension moment–angle relationship in adults and children. Eur J Appl Physiol. 2009;106:849–856. doi: 10.1007/s00421-009-1088-4. [DOI] [PubMed] [Google Scholar]
- Oleson M, Adler D, Goldsmith P. A comparison of forefoot stiffness in running and running shoe bending stiffness. J Biomech. 2005;38:1886–1894. doi: 10.1016/j.jbiomech.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng. 2010;12:401–433. doi: 10.1146/annurev-bioeng-070909-105259. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Zatsiorsky VM. Tendon action of two-joint muscles: transfer of mechanical energy between joints during jumping, landing, and running. J Biomech. 1994;27:25–34. doi: 10.1016/0021-9290(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rassier DE, MacIntosh BR, Herzog W. Length dependence of active force production in skeletal muscle. J Appl Physiol. 1999;86:1445–1457. doi: 10.1152/jappl.1999.86.5.1445. [DOI] [PubMed] [Google Scholar]
- Refshauge KM, Chan R, Taylor JL, et al. Detection of movements imposed on human hip, knee, ankle and toe joints. J Physiol. 1995;488:231–241. doi: 10.1113/jphysiol.1995.sp020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda M, Takahara Y, Yagata Y, et al. Measurement of the muscle power of the toes in female marathon runners using a toe dynamometer. Acta Med Okayama. 1999;53:189–191. doi: 10.18926/AMO/31617. [DOI] [PubMed] [Google Scholar]
- Stefanyshyn DJ, Nigg BM. Dynamic angular stiffness of the ankle joint during running and sprinting. J Appl Physiol. 1998;14:292–299. doi: 10.1123/jab.14.3.292. [DOI] [PubMed] [Google Scholar]
- Stefanyshyn DJ, Nigg BM. Influence of midsole bending stiffness on joint energy and jump height performance. Med Sci Sports Exerc. 2000;32:471–476. doi: 10.1097/00005768-200002000-00032. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, Roy RR, Powell PL, et al. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275–283. [PubMed] [Google Scholar]
- Williams M, Stutzman L. Strength variation through the range of joint motion. Phys Ther Rev. 1959;39:145–152. doi: 10.1093/ptj/39.3.145. [DOI] [PubMed] [Google Scholar]
- Winter DA. Energetics of the mechanics of human locomotion. J Am Osteopath Assoc. 1980;80:295–297. [PubMed] [Google Scholar]
- Winter DA. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin Orthop Relat Res. 1983;175:147–154. [PubMed] [Google Scholar]
- Winter DA, Sienko SE. Biomechanics of below-knee amputee gait. J Biomech. 1988;21:361–367. doi: 10.1016/0021-9290(88)90142-x. [DOI] [PubMed] [Google Scholar]
- Woittiez RD, Huijing PA, Boom HB, et al. A three-dimensional muscle model: a quantified relation between form and function of skeletal muscles. J Morphol. 1984;182:95–113. doi: 10.1002/jmor.1051820107. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle coordination of movement: a perspective. J Biomech. 1993;26(Suppl 1):109–124. doi: 10.1016/0021-9290(93)90083-q. [DOI] [PubMed] [Google Scholar]


