Abstract
The aim of this study was to investigate the initiation and progression of autoimmune damage in the lesions of labial salivary glands (LSGs) from primary Sjögren's syndrome (SS) patients by examining the selective localization of T helper (Th) subsets such as Th1, Th2, Th17 regulatory T cells (Tregs) and follicular T helper cells (Tfh). The expression of cytokines and transcription factors associated with these Th subsets in the LSGs from 54 SS patients and 16 healthy controls was examined using real-time polymerase chain reaction (PCR) and immunostaining. Additionally, infiltrating lymphocytes without germinal centre (GC-) and with GC (GC+) in the LSGs specimens from eight SS patients were extracted selectively by laser capture microdissection (LCM). The mRNA expression of these molecules was compared between the two sample groups of GC- and GC+ by real-time PCR. The mRNA expression of cytokines and transcription factors of all T helper (Th) subsets in the LSGs from the SS patients was increased significantly in comparison with controls. In LSGs from the SS patients, Th2 and Tfh was associated closely with strong lymphocytic infiltration; however, Th1, Th17 and Tregs was not. In the selectively extracted lesions of LSGs, Th1 and Th17-related molecules were detected strongly in the GC-, while Th2 and Tfh-related molecules were detected in the GC+. In contrast, no significant association with strong lymphocytic infiltration was observed in Treg-related molecules. These results indicate that SS has selective localization of Th subsets such as Th1, Th2, Th17 and Tfh in the LSGs, which is associated closely with disease severity and/or status. SS might be initiated by Th1 and Th17 cells, and then progressed by Th2 and Tfh cells via GC formation.
Keywords: cytokine, laser capture microdissection, Sjögren's syndrome, T helper subsets, transcription factor
Introduction
Sjögren's syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration into the salivary and lacrimal glands with concomitant destruction of the glandular tissue and autoantibody production. In approximately 5% of patients the disease may progress to the development of B cell lymphoma, possibly accompanied by hypergammaglobulinaemia and immunodeficiency [1], in close association with B cell accumulation [2,3] and germinal centre (GC) formation [4] in salivary glands. Histologically, SS is characterized by extensive lymphocytic infiltration of the salivary glands [5], and the majority of infiltrating cells are T cells, predominantly CD4+ T cells but also CD8+ T cells, B cells, plasma cells and macrophages [6]. Although it is generally thus accepted that CD4+ T helper (Th) cells play a crucial role in the pathogenesis of SS, the pathological role of CD4+ T cells in SS remains to be elucidated.
Several studies indicate that the Th cell population is comprised of functionally distinct subsets characterized by specific patterns of cytokines and transcription factors [7,8], and that at least six subsets exist – Th0, Th1, Th2, Th17, regulatory T (Treg) and follicular helper T (Tfh) cells. Th1 cells induced by interleukin (IL)-12 are mainly responsible for cell-mediated immunity, while Th2 cells induced by IL-4 are responsible for humoral immunity. These subsets are then controlled mutually by their own cytokines. Several studies have reported that some autoimmune diseases or allergic diseases are caused by the collapse of Th1/Th2 balance. In contrast, Th0 cells are produced by both Th1 and Th2 cytokines and are considered to be precursors of Th1 and Th2 cells. Treg cells are essential for the maintenance of immunological self-tolerance and immune homeostasis. Furthermore, Th17 cells have been shown to play a crucial role in the induction of autoimmunity and allergic inflammation [9]. Recently, Tfh cells contribute to impaired B cell differentiation and humoral immunity in conditions of immunodeficiency [7]. These Th subsets are generally considered to maintain the balance and homeostasis of the immune system, and possibly to induce various diseases by their impaired regulation. Importantly, the unique interactions and cross-talk between these Th subsets appear to be involved intimately in autoimmunity [8,10–12]. Consequently, an understanding of the presence and distribution of local Th subsets in the salivary glands from SS patients is relevant in oreder to clarify the mechanisms of the onset and progression of SS.
As glandular lymphocytic infiltration is a progressive feature in SS, in some patients ectopic GC can occur in the labial salivary glands (LSGs) [13]. Ectopic GC was defined as B and T cell follicles with follicular dendritic cell networks, high endothelial venules and clusters of proliferating cells. SS patients with ectopic GC in the LSGs coincided with aberrations of serum anti-Ro/SSA, anti-La/SSB and immunoglobulin (Ig)G levels in comparison with without GC [14]. Ectopic GC formation in the LSGs was involved in the pathogenesis of SS [15]. GCs have been recognized as important loci for the maturation of B cells and the generation of B cell lymphomas [16]. Furthermore, we have reported that Th2 cells are involved in the progression of the disease process in SS, especially local B cell activation [17]. Recently, Theander et al. [4] reported that the detection of GC-like structures (B cell accumulation) in the LSGs biopsy specimens from primary SS patients is proposed as a highly predictive and easy-to-obtain marker for B cell lymphoma development. Therefore, elucidating the mechanisms leading to ectopic GC formation may be of critical importance in clarifying further the pathogenesis of the disease and the possible development of salivary gland lymphomas. However, we are aware of no published reports that describe ectopic GC formation and the selective localization of Th subsets at any lesions in SS. We thus focused on the infiltrating lymphocytes, especially Th subsets, around ductal epithelial cells and ectopic GC in the LSGs, and adopted a laser capture microdissection (LCM) technique to obtain tissue samples exclusively from specific regions of interest [18,19].
