Abstract
A.SW and B10.S mice share the same major histocompatibility complex (MHC) haplotype (H-2s). However, A.SW mice are susceptible to experimental autoimmune myocarditis (EAM) and develop severe disease after immunization with myosin, whereas B10.S mice are resistant. We found that naive A.SW mice have intrinsically increased total CD4+ T cell counts and increased proportions of CD4+ T cells in their spleens compared to B10.S mice. Among total CD4+ T cells, naive A.SW mice have a lower relative frequency of forkhead box protein 3 (FoxP3+)CD25+ regulatory T cells (Tregs). A.SW mice also had a higher proportion of CD4+ T cells and a lower proportion of Tregs in their hearts and spleen during EAM, with greater T cell activation and proliferation, compared to B10.S mice. These differences in the T cell compartment were not antigen-specific, as ovalbumin/complete Freund's adjuvant (OVA/CFA) or CFA immunization elicited the same differences in CD4+ T cells and Tregs between A.SW and B10.S mice. Moreover, A.SW mice had more T helper type 17 (Th17) cells and B10.S had more Th1 cells in their hearts. The higher percentage of CD4+ T cells and their enhanced potential to differentiate towards the Th17 pathway was also observed in naive A.SW mice. Interleukin (IL)-6 is required for Th17 induction. Interestingly, IL-6Rα expression was greater on naive A.SW CD4+ T cells, compared to B10.S CD4+ T cells, indicating that this intrinsic difference, together with a relatively lower Treg proportion of CD4+ T cells, might lead to heightened Th17 responses and greater susceptibility to autoimmunity in A.SW mice.
Keywords: autoimmunity, experimental autoimmune myocarditis, IL-6 receptor, Th1, Th17
Introduction
Susceptibility to experimental autoimmune myocarditis (EAM) is genetically determined not only by major histocompatibility complex (MHC) [1], but influenced strongly by non-MHC genes [2,3]. We used two mouse strains (A.SW and B10.S) that share the same MHC haplotype (H-2s) to elucidate the role of non-MHC genes. A.SW mice are susceptible to myocarditis, whereas B10.S mice are resistant [4,5].
Autoimmune myocarditis is mediated by CD4+ T cells. Transfer of CD4+ T cells to severe combined immunodeficient (SCID) mice produced disease, while depletion of CD4+ T cells ameliorated EAM [6]. We have found recently that both T helper 1 (Th1) and Th17 cells infiltrate hearts of wild-type (WT) BALB/c mice after myocarditis induction [7]. About 20% of interleukin (IL)-17A+CD4+ T cells in the hearts of WT BALB/c mice on day 21 of EAM co-produce tumour necrosis factor (TNF)-α and IL-6, reinforcing the highly proinflammatory phenotype of these cells [7]. Both Th1 and Th17 myosin-specific cells are able to induce myocarditis; however, neither IL-17A nor interferon (IFN)-γ is essential for EAM development, as knock-out mice for either of these cytokines still develop myocarditis [7]. In addition, mice deficient in retinoic acid-related orphan receptor (ROR)-γt, the transcription factor essential for differentiation of Th17 cells and production of all Th17 cytokines, were resistant to EAM development [8]. Th17 cell differentiation in vitro depends on the presence of transforming growth factor (TGF)-β and IL-6 [9,10]. IL-6 is considered an important mediator of the inflammatory response in many autoimmune diseases, such as systemic lupus erythematosus [11], rheumatoid arthritis [12] and cardiac myxomas, which are benign tumours often accompanied with autoimmunity clearance during Coxsackie-induced myocarditis [13]. IL-6 is essential for EAM development; however, IL-6 is essential for virus clearance during CVB3-induced myocarditis [14,15].
In this report, we concentrate on differences between A.SW and B10.S mice in the CD4+ T cell-mediated immune responses with particular attention to imbalances of Th1, Th17 and regulatory T cells (Tregs) that may affect susceptibility to EAM.
Materials and methods
Mice, EAM induction and evaluation
A.SW and B10.S mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA), housed and bred in our facilities under specific pathogen-free conditions. The experiments reported herein were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals[16]. All procedures were approved by the Johns Hopkins Animal Care and Use Committee. EAM was induced as described previously [2]. Briefly, myosin (300 µg/mouse/injection) emulsified in complete Freund's adjuvant (CFA; Sigma-Aldrich, St Louis, MO, USA) supplemented with Mycobacterium tuberculosis strain H37Ra (total 5 mg/ml) was injected subcutaneously (s.c.) on days 0 and 7, respectively. On day 0, mice also received an intraperitoneal (i.p.) injection of 500 ng of pertussis toxin (Sigma-Aldrich). Mock-immunized mice injected with phosphate-buffered saline (PBS)/CFA and pertussis toxin were used as controls, as were naive unimmunized animals. Myocarditis severity was evaluated at the peak of disease on day 21. Hearts were cut and stained with haematoxylin and eosin (H&E), according to standard protocol (Histoserv, Inc., Germantown, MD, USA) [17]. The degree of EAM was scored based on the percentage of the area of leucocyte-infiltrated myocardium, as follows: grade 0 = no infiltration, grade 1 = < 10%, grade 2 = 10–30%, grade 3 = 30–50%, grade 4 = 50–90% and grade 5 ≥ 90% [2].
Flow cytometry analysis
Isolated splenocytes were stained with fluorochrome-conjugated antibodies against CD3, CD4, CD8α, CD19, CD11b, CD11c, F4/80 and DX5 (eBioscience, San Diego, CA, USA).
For intracardiac flow cytometry, the aorta was cannulated and hearts were perfused at a constant flow of 14 ml/min with cold PBS (Biofluids, Rockville, MD, USA) for 2 min followed by collagenase II (1 mg/ml; Sigma-Aldrich) and protease XIV (0·5 mg/ml; Sigma-Aldrich) for 5 min at 37°C, as described previously [18,19]. Isolated cells were restimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (250 ng/ml) (Sigma-Aldrich) and GolgiStop (BD Biosciences) were added for 5 h. Cells were stained with fluorochrome-conjugated antibodies against CD4, CD25 and CD45 and fixed according to manufacturing protocols (BD Biosciences). Cells were then stained with antibodies to forkhead box protein 3 (FoxP3), IL-17A and IFN-γ (eBiosciences).
Cells were acquired by the fluorescence activated cell sorter (FACS)Calibur flow cytometer and LSRII flow cytometer (eBiosciences, Franklin Lakes, NJ, USA) and data were analysed using FlowJo 7·5 (Treestar Software Ashland, OR, USA).
Proliferation assay
For measurement of proliferation in vivo, all mice were given one i.p. injection of 2 mg bromodeoxyuridine (BrdU) solution (eBiosciences, Franklin Lakes, NJ, USA). BrdU incorporation was examined 24 h post-injection by staining with antibodies against BrdU, CD4 and CD8α.
In-vitro differentiation of Th cells
In-vitro differentiation of naive CD4+ T cells to different Th subtypes has been described previously [20]. Briefly, naive CD4+ T cells were isolated from spleens of A.SW and B10.S mice using the CD4+CD62L+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were plated at 2 × 105/200 µl in 96-well, flat-bottomed plates and stimulated with 2 µg/ml plate-bound anti-CD3 and 1 µg/ml soluble anti-CD28 monoclonal antibodies (mAbs) (BD Pharmingen). Th1 cells were induced by the addition of 10 ng/ml recombinant (r)IL-12p70 (R&D, Minneapolis, MN, USA) to the culture. Th17 cells were induced by adding a combination of 20 ng/ml rIL-6 + 1 ng/ml human rTGF-β1 + 10 µg/ml anti-IFN-γ mAb (R&D) to the culture. Cells were analysed by flow cytometry 4 days later using intracellular staining for IL-17A and IFN-γ.
Statistical analysis
Significant differences (P < 0·05) were calculated with Prism version 5·0 software (GraphPad, La Jolla, CA, USA) using a Students' two-tailed t-test, with the exception of the EAM score, for which a Mann–Whitney U-test was used.
Results
A.SW mice develop severe myosin-induced myocarditis while B10.S mice are resistant
Unlike most other autoimmune diseases, in myocarditis non-MHC genes seem to have a greater impact on disease susceptibility than do MHC genes [4]. When we induced myocarditis in A.SW and B10.S mice congenic at the MHC locus (H-2s) by immunization with cardiac myosin in CFA, A.SW mice developed severe myocardial infiltration, composed mainly of mononuclear cells with extensive myocyte necrosis. In contrast, B10.S mice developed minimal myocardial infiltration with a few small, circumscribed foci of infiltrating cells and a significantly lower histology score (Fig. 1a,b). The heart weight to body weight (HW/BW) ratio of A.SW mice with EAM was significantly higher than that of immunized B10.S mice hearts (Fig. 1c). Thus, non-MHC genes participate in regulating susceptibility to EAM.
Fig. 1.

A.SW mice develop more severe experimental autoimmune myocarditis (EAM) than B10.S mice. (a) Heart sections were stained by haematoxylin and eosin (H&E) for histological analysis in ×5 (left) and ×100 (right) magnification under light microscopy at day 21: examples of A.SW and B10.S complete Freund's adjuvant (CFA) alone mock-immunized controls and A.SW and B10.S hearts immunized with myosin/CFA. Myocarditis severity (b) and heart weight/body weight ratios (c) are shown for A.SW and B10.S mice immunized with CFA alone or with myosin/CFA. Symbols denote individual mice and lines represent the median value. Statistics are by Mann–Whitney U-test. Data are representative of three independent experiments with four to 11 mice per group.
Naive A.SW mice have a higher frequency of CD4+ T cells in their spleens compared to B10.S mice
Intrinsic differences in the immune system between mouse strains may influence susceptibility to EAM. Therefore, we assessed various leucocyte populations in the spleens of naive A.SW and B10.S mice. There were no significant differences in the total numbers of cells in the spleens of naive A.SW and B10.S mice, although A.SW mice had a tendency towards increased splenocyte numbers (Fig. 2a). The total number of CD4+ T cells was increased in naive A.SW spleens (Fig. 2c, left). To ensure that the increase in absolute numbers of CD4+ T cells is not due to the tendency of A.SW mice to have more splenocytes, we also calculated the relative proportion of CD4+ T cells. We found that a greater proportion of A.SW spleens was taken up by CD4+ T cells compared to B10.S mice (Fig. 2c, right). The absolute number of CD25+FoxP3+CD4+ Treg cells was not significantly different, despite a tendency towards increase in A.SW mice. However, the relative proportion of Tregs was decreased significantly in naive A.SW spleens, compared to naive B10.S mice (Fig. 2d). Similarly, the proportion of CD8+ T cells was also significantly lower in naive A.SW mice (Fig. 2b), with no difference in absolute counts (not shown).
Fig. 2.