Patients and methods
Patients
Fifty-four patients with primary SS (51 female and three male) referred to the Department of Oral and Maxillofacial Surgery, Kyushu University Hospital, which is a tertiary care centre, between 2007 and 2011 were included into the study. The patients ranged in age from 21 to 88 years [mean ± standard deviation (s.d.) age: 61·2 ± 11·5 years]. Medical records were reviewed retrospectively after diagnosis. All fulfilled the diagnostic criteria for definite SS proposed by the Research Committee on SS of the Ministry of Health and Welfare of the Japanese Government (1999) [20], and the diagnosis was also based on the diagnostic criteria proposed by the American–European Consensus Group criteria for SS [21]. Each patient exhibited objective evidence of salivary gland involvement based on the presence of subjective xerostomia and a decreased salivary flow rate, abnormal findings on parotid sialography and focal lymphocytic infiltrates in the LSGs. There was no documented history of treated with steroids, human immunodeficiency virus (HIV), infection with hepatitis B virus, hepatitis C virus, sarcoidosis and any other immune depressants in any of the patients. None of the patients with SS had evidence of malignant lymphoma at the time of the study. The prevalence of anti-SS-A/Ro, anti-SS-B/La and anti-nuclear antibodies were, respectively, 75·7, 31·2 and 78·2%. The LSG biopsies were performed as described by Greenspan et al. [22].
Sixteen patients with mucoceles (14 female and two male) who had no clinical or laboratory evidence of systemic autoimmune disease for use were chosen as a control group. The patients ranged in age from 37 to 66 years (mean age: 52·3 years; 56·8 ± 16·3 years). All control LSGs were histologically normal.
Informed consent, which was approved by the Ethics Committee of Kyushu University, Japan, was obtained from all the patients and healthy controls included in the study.
Histological study of LSGs
A histological study was performed in the LSG specimens from 54 patients in whom enough LSGs were available. Four-µm formalin-fixed and paraffin-embedded sections of LSG specimens were prepared and stained with haematoxylin and eosin (H&E) for conventional histological examination. The degree of lymphocytic infiltration in the specimens was judged by focus scoring [22,23]. One standardized score is the number of focal inflammatory cell aggregates containing 50 or more mononuclear cells in each 4-mm2 area of salivary gland tissue [24]. Ectopic GC are defined only by H&E stainings as a well-circumscribed chronic inflammatory cell infiltrate consisting of at least 50 mononuclear cells presenting with a densely packed dark zone and less densely packed light zone, as described previously by Jonsson et al. [14]. Only a few of these structures defined as such correspond, in fact, to real GC.
RNA extraction and complementary DNA (cDNA) synthesis
Total RNA was prepared from the whole LSGs by the acidified guanidinium–phenol–chloroform method, as described previously [25,26]. Three micrograms of the total RNA preparation was then used for the synthesis of cDNA. Briefly, RNA was incubated for 1 h at 42°C with 20 U of RNasin ribonuclease inhibitor (Promega, Madison, WI, USA), 0·5 µg of oligo-(dT) 1218 (Pharmacia, Uppsala, Sweden), 0·5 mM of each deoxyribonucleotide triphosphate (dNTP) (Pharmacia), 10 mM of dithiothreitol (DTT) and 100 U of RNase H reverse transcriptase (Life Technologies, Gaithersburg, MD, USA).
Tissue sampling by LCM
LCM was performed using a Leica Microsystems Japan (AS-LMD; Leica Microsystems Japan, Tokyo, Japan), as described previously [18,19]. In preparation for LCM, 13-µm-thick frozen sections were cut from these LSGs. The sections were first treated with 0·5 M ethylenediamine tetraacetic acid (EDTA) for 3 min, dehydrated with graded concentrations of ethanol, stained with HistoGene Staining Solution [LCM Frozen Section Staining Kit (Arcturus®; Applied Biosytstems, cat. no. KIT0401NS, Foster City, CA, USA)] for 45 s. After washing in distilled water, they were dehydrated with 100% ethanol, then clarified with xylene and air-dried with a fan for 30 s. All reagents were prepared RNase-free, and the entire process was completed within 20 min to minimize RNA degradation. Briefly, the tissue area of interest was positioned and cut out using a focused pulsed laser beam. Dissected areas were collected in the cap of a microcentrifuge tube via laser pressure catapulting. The cap was filled with 30 µl QIAzol lysis reagent (Qiagen, cat. no. 79306, Valencia, CA, USA).
RNA extraction from microdissected samples and cDNA synthesis
Total RNA was extracted independently from the LCM samples by using the RNeasy Mini Kit (Qiagen). Three micrograms of the isolated total RNA preparation was then used for the synthesis of cDNA, as described previously [25,26].