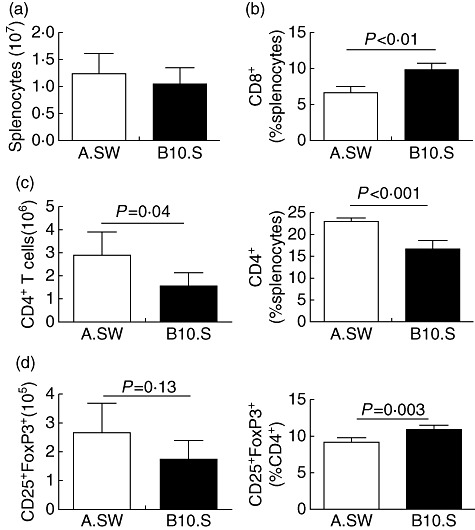
Naive A.SW mice increased relative frequency of CD4+ T cells compared to B10.S spleens. (a) Number of total splenocytes in naive A.SW and B10.S mice. (b) The percentage of CD8+ T cells in the spleen of naive A.SW and B10.S mice. (c) The absolute count and relative proportion of CD4+ T cells in the spleen of naive A.SW and B10.S mice. (d) The total counts and percentages of CD4+-gated CD25+forkhead box protein 3 (FoxP3+) T cells in the spleen of naive A.SW and B10.S mice. Data are presented as the mean ± standard deviation. Statistics are by two-tailed Student's t-test. Data depict one representative experiment of three conducted with five mice per group.
We did not find any significant differences in the frequencies or absolute numbers of CD19+ B cells, CD11b+F4/80– monocytes, CD11b+F4/80+ macrophages, CD11chi dendritic cells, DX5+CD3– natural killer (NK) cells or DX5+CD3+ NK T cells in spleens of naive A.SW and B10.S mice (data not shown). Thus, naive A.SW mice have more CD4+ T cells in relative proportion as well as in absolute numbers, while CD4+CD25+FoxP3+ Treg cells are relatively decreased in naive A.SW mice.
A.SW mice have an increased frequency of CD4+ T cells in the spleen and heart compared to B10.S mice during EAM
To examine whether differences in T cell profiles between A.SW and B10.S mice are also apparent during the development of EAM, we examined CD4+ T cell, CD4+ CD25+ FoxP3+ Treg and CD8+ T cell changes in the spleens of A.SW and B10.S mice at different times after induction of EAM. There were no significant differences in the total number of splenocytes in A.SW and B10.S mice on days 0, 7, 14 and 21 of EAM (not shown). The two strains showed similar kinetics of total cell expansion, with the highest numbers of splenocytes on day 14 after immunization (Fig. 3a). The relative proportion (Fig. 3b) as well as total number (not shown) of CD4+ T cells was significantly higher during the whole course of disease in A.SW, compared to B10.S mice. The only exception was on day 21, when the total CD4+ count was not different between A.SW and B10.S spleens (not shown); CD4+ T cells still constituted a greater proportion of A.SW spleens at this time-point (Fig. 3b). We did not observe the same pattern in CD8+ T cells (not shown). The total number of Tregs was increased significantly in A.SW spleens on day 7 of EAM compared to B10.S mice (not shown). However, when we compared the relative proportion of Tregs as a proportion of CD4+ T cells during EAM, we found that similar to naive mice, A.SW spleens had a significantly lower proportion of Tregs on days 14 and 21 of EAM (Fig. 3d). To assess in-vivo proliferation, we injected BrdU into myosin-immunized A.SW and B10.S mice 1 day before killing (day 20) and analysed CD4+ T cell proliferation on day 21. The number of CD4+BrdU+ T cells in A.SW mice was increased significantly compared to B10.S mice (Fig. 3e).
Fig. 3.
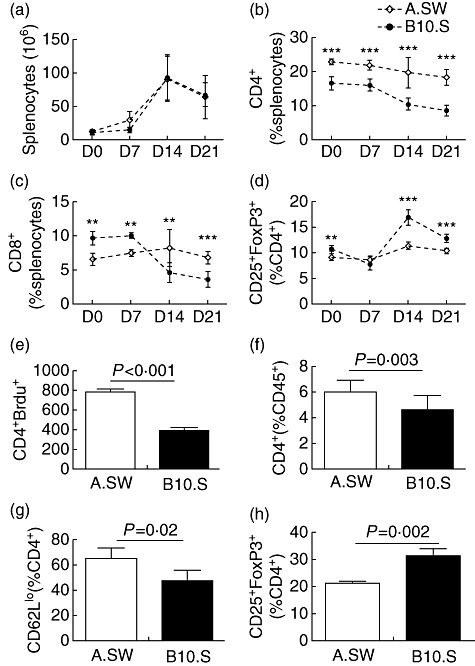
A.SW mice have an increased frequency of CD4+ T cells in the spleen and heart during experimental autoimmune myocarditis (EAM) development, compared to B10.S mice. (a) The kinetics of number of splenocytes in A.SW and B10.S mice from days 0 to 21 after immunization. (b) The kinetics of the relative frequency of CD4+ T cells in the spleen of A.SW and B10.S mice from days 0 to 21 after immunization. (c) The kinetics of the relative frequency of CD8+ T cells in the spleen of A.SW and B10.S mice from days 0 to 21 after immunization. (d) The kinetics of the relative frequency of regulatory T cells (Tregs) in the spleen of A.SW and B10.S mice from days 0 to 21 after immunization. (e) CD4+ T cells incorporating bromodeoxyuridine (BrdU) on day 20 of EAM in A.SW and B10.S mice. The total count of CD4+ T cells incorporating BrdU differed significantly between A.SW and B10.S (P < 0·001). (f) Frequency of CD4+ T cells in the hearts of A.SW and B10.S mice during EAM. (g) Activated CD4+ T cells in the heart were determined by surface CD62Llo expression in A.SW and B10.S mice. (h) The relative frequency of CD25+forkhead box protein 3 (FoxP3+) regulatory T cells (Tregs) gated on CD4+ T cells in the heart of A.SW and B10.S mice on day 21 after immunization. Data are presented as the mean of each group ± standard deviation. Shown are representative data from three independent experiments.
We examined heart-infiltrating T cells on day 21 of EAM at the peak of the disease. A.SW mice showed a greater frequency of CD4+ T cells in their hearts, as assessed by flow cytometry (Fig. 3f). A.SW heart-infiltrating CD4+ T cells expressed lower levels of CD62L when compared with B10.S CD4+ T cells, indicating that they were more activated (Fig. 3g). We also found a lower relative frequency of CD25+FoxP3+ Treg cells in the hearts of A.SW mice as a proportion of cardiac CD4+ T cells (Fig. 3h). Thus, A.SW mice had increased percentages of CD4+ T cells during the whole course of EAM in their spleen and hearts, and had decreased frequencies of Tregs in their spleens and hearts. Additionally, A.SW CD4+ T cells were more activated and proliferated more vigorously during EAM than comparable B10.S CD4+ T cells.
The increased frequency of CD4+ T cells in A.SW mice is not myosin-specific
To investigate if the increased percentage of CD4+ T cells and decreased frequency of Tregs could be observed after in-vivo injection with a different antigen, we immunized both strains of mice with either 100 µg ovalbumin (OVA) emulsified with CFA or CFA alone on days 0 and 7, and isolated their splenocytes on day 14. Interestingly, OVA immunization increased total numbers of splenocytes significantly in B10.S mice, compared to A.SW mice. However, the expansion did not affect the T cell compartment, as there were no differences in total CD4+ T cells or total Tregs between the two strains. The expansion of B10.S splenocytes was due to the increased number of myeloid cells. Immunization with CFA alone did not change the total number of splenocytes or the total number of CD4+ T cells in either strain (not shown).
However, similar to EAM and naive mice, A.SW mice immunized with OVA/CFA had increased percentages of spleen CD4+ T cells (Fig. 4a) and lower frequencies of Tregs (Fig. 4b). We also observed the same trends in mock-immunized A.SW mice given CFA alone (Fig. 4c,d). Thus, the greater percentage of CD4+ T cells and decreased percentages of Tregs out of CD4+ T cells in A.SW mice is an intrinsic feature of the immune response of that strain and is independent of which antigen is used.
Fig. 4.
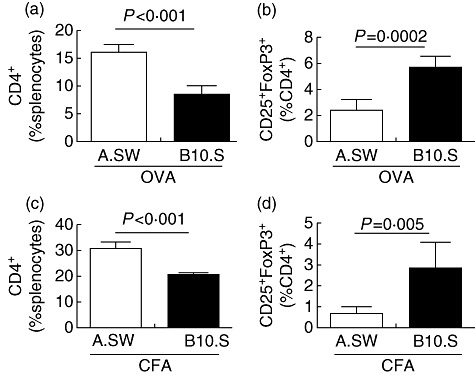
The increased relative frequency of CD4+ T cells in A.SW mice is not antigen-specific. A.SW and B10.S mice were immunized with ovalbumin (OVA) emulsified in complete Freund's adjuvant (CFA). The relative frequency of CD4+ T cells (a) and regulatory T cells (Tregs) (b) were measured by flow cytometry on day 14. A.SW and B10.S mice were immunized with CFA only. The relative frequency of CD4+ T cells (c) and Tregs (d) was measured by flow cytometry on day 14. Data are presented as the mean of each group ± standard deviation. Shown are representative data from three independent experiments.
Heart-infiltrating A.SW CD4+ T cells produce more IL-17A and less IFN-γ compared to B10.S CD4+ T cells
Differentiation of naive CD4+ T cells into functionally distinct Th1 or Th17 cell subsets may affect the pathogenic outcome of an autoimmune response. We measured intracardiac IL-17A- and IFN-γ-producing CD4+ T cells by flow cytometry on day 21 of EAM. The frequencies and absolute numbers of IL-17A+CD4+ T cells were significantly greater in the hearts of A.SW mice compared to B10.S mice (Fig. 5a–c). In contrast, B10.S mice demonstrated increased percentages and absolute numbers of IFN-γ+CD4+ T cells compared to A.SW mice (Fig. 5a–c). Levels of IL-23, a key cytokine for maintaining Th17 cells, were higher in the heart tissue of A.SW than B10.S mice on day 21 (Fig. 5d). Thus, A.SW mice exhibit greater Th17 responses in their hearts during EAM, while B10.S mice have a stronger Th1 response.
Fig. 5.