To determine the minimum amount of cells necessary to obtain enough RNA for a stable real-time quantitative polymerase chain reaction (PCR), infiltrating lymphocytes in the LSG specimens were microdissected and used for RNA isolation, cDNA synthesis and real-time PCR. For quantification with real-time PCR, it is recommended not to use an amplification crossing-point over 40 cycles. Applying this rule, for quantification purposes at least 15 frozen sections for each patient are recommended, depending on cell density. mRNA expression for all experiments was detected by PCR (date not shown).
Quantitative estimation of mRNA by real-time PCR
Quantitative cDNA amplification from the whole LSGs and the microdissected samples was performed according to the manufacturer's instructions and previous reports [25,26]. The cDNA of the cytokines and transcription factors were analysed by real-time PCR using Light Cycler Fast Start DNA Master SYBR Green 1 (Roche Diagnostics, Mannheim, Germany) in a Light Cycler real-time PCR instrument (version 3·5; Roche Diagnostics). Each Th subset expressed transcription factors T box transcription factor (T-bet), GATA3, retinoic acid-related orphan receptor C2 (RORC2) [27], forkhead box protein 3 (FoxP3) and B cell lymphoma 6 (Bcl-6), representing Th1, Th2, Th17, Treg and Tfh cells, respectively [8]. In this study, the cytokines and transcription factors examined were IL-4, IL-5, IL-10, IL-12, IL-17, IL-21, interferon (IFN)-γ, transforming growth factor (TGF)-β, T-bet, GATA3, RORC2, FoxP3 and Bcl-6.
In order to provide a meaningful comparison between different individuals or samples, we calculated the relative amounts of the PCR products of these molecules to the amounts of β-actin PCR products (for the standardization of total cellular mRNA) in each sample, as described previously [17,26].
Immunohistochemical analysis
For immunohistochemical analysis, 4-µm formalin-fixed and paraffin-embedded sections were prepared and stained by a conventional avidin–biotin complex technique, as described previously [26,28]. The polyclonal antibodies used to analyse the cytokines were anti-IL-4 (clone: ab9622; Abcam, Cambridge, UK), anti-IFN-γ (clone: ab9657; Abcam), anti-IL-17 (clone: sc-7927; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-IL-21 (LS-C401; LifeSpan BioScience, LSBio, Seattle City, WA, USA) were used as control rabbit polyclonal antibodies. The mouse monoclonal antibodies used to analyse transcription factors were anti-FoxP3 (clone: mAbcam 22510; Abcam), anti-Bcl-6 (clone: ab9479; Abcam) and anti-CXC chemokine receptor 5 (CXCR5) (clone: ab89259; Abcam). The sections were incubated sequentially with primary antibodies, biotinylated anti-mouse IgG secondary antibodies (Vector Laboratories, Burlingame, CA, USA), avidin–biotin–horseradish peroxidase complex (Vector Laboratories) and 3,3′-diaminobenzidine (Vector Laboratories). Mayer's haematoxylin was used for counterstaining. Photomicrographs were obtained using a light microscope equipped with a digital camera (CoolSNAP; Photometrics, Tucson, AZ, USA).
Statistical analysis
The statistical significance of the differences between the groups was determined by the Mann–Whitney U-test. All statistical analyses in this study were performed using jmp software (version 8; SAS Institute, Tokyo, Japan). A P-value of less than 0·05 was considered statistically significant.
Results
The mRNA expression levels of cytokines and transcription factors detected in whole LSGs
mRNA expression levels of IL-4, IL-5, IL-10, IL-17, IL-21, IFN-γ, T-bet, GATA3, RORC2, FoxP3 and Bcl-6 in whole LSGs from the 54 SS patients were significantly higher than those from the 16 controls (Fig. 1a). The 54 SS patients were then divided into two groups: one group of patients with weak lymphocytic infiltration of LSGs (n = 26; focus score range: 1–6; mean ± s.d., 3·1 ± 1·9) and a second group of patients with strong lymphocytic infiltration (n = 28; focus score range 7–12; mean ± s.d., 8·6 ± 2·2). mRNA expression levels of IL-4, Bcl-6 and GATA3 in whole LSGs with strong lymphocytic infiltration were significantly higher than in those with weak lymphocytic infiltration (Fig. 1b). In contrast, the mRNA expression levels of IL-5, IL-10, IL-12, IL-17, IL-21, IFN-γ, TGF-β, T-bet, RORC2 and FoxP3 in whole LSGs showed no relationship to the degree of lymphocytic infiltration.
Fig. 1.

(a) mRNA expression levels of cytokines and transcription factors were compared in the whole labial salivary glands (LSGs) from Sjögren's syndrome (SS) patients (n = 54) and controls (n = 16). T helper type 1 (Th1): interferon (IFN)-γ, interleukin (IL)-12 and T-bet; Th2: IL-4, IL-5 and GATA3; Th17: IL-17 and retinoic acid-related orphan receptor C2 (RORC2); regulatory T cells (Tregs) type: IL-10, transforming growth factor (TGF)-β and forkhead box protein 3 (FoxP3); Tfh type: IL-21 and B cell lymphoma 6 (Bcl-6) were estimated quantitatively, as described in the Patients and methods section. (b) mRNA expression levels in whole LSGs from SS patients were associated with the degree of lymphocytic infiltration, scored as weak (n = 26) or strong (n = 28). Bars represent means and standard deviations. Significant differences between groups were determined by Mann–Whitney U-tests (*P < 0·05; **P < 0·01).
Histological study and LCM of the LSGs
The histological findings were examined in the LSG specimens from the 54 SS patients in whom enough LSGs were available. Formalin-fixed LSGs from these patients were screened for the ectopic GC. Among the screened patients, the 12 SS patients (22%) were found to have the structures. For diagnostic purposes, at least five minor salivary glands were recommended [13]. All the LSGs from SS patients showed periductal lymphocytic infiltration with atrophy or severe destruction of the acini. Eight of our 12 SS patients had ectopic GC formation in the frozen LSGs. The remaining four SS patients did not have this structure. We thus selected frozen LSGs with ectopic GC formation from the eight SS patients, the clinical characteristics of which are summarized in Table 1. These frozen specimens from the eight SS patients were available for LCM. In these LSG specimens, the infiltrating lymphocytes in/around ducal epithelial cells without ectopic GC were defined as GC-, while with ectopic GC were defined as GC+ (Fig. 2a). As shown in Fig. 2b, GC- and GC+ from the same LSGs specimens were isolated by LCM. Total RNA was isolated independently from the LCM samples. Isolated total RNA was reverse-transcribed to generate cDNA. As mRNA expression isolated by LCM is a very small quantity (20–50 ng/µl), it is detected by PCR (Fig. 2c).
Table 1.
Clinical characteristics of eight patients (focus score 7–12) with primary Sjögren's syndrome (SS)
| Autoantibody | Immunoglobulin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) | Sex | Lymphocytic infiltration† | RF (20–≥ U/ml) | ANA (titre) | DNA (10–> U/ml) | SS-A/Ro (10–>) | SS-B/La (15–>) | IgG (872–1815 mg/dl) | IgA (95–405 mg/dl) | IgM (59–269 mg/dl) |
| 1 | 26 | F | 10 | 25 | > 1280 | ND | > 256 | > 15 | > 1815 | Negative | Negative |
| 2 | 44 | F | 9 | < 20 | Negative | Negative | 104 | 29 | 1517 | 193 | 174 |
| 3 | 65 | F | 9 | n.d. | 80 | n.d. | 64 | < 15 | n.d. | n.d. | n.d. |
| 4 | 52 | F | 11 | 40 | > 1280 | < 5 | > 256 | 32 | 2206 | 270 | 107 |
| 5 | 48 | F | 10 | 35 | 320 | < 5 | 16 | < 15 | n.d. | n.d. | n.d. |
| 6 | 65 | F | 9 | n.d. | 160 | 2 | < 10 | < 15 | 1338 | n.d. | 62 |
| 7 | 53 | F | 11 | n.d. | n.d. | n.d. | < 10 | < 15 | n.d. | n.d. | n.d. |
| 8 | 59 | F | 12 | n.d. | n.d. | n.d. | > 256 | < 15 | n.d. | n.d. | n.d. |
The degree of lymphocytic infiltration in the labial salivary glands (LSGs) was graded from 1 to 12 by focus scoring. Ig: immunoglobulin; F: female; n.d.: not done; RF: rheumatoid factor; ANA: anti-nuclear antibodies.
Fig. 2.