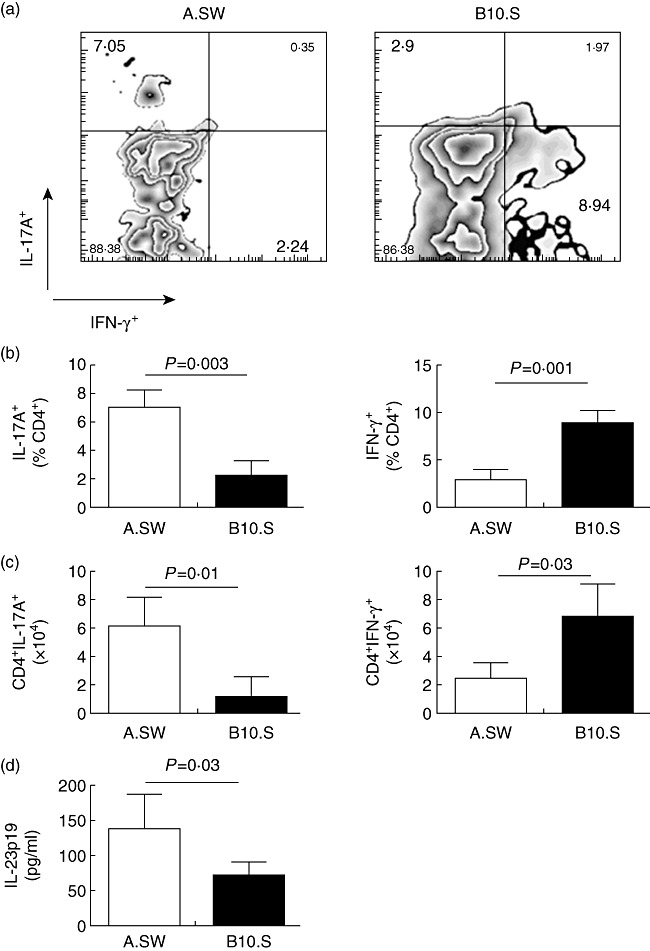
Heart-infiltrating A.SW CD4+ T cells produce more interleukin (IL)-17A and less interferon (IFN)-γ compared to B10.S CD4+ T cells. (a) Representative bivariate dot-plots of IL-17A and IFN-γ staining from A.SW and B10.S mice. (b) Relative frequencies of IL-17A+ T helper type 17 (Th17) cells and IFN-γ+ Th1 cells as a proportion of CD4+-gated cells were analysed by flow cytometry. (c). The absolute counts of CD4+IL-17A+ and CD4+IFN-γ+ cells were analysed by flow cytometry. (d) IL-23 was higher in the heart tissue of A.SW mice than B10.S by enzyme-linked immunosorbent assay (ELISA). Data are presented as the mean ± standard deviation. Statistics are by two-tailed Student's t-test. Data depict one representative experiment of three conducted with five mice per group.
Naive A.SW CD4+ T cells differentiate towards Th17 cells and express more IL-6Ra than B10.S CD4+ T cells
To determine whether the differences in CD4+ T cell differentiation in EAM-susceptible and -resistant mice represent an intrinsic difference in the ability of CD4+ T cells to commit to the Th1 or Th17 lineages, we differentiated naive spleen-derived A.SW and B10.S CD4+ T cells towards Th1 or Th17 pathways in vitro. Isolated naive CD4+ T cells were stimulated with anti-CD3 + anti-CD28 antibodies and exogenous cytokines for 4 days. In response to Th17 conditioning, a greater proportion of CD4+ T cells from A.SW mice produced IL-17A and expressed ROR-γt compared to B10.S mice (Fig. 6a–c). In response to Th1 conditioning, a greater fraction of B10.S CD4+ T cells expressed IFN-γ compared to A.SW CD4+ T cells (Fig. 6a,b). IL-6 is critical for the development of Th17 cells; therefore, we investigated whether IL-6Rα expression contributes to the increased tendency of A.SW CD4+ T cells to differentiate towards a Th17 phenotype. The proportion, as well as the mean fluorescence index of IL-6Rα-expressing naive A.SW CD4+ T cells, was higher than in B10.S CD4+ T cells (Fig. 6d).
Fig. 6.
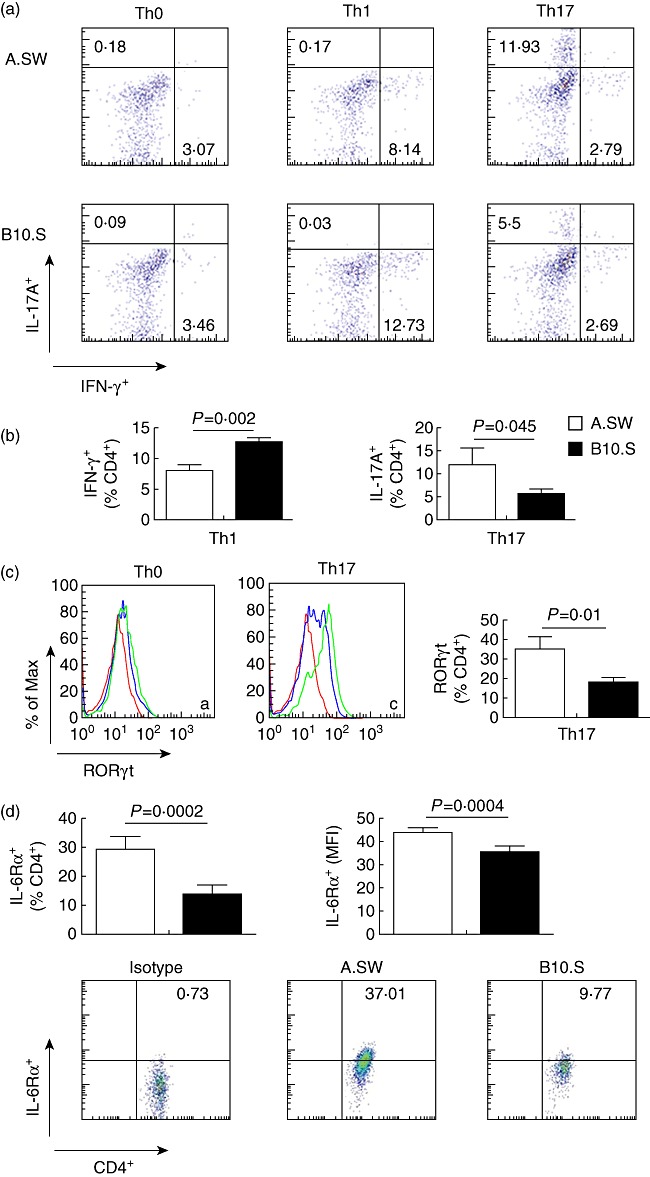
Naive A.SW CD4+ T cells differentiate more towards T helper type 17 (Th17) phenotype and express more interleukin (IL)-6Ra than B10.S CD4+ T cells. (a) Naive CD4+ T cells from A.SW and B10.S mice were cultured in Th1 or Th17-polarizing conditions. (b) The relative frequency of IL-17A+ cells gated on CD4+ T cells was higher in A.SW than B10.S in Th17-polarized conditions. The relative frequency of interferon (IFN)-γ+ cells gated on CD4+ T cells was lower in A.SW than B10.S in the Th1-skewing condition. (c) The expression of retinoic acid-related orphan receptor (ROR)-γt on naive CD4+ T cells or Th17-polarized cells from A.SW and B10.S mice (red line: isotype control, blue line: B10.S; green line: A.SW). The percentage of ROR-γt expressed on CD4+ T cells is shown. (d) Expression of IL-6Rα on naive CD4+ T cells from blood of A.SW and B10.S mice was measured by flow cytometry: the frequencies of IL-6Rα-positive events, gated on CD4+ T cells, was significantly higher in A.SW than B10.S; mean fluorescence index (MFI) of IL-6Rα, gated on CD4+ T cells, was significantly higher in A.SW than B10.S; representative dot-plots of isotype control, A.SW, B10.S. Data are presented as the mean ± standard deviation. Data depict one representative of three independent experiments.
Discussion