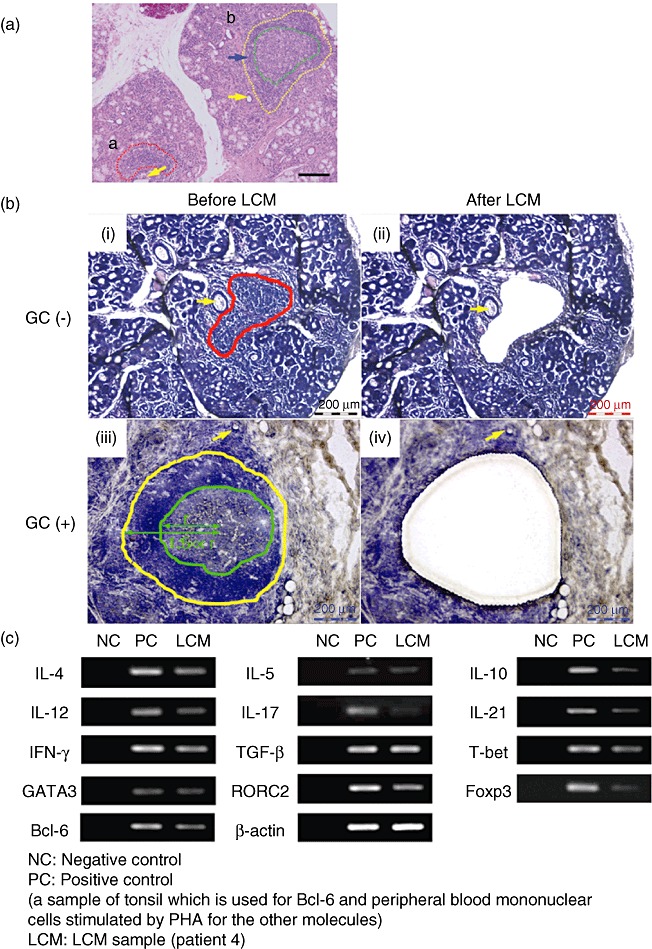
Representative results from patient 4. (a) Haematoxylin and eosin-stained labial salivary glands (LSGs). (i) Lymphocyte infiltration (enclosed by red broken line) around the ducts (yellow arrow). (ii) Secondary lymphoid follicles (enclosed by yellow broken line) show lymphocytic infiltration around the ectopic germinal centre (GC) (blue arrow) and the ectopic GC (enclosed by green broken line). Scale bars, 100 µm (original magnification ×100). (b) LSG specimens before/after laser capture microdissection (LCM). A defined area of infiltrating lymphocytes was marked using AS LMD microdissection software (Leica Microsystems) before LCM (i, iii). The preselected area was microdissected using a guided laser beam after LCM (ii, iv). The yellow arrow shows ductal epithelial cells (i–iv). (c) Secondary lymphoid follicles (enclosed by yellow line) showing lymphocytic infiltration around the ectopic GC, and the ectopic GC (enclosed by green broken line). Infiltrating lymphocytes in/around the GC were collected from a distance of approximately 1·5 GC radii (r) by LCM. Care was taken to avoid capturing epithelial cells (ii, iv). Scale bars, 200 µm (original magnification ×200). (c) Polymerase chain reaction (PCR) products from LCM specimens.
Comparison of the mRNA expression levels of cytokines and transcription factors between GC- and GC+
As shown in Fig. 3a,c, the mRNA expression of IL-12, IL-17, IFN-γ, T-bet and RORC2 in the GC- was significantly higher than that in the GC+. As shown in Fig. 3b,e, the mRNA expression of IL-4, IL-21, GATA3 and Bcl-6 in the GC+ was significantly higher than that in the GC-. As shown in Fig. 3d, the mRNA expression of IL-10, TGF-β and FoxP3 showed no statistically significant difference.
Fig. 3.

Comparison of mRNA expression patterns of cytokines and transcription factors in germinal centre (GC)- and GC+ from Sjögren's syndrome (SS) patients (n = 8), using laser capture microdissection (LCM). Real-time polymerase chain reaction (PCR) products of interleukin (IL)-4, IL-5, IL-10, IL-12, IL-17, IL-21, interferon (IFN)-γ, transforming growth factor (TGF)-β, T-bet, GATA3, retinoic acid-related orphan receptor C2 (RORC2), forkhead box protein 3 (FoxP3) and Bcl-6 were estimated quantitatively, as described in the Patients and methods section. Bars show means and standard deviations. Significant differences between groups were determined by Mann–Whitney U-tests (*P < 0·05; **P < 0·01).
Immunohistochemical analysis of cytokines and transcription factors in the LSGs
As shown in Fig. 4a, the specimens were examined immunohistochemically to evaluate the distributions of these proteins in the LSGs from SS patients and healthy controls. Expression of IFN-γ and IL-17 was detected slightly in ductal epithelial cells in LSGs from healthy controls (Fig. 4a,e), but was detected more strongly in/around the ductal epithelial cells rather than the ectopic GC from SS patients (Fig. 4b–d,f–h). Expression of IL-4 was detected in acinar cells in LSGs from SS patients and healthy controls (Fig. 4i,j,k), but was detected more strongly in/around the ectopic GC rather than in/around the ductal epithelial cells from SS patients (Fig. 4j–l). Expression of FoxP3 was not detected in the LSGs from healthy controls (Fig. 4m), but was detected in the diffuse-infiltrating lymphocytes of LSGs from SS patients (Fig. 4n–p). However, expression of FoxP3 in LSGs from SS patients showed no difference between GC- and GC+. Expression of Bcl-6 was not detected in LSGs from healthy controls (Fig. 4q), but was detected in/around the ectopic GC (Fig. 4r–t). As shown in Fig. 4b, expression of Bcl-6, IL-21 and the chemokine receptor CXCR5 in Tfh was detected in/around the ectopic GC in the LSGs from the same SS patients. Expression of CXCR5 was detected especially around the ectopic GC (Fig. 4u–w).
Fig. 4.

Distribution of T helper (Th) subsets in the lesions of labial salivary glands (LSGs). Immunostaining with anti-interferon (IFN)-γ (a–d), anti-interleukin (IL)-4 (e–h), anti-IL-17 (i–l), anti-forkhead box protein 3 (FoxP3) (m–p), anti-Bcl-6 (q–t, u) and anti-CXC chemokine receptor 5 (CXCR5) (v) monoclonal antibodies in LSGs from representative patients with Sjögren's syndrome (SS) (b–d, f–h, j–l, n–p, r–t, u, v) and healthy subjects (a, e, i, m, q) (brown). Counterstaining with Mayer's haematoxylin (blue). Arrows indicate key features of infiltrating cells. Representative sections from SS patient 6 are shown. Scale bars, 100 µm (original magnifications ×100, ×200, ×300).
Discussion
The findings of this study are: (1) the expression of all Th subset-related molecules in whole LSGs from SS patients was higher than those in the controls. (2) The expression of Th2 and Tfh-related molecules was associated closely with strong lymphocytic accumulation in whole LSGs from SS patients. (3) In the selectively extracted lesions of LSGs, expression of Th1- and Th17-related molecules in infiltrating lymphocytes without ectopic GC was higher than those with ectopic GC. In contrast, expression of Th2 and Tfh-related molecules in infiltrating lymphocytes with ectopic GC was higher than in those without ectopic GC.
Analyses over time of the mRNA expression of cytokines and transcription factors in whole LSGs performed neither a prospective cohort study nor multiple biopsies. From a practical viewpoint, it is difficult to evaluate the initiation and progression of SS. The acquisition of lymphoid features by inflammatory foci in the LSGs of SS is associated critically with enlargement of the inflammatory foci and with secondary lymphoid follicles [29]. Therefore, in order to evaluate accurately the propagation of the disease process, in the same SS patients a novel strategy was thus used to compare the expression of cytokines and transcription factors in the infiltrating lymphocytes with/without ectopic GC in the LSGs specimens by using LCM.
In SS-susceptible mice, the elimination of Th1 cells ameliorated all pathological and clinical signs of the disease [30]. Our previous study suggested a model of the pathogenesis of SS [17]. The mutual stimulation of Th1 cells and the target organ via the production of various cytokines plays a key role in the induction and/or maintenance of the disease and results in the eventual destruction of the target organ. Recently, CD4+ Th17 cells have been shown to be tissue-seeking and involved intimately in the initiation of SS [31,32]. The results of the present study concerning lymphocyte subsets and cytokine production in the LSGs are consistent with this model. Youinou et al. [33] reported that Th17 cells orchestrate autoreactive GCs. Our results were consistent with this report. However, in the selectively extracted lesions of LSGs, expression of Th17-related molecules in infiltrating lymphocytes without the ectopic GC was higher than in those with the ectopic GC. Interestingly, Th17/Th1 cells reportedly co-express IFN-γ with IL-17 [34], and such a subset has been identified in the gut in Crohn's disease [35]. Both Th1 and Th17 cells together were involved in the pathogenesis of SS [36], and there was an early induction of a CD4+ Th1/Th17 pathway leading to systemic release of IL-17 in mice [31]. Our observations suggest that both Th1 and Th17 cells around the ductal epithelial cells might be of critical importance in the initiation of SS.
Mitsiasis et al. [37] have reported that the balance between Th1 and Th2 shifted in favour of the former in LSG with a high infiltration score. Our results are consistent with these results. In our present data, mRNA expression of Th1 cytokines in the LSG with both weak and strong lymphocytic infiltration from SS patients was also significantly higher than that from controls. Therefore, these results suggest that Th1 cytokines play a key role in the induction and maintenance of the disease. Conversely, we reported that the levels of mRNA for both Th1 and Th2 cytokines and chemokines in LSGs with strong lymphocytic infiltration from patients with SS were significantly higher than in controls [26]. As described above, Theander et al. [4] reported that the ectopic GC-like structures, including ectopic GC in the LSGs, was involved in the development of malignant lymphoma in primary SS. We thus speculate that additional Th2 cells play a role in the lympho-aggressiveness of the disease. This paper focused on infiltrating lymphocytes, particularly Th subsets, around the ductal epithelial cells and ectopic GCs in LSGs, using a LCM technique to obtain tissue samples exclusively from specific regions of interest. We have positive results indicating that expression of Th2 cytokines in infiltrating lymphocytes with the ectopic GC was higher than that around the ductal epithelial cells. From the results obtained in this study, we speculated that Th2 cells might be involved in the progression of the disease, especially in the growth and activation of ectopic GC formation. Furthermore, several studies on autoimmune diseases in mice and humans have indicated a pathogenic role for Th1 cells and a possible protective role for Th2 cells [38,39]. Our results, showing that expression of Th1-related molecules in infiltrating lymphocytes with the ectopic GC was lower than without GC, and that of Th2-related molecules with GC was higher than without GC, are consistent with this report. Previous studies have reported that Th2 effector cells was important role for GC formation [40], and that Th2 cytokines induced infiltrating B lymphocytes to produce autoreactive antibodies [41]. Furthermore, the salivary gland environment in SS, in association with tissue trophic viruses such as the Epstein–Barr virus [42], cytomegalovirus [43] and retrovirus [44], might increase the risk of pseudolymphoma and hypergammaglobulinaemia promotion, and might hasten the progression to B cell malignant lymphoma. Considering the possible role of Th2 cells in the induction of B cell abnormalities, these cells might have a harmful (rather than a protective) effect on SS. Conversely, several studies have reported that expression of the chemokine receptor CXCR5 allows Tfh to home to the B cell follicle [45], IL-21 was increased in serum from SS patients [46] and high levels of IL-21 receptor were present at the surface of most B cells [7]. Furthermore, mice that lack the receptors for both IL-4 and IL-21 have greatly reduced IgG responses, indicating that IL-21 co-operates with IL-4 to regulate humoral immune responses [47]. In the LSG lesions, Tfh cells in infiltrating lymphocytes in/around the ectopic GC was significantly higher than that around the ductal epithelial cells (Figs 3 and 4). Our results strongly support that Th2 and Tfh cells are involved in the progression of the disease process as a lympho-aggressive disorder, particularly growth and activation of the ectopic GC formation.