As we have demonstrated in this and previous reports, A.SW mice are more susceptible to EAM than are B10.S mice, even though they share the same H-2S MHC haplotype [3]. Greater susceptibility of A.SW mice to an autoimmune disease, compared to B10.S mice, is not unique to EAM, because similar differences in susceptibility in the same mouse strains were reported in a scleroderma model [21,22].
Using bone marrow chimeric mice, we have shown previously that genetic susceptibility of myocarditis is controlled by donor-derived haematopoietic cells, and that T and B lymphocytes were indispensable for transferring the susceptible phenotype to disease-resistant recipients [17]. To continue with this line of research, we performed a comprehensive flow cytometric analysis of splenocytes from naive A.SW and B10.S mice. The results revealed that A.SW mice have a tendency towards an increase of total number of splenocytes in naive as well as in myosin-, OVA- or CFA-only immunized animals. We found no differences in percentages of monocytes, macrophages, dendritic cells, B cells, NK cells or NK T cells in the spleens of naive A.SW and B10.S mice. We have observed increased total numbers of CD4+ T cells in spleens of naive A.SW mice. Absolute counts of CD4+CD25+FoxP3+ Tregs were not significantly different, although we saw a tendency towards increased Tregs in A.SW mice. Due to the tendency for differences in the baseline of number of splenocytes between the two strains, we analysed the relative frequency of total CD4+ T cells and Tregs. Naive A.SW spleens had a significantly greater frequency of CD4+ T cells (as a percentage of total splenocytes) and a decreased percentage of FoxP3+CD25+ cells among total CD4+ T cells. Several publications have demonstrated the ability of regulatory cells to abrogate the severity of myocarditis in murine models. Huber et al. showed that transgenic mice expressing the TNF-α gene under the cardiac myosin promoter develop dilated cardiomyopathy that could be prevented by adoptive transfer of CD4+CD25+ cells [23]. Adoptive transfer of CD4+CD25+ Tregs was shown to protect mice from Coxsackievirus B3-induced myocarditis [24]. Depleting T cells that highly expressed glucocorticoid-induced TNFR family-related gene/protein (GITRhigh), a marker of regulatory cells, transferred to BALB/c nude mice induced fatal autoimmune myocarditis [25]. Others have published indirect evidence that an increased proportion of Tregs correlates with reduced severity of EAM [26,27]. The increased percentage of CD4+ T cells in A.SW mice and decreased frequency of Tregs compared to B10.S mice were also observed during several time-points throughout the development of EAM.
Similar to findings in the spleen, A.SW hearts had a greater proportion of CD4+ T cells than did B10.S hearts on day 21 of EAM. However, we found a lower frequency of CD4+CD25+FoxP3+ Tregs in the hearts of A.SW mice. EAM-susceptible A.SW mice not only had increased frequencies of CD4+ T cells compared to resistant B10.S mice, but their CD4+ T cells were more activated and proliferated more vigorously to cardiac myosin. These T cell differences are not limited to myocarditis, as A.SW mice immunized with different antigen, OVA or injected with CFA adjuvant only had increased percentages of CD4+ T cells and decreased percentages of Tregs. Therefore, the increased CD4+ T cells/Treg ratio is an intrinsic characteristic of A.SW mice and could contribute to their heightened susceptibility to T cell-mediated autoimmune diseases such as EAM.