Kolkowski et al. revealed that salivary glands in SS consistently express IL-10 and TGF-β[17,48]. Our results obtained in the present study are in accord with this report. Other studies have reported that the remarkable reduction of Treg in LSGs and peripheral blood might be involved in the pathogenesis of salivary gland destruction in SS [49]. Contrary to this study, Gottenberg et al. reported that Treg cell numbers were increased in the peripheral blood of patients with SS [50]. Therefore, it is unclear whether or not Tregs are involved in the pathogenesis of SS. Recently, the immunoregulatory role of CD4+CD25+ T cells might be different at each stage of the disease process in rheumatoid arthritis [51]. However, our results indicated that FoxP3 mRNA expression showed no relationship to the degree of lymphocytic infiltration in whole LSGs from SS patients, between weak and strong lymphocytic infiltration (Fig. 1b). The lower levels of Treg cells in/around the ectopic GC might not result in ectopic GC formation (Figs 3 and 4). Furthermore, recent studies, in which SS patients were divided into three groups (mild, intermediate and severe lymphocytic infiltration in the LSGs), reported that FoxP3+ Treg cells at the LSGs of severe SS stage was reduced in comparison with that of intermediate stage SS [3,52]. Our previous study, which concerned the frequency of reduced Treg in the severe stage of LSGs from SS patients, is consistent with this report [26]. Furthermore, increased levels of Treg cells in the whole LSGs from SS patients might suggest that negative feedback is more active in the LSGs from SS patients than in healthy subjects (Fig. 1a). Therefore, these results suggest that Tregs might be not involved in the initiation, growth and activation of ectopic GC formation of SS. However, the immunoregulatory role of Treg might be different at each stage of disease status in SS [52]. Conversely, we have also investigated whether Mikulicz's disease, which apparently differs from SS, was a unique IgG4-related disease characterized by Th2 and regulatory immune reactions [26].
Furthermore, we also examined the mRNA expression of Th1-, Th2-, Th17- and Treg-related molecules in peripheral blood mononuclear cells (PBMCs) from SS patients and controls. The mRNA expression of IFN-inducible protein 10 (IP-10) and CXCR3 in PBMCs from SS patients was slightly higher than that from controls, but that of the other molecules was not. In addition, the mRNA expression of all these molecules in the PBMCs showed no significant relationship to the degree of lymphocytic infiltration in the LSGs from the SS patients. These results indicate that few T cells involved in the pathogenesis of SS circulate in the periphery (manuscript in preparation).
In conclusion, we provide new evidence concerning the selective localization of Th subsets in LSGs from SS patients. In addition, it is still necessary to elucidate the mechanisms underlying Th2 and Tfh cell induction to provide functional evidence on the direct role of Th2 and Tfh cell progression, which might lead eventually to the creation of therapeutic strategies for inhibiting the disease progression. A more thorough understanding of the complex mechanisms of the disease, especially those involved in these Th subsets, might lead to pharmacological strategies to disrupt the cytokine network as a further means of inhibiting the initiation and/or progression of SS as a lympho-aggressive disorder.
Although our present study focused on Th subsets, B cells also play a key role in the formation of ectopic GC, and we are thus trying to evaluate the progression of SS more thoroughly (manuscript in preparation).
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (22791990) and the Research Program of Intractable Disease provided by the Ministry of Health, Labor, and Welfare of Japan.
Disclosure
The authors have declared no conflicts of interest concerning this paper.