In several autoimmune disease models, it has been shown that both Th1 and Th17 cells are able to induce autoimmune disease, although Th17 cells often induce more severe pathology [28,29]. We and others have shown that IFN-γ is protective in EAM, possibly through down-regulating Th17 responses [30–32]. Several studies have found that IL-17A is not essential for EAM induction; however, it supports the pathogenic role of Th17 cells in EAM [7,33,34]. Primed CD4+ T cells that were conditioned towards a Th17 phenotype transfer more severe EAM than Th1-polarized cells in BALB/c mice (our unpublished data). Similarly, transfer of Th17 cells appeared to induce more severe experimental autoimmune encephalomyelitis compared with transfer of Th1 cells [35]. Despite the fact that both Th1 and Th17 cells are present in heart infiltrates in equal numbers during EAM, Th17 cells displayed a more pathogenic phenotype, as evidenced by their increased production of TNF-α and IL-6. IL-17A is essential for cardiac remodelling, fibrosis and development of dilated cardiomyopathy [7]. In the current study, intracellular staining of CD4+ T cells from hearts on day 21 of EAM showed that CD4+ T cells from A.SW produced less IFN-γ and more IL-17A compared to CD4+ T cells from B10.S hearts.
To examine whether these differences in cytokine production account for the different abilities of A.SW and B10.S CD4+ T cells to develop into Th1 or Th17 cells, we performed several in-vitro experiments. We purified CD4+ T cells from naive A.SW and B10.S mice, and differentiated them towards Th1 or Th17 pathways. Similar to our EAM data, fewer A.SW CD4+ T cells expressed IFN-γ in Th1-inducing conditions and significantly more IL-17A in Th17-inducing conditions, compared with B10.S CD4+ T cells. Moreover, Th17-polarized cells from A.SW mice expressed higher levels of ROR-γt, a critical transcription factor for Th17 cell development. Our data support the hypothesis that A.SW CD4+ T cells are intrinsically more responsive under Th17-inducing conditions and differentiate in greater numbers towards the Th17 lineage. In contrast, B10.S CD4+ T cells differentiate preferentially into Th1 cells. A similar example of MHC-independent differences in Th responses can be seen between Lewis and Brown Norway rats. Lewis rats are susceptible to Th1-mediated autoimmune disease while Brown Norway rats are highly susceptible to Th2-mediated autoimmune disease, despite sharing the same MHC haplotype [36].
The ability to respond preferentially to an insult with either Th17 or Th1 cell responses might predispose an individual to develop certain types of autoimmunity, or perhaps influence disease outcomes. For instance, plasma levels of IL-6, a cytokine that promotes the development of Th17 cells, are higher in patients with systemic lupus erythematosus than in healthy subjects [37]. IL-6 overproduction has been found in several mouse models of autoimmunity, such as collagen-induced arthritis, antigen-induced arthritis and experimental autoimmune encephalitis [38]. Th17 cells can be induced in-vitro from naive CD4+ T cells in the presence of IL-6 and TGF-β[39–42]. To determine why naive A.SW CD4+ T cells differentiate more readily towards a Th17 phenotype, we analysed the expression of IL-6Rα and TGF-βRI on naive CD4+ T cells isolated from spleens of A.SW and B10.S mice. We observed greater IL-6Rα expression on naive A.SW CD4+ T cells at the transcriptional as well as the protein level. No differences were found in TGF-βRI expression (not shown). We speculate that the greater tendency of A.SW CD4+ T cells to differentiate towards the Th17 phenotype may be determined by increased expression of IL-6Rα, and thus higher T cell responsiveness to IL-6. It has been shown that diminished Th17 responses result from down-regulation of IL-6Rα expression on CD4+ T cells [43]. Differences in IL-6Rα expression may have other consequences. IL-6 is an essential cytokine for EAM development, as IL-6-deficient mice are completely resistant to the induction of EAM [15]. In addition to Th17 differentiation, IL-6 also promotes the survival and proliferation of effector and memory T cells [44].
We have extended here our previous findings showing that genetic susceptibility to EAM was mediated by T lymphocytes [17]. We showed that intrinsic differences in the frequency of CD4+ T cells and Tregs and their ability to receive cytokine instruction to differentiate towards specific T helper subtypes could represent a factor in the susceptibility of A.SW and B10.S mice to autoimmune myocarditis. Our data suggest that higher percentages of CD4+ T cells in A.SW mice, together with the tendency of their CD4+ T cells to differentiate towards a Th17 phenotype, via greater expression of IL-6Rα on their CD4+ T cells and lower relative frequency of Treg, contributes to the heightened susceptibility of A.SW mice to autoimmune myocarditis.
Acknowledgments
This work was supported by NIH/NHLBI grants R01 HL70729 and R01HL67290. Daniela Cihakova is supported by W. W. Smith Charitable Trust grant 111745. The authors would like to express their gratitude to R. Lee Blosser and Ada Tam for their expert assistance with flow cytometric analysis, and Dongfeng Zheng for technical help. We would also like to thank Dr Karl L. Womer and Ruizhen Chen for critical comments. Ping Chen received a joint PhD student fellowship from the China Scholarship Council and the Johns Hopkins Research Center for Autoimmune Diseases. The views expressed in this paper are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
Disclosures
The authors have no competing financial interests to report.