References
- 1.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjogren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjogren's Syndrome. Arthritis Rheum. 1999;42:1765–72. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura S, Ikebe-Hiroki A, Shinohara M, et al. An association between salivary gland disease and serological abnormalities in Sjogren's syndrome. J Oral Pathol Med. 1997;26:426–30. doi: 10.1111/j.1600-0714.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 3.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun. 2010;34:400–7. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren's syndrome. Ann Rheum Dis. 2011;70:1363–8. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox RI. Sjogren's syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 6.Price EJ, Venables PJ. The etiopathogenesis of Sjogren's syndrome. Semin Arthritis Rheum. 1995;25:117–33. doi: 10.1016/s0049-0172(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 7.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 8.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 9.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 10.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson MV, Skarstein K, Jonsson R, Brun JG. Serological implications of germinal center-like structures in primary Sjogren's syndrome. J Rheumatol. 2007;34:2044–9. [PubMed] [Google Scholar]
- 15.Le Pottier L, Devauchelle V, Fautrel A, et al. Ectopic germinal centers are rare in Sjogren's syndrome salivary glands and do not exclude autoreactive B cells. J Immunol. 2009;182:3540–7. doi: 10.4049/jimmunol.0803588. [DOI] [PubMed] [Google Scholar]
- 16.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–62. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama Y, Nakamura S, Matsuzaki G, et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 1996;39:1376–84. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RA. Laser capture microdissection of mammalian tissue. J Vis Exp. 2007;8:309. doi: 10.3791/309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto A, Tarner IH, Bohle RM, et al. Analysis of vascular gene expression in arthritic synovium by laser-mediated microdissection. Arthritis Rheum. 2007;56:1094–105. doi: 10.1002/art.22450. [DOI] [PubMed] [Google Scholar]
- 20.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004;14:425–34. doi: 10.3109/s10165-004-0338-x. [DOI] [PubMed] [Google Scholar]
- 21.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 23.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjogren's syndrome. Arthritis Rheum. 1994;37:869–77. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 24.Szodoray P, Alex P, Jonsson MV, et al. Distinct profiles of Sjogren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol. 2005;117:168–76. doi: 10.1016/j.clim.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Nakamura S, Ohyama Y, et al. Accumulation of common T cell clonotypes in the salivary glands of patients with human T lymphotropic virus type I-associated and idiopathic Sjogren's syndrome. J Immunol. 2000;164:2823–31. doi: 10.4049/jimmunol.164.5.2823. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka A, Moriyama M, Nakashima H, et al. Th2 and regulatory immune reactions contributes to IgG4 production and the initiation of Mikulicz's disease. Arthritis Rheum. 2012;64:254–63. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 27.Burgler S, Mantel PY, Bassin C, Ouaked N, Akdis CA, Schmidt-Weber CB. RORC2 is involved in T cell polarization through interaction with the FOXP3 promoter. J Immunol. 2010;184:6161–9. doi: 10.4049/jimmunol.0903243. [DOI] [PubMed] [Google Scholar]
- 28.Hiroki A, Nakamura S, Shinohara M, et al. A comparison of glandular involvement between chronic graft-versus-host disease and Sjogren's syndrome. Int J Oral Maxillofac Surg. 1996;25:298–307. doi: 10.1016/s0901-5027(06)80062-7. [DOI] [PubMed] [Google Scholar]
- 29.Barone F, Bombardieri M, Manzo A, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren's syndrome. Arthritis Rheum. 2005;52:1773–84. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 30.Cha S, Brayer J, Gao J, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren's syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. Am J Pathol. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youinou P, Pers JO. Disturbance of cytokine networks in Sjogren's syndrome. Arthritis Res Ther. 2011;13:227–36. doi: 10.1186/ar3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. F-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 35.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 37.Mitsias DI, Tzioufas AG, Veiopoulou C, et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–8. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–505. [PubMed] [Google Scholar]
- 39.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson-Lindbom B, Ingvarsson S, Ingvarsson S, Borrebaeck CAK, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol. 2003;171:1657–66. doi: 10.4049/jimmunol.171.4.1657. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7:1299–308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 42.Schuurman HJ, Schemmann MH, de Weger RA, Aanstoot H, Hene R. Epstein–Barr virus in the sublabial salivary gland in Sjogren's syndrome. Am J Clin Pathol. 1989;91:461–3. doi: 10.1093/ajcp/91.4.461. [DOI] [PubMed] [Google Scholar]
- 43.Shillitoe EJ, Daniels TE, Whitcher JP, Vibeke Strand C, Talal N, Greenspan JS. Antibody to cytomegalovirus in patients with Sjogren's syndrome. As determined by an enzyme-linked immunosorbent assay. Arthritis Rheum. 1982;25:260–5. doi: 10.1002/art.1780250303. [DOI] [PubMed] [Google Scholar]
- 44.Talal N, Dauphinee MJ, Dang H, Alexander SS, Hart DJ, Garry RF. Detection of serum antibodies to retroviral proteins in patients with primary Sjogren's syndrome (autoimmune exocrinopathy) Arthritis Rheum. 1990;33:774–81. doi: 10.1002/art.1780330603. [DOI] [PubMed] [Google Scholar]
- 45.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 46.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 48.Kolkowski EC, Reth P, Pelusa F, et al. Th1 predominance and perforin expression in minor salivary glands from patients with primary Sjogren's syndrome. J Autoimmune. 1999;13:155–62. doi: 10.1006/jaut.1999.0289. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Li X, Qian L, et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren's syndrome. J Rheumatol. 2007;34:2438–45. [PubMed] [Google Scholar]
- 50.Gottenberg JE, Lavie F, Abbed K, et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun. 2005;24:235–42. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 51.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 52.Christodoulou MI, Kapsogeorgou EK, Kapsogeorgou EK, et al. Foxp3+ T-regulatory cells in Sjogren's syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol. 2008;173:1389–96. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]