References
- 1.Li HS, Ligons DL, Rose NR. Genetic complexity of autoimmune myocarditis. Autoimmun Rev. 2008;7:168–73. doi: 10.1016/j.autrev.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guler ML, Ligons DL, Wang Y, et al. Two autoimmune diabetes loci influencing T cell apoptosis control susceptibility to experimental autoimmune myocarditis. J Immunol. 2005;174:2167–73. doi: 10.4049/jimmunol.174.4.2167. [DOI] [PubMed] [Google Scholar]
- 3.Ligons DL, Guler ML, Li HS, et al. A locus on chromosome 1 promotes susceptibility of experimental autoimmune myocarditis and lymphocyte cell death. Clin Immunol. 2009;130:74–82. doi: 10.1016/j.clim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose NR, Neumann DA, Herskowitz A, et al. Genetics of susceptibility to viral myocarditis in mice. Pathol Immunopathol Res. 1988;7:266–78. doi: 10.1159/000157122. [DOI] [PubMed] [Google Scholar]
- 5.Neu N, Rose NR, Beisel KW, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–6. [PubMed] [Google Scholar]
- 6.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–7. [PubMed] [Google Scholar]
- 7.Baldeviano GC, Barin JG, Talor MV, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–55. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T, Iwakura T, Matsui K, et al. IL-6-mediated Th17 differentiation through RORgammat is essential for the initiation of experimental autoimmune myocarditis. Cardiovasc Res. 2011;91:640–8. doi: 10.1093/cvr/cvr148. [DOI] [PubMed] [Google Scholar]
- 9.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–6. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Xing M, Ji X, et al. Interferon-alpha and interleukin-6 in SLE serum induce the differentiation and maturation of dendritic cells derived from CD34+ hematopoietic precursor cells. Cytokine. 2010;50:195–203. doi: 10.1016/j.cyto.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Take Y, Nakata K, Hashimoto J, et al. Specifically modified osteopontin in rheumatoid arthritis fibroblast-like synoviocytes supports interaction with B cells and enhances production of interleukin-6. Arthritis Rheum. 2009;60:3591–601. doi: 10.1002/art.25020. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto T. Interleukin-6 and its receptor in autoimmunity. J Autoimmun. 1992;5(Suppl. A):123–32. doi: 10.1016/0896-8411(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 14.Poffenberger MC, Straka N, El Warry N, et al. Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis. PLoS ONE. 2009;4:e6207. doi: 10.1371/journal.pone.0006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson U, Kurrer MO, Schmitz N, et al. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–5. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animals Resources NRC. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 17.Li HS, Ligons DL, Rose NR, et al. Genetic differences in bone marrow-derived lymphoid lineages control susceptibility to experimental autoimmune myocarditis. J Immunol. 2008;180:7480–4. doi: 10.4049/jimmunol.180.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenzo JC, Fairweather D, Cull V, et al. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J Mol Cell Cardiol. 2002;34:629–40. doi: 10.1006/jmcc.2002.2003. [DOI] [PubMed] [Google Scholar]
- 19.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–9. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 20.Oyamada A, Ikebe H, Itsumi M, et al. Tyrosine kinase 2 plays critical roles in the pathogenic CD4 T cell responses for the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:7539–46. doi: 10.4049/jimmunol.0902740. [DOI] [PubMed] [Google Scholar]
- 21.Havarinasab S, Haggqvist B, Bjorn E, et al. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol Appl Pharmacol. 2005;204:109–21. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110(Suppl. 5):877–81. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber SA, Feldman AM, Sartini D. Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-alpha transgenic mice. Circ Res. 2006;99:1109–16. doi: 10.1161/01.RES.0000249405.13536.49. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Fukuoka M, Li G, et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta–coxsackie–adenovirus receptor pathway. Circulation. 2010;121:2624–34. doi: 10.1161/CIRCULATIONAHA.109.893248. [DOI] [PubMed] [Google Scholar]
- 25.Nishioka T, Shimizu J, Iida R, et al. CD4+CD25+Foxp3+ T cells and CD4+CD25–Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Li S, Tian W, et al. Immunoregulatory effects of alpha-GalCer in a murine model of autoimmune myocarditis. Exp Mol Pathol. 2011;91:636–42. doi: 10.1016/j.yexmp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Wei L, Wei-Min L, Cheng G, et al. Upregulation of CD4+CD25+ T lymphocyte by adenovirus-mediated gene transfer of CTLA4Ig fusion protein in experimental autoimmune myocarditis. Autoimmunity. 2006;39:289–98. doi: 10.1080/08916930600758035. [DOI] [PubMed] [Google Scholar]
- 28.Nakae S, Nambu A, Sudo K, et al. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 29.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Afanasyeva M, Georgakopoulos D, Belardi DF, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2005;102:180–5. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afanasyeva M, Georgakopoulos D, Fairweather D, et al. Novel model of constrictive pericarditis associated with autoimmune heart disease in interferon-gamma-knockout mice. Circulation. 2004;110:2910–7. doi: 10.1161/01.CIR.0000147538.92263.3A. [DOI] [PubMed] [Google Scholar]
- 32.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valaperti A, Marty RR, Kania G, et al. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J Immunol. 2008;180:2686–95. doi: 10.4049/jimmunol.180.4.2686. [DOI] [PubMed] [Google Scholar]
- 34.Sonderegger I, Iezzi G, Maier R, et al. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J Exp Med. 2008;205:2281–94. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jager A, Dardalhon V, Sobel RA, et al. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournie GJ, Cautain B, Xystrakis E, et al. Cellular and genetic factors involved in the difference between Brown Norway and Lewis rats to develop respectively type-2 and type-1 immune-mediated diseases. Immunol Rev. 2001;184:145–60. doi: 10.1034/j.1600-065x.2001.1840114.x. [DOI] [PubMed] [Google Scholar]
- 37.Shah K, Lee WW, Lee SH, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assier E, Boissier MC, Dayer JM. Interleukin-6: from identification of the cytokine to development of targeted treatments. Joint Bone Spine. 2010;77:532–6. doi: 10.1016/j.jbspin.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Korn T, Oukka M, et al. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurence A, O'Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–5. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 41.Wei L, Laurence A, Elias KM, et al. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris TJ, Grosso JF, Yen HR, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Zhang M, Liao M, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–42. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 44.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–7. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]


