Abstract
Human infection with Trypanosoma cruzi leads to Chagas disease, which presents as several different clinical conditions ranging from an asymptomatic form to a severe dilated cardiomyopathy. Several studies have demonstrated that T cells play a critical role in the development of cardiac pathology, as well as in immunoregulation during chronic disease. However, the mechanisms that drive protective or pathogenic T cell response are not known. We have shown that CD4+ T cells from chagasic patients preferentially express T cell receptor (TCR) β-chain variable region (Vβ) 5. The aim of this work was to determine whether T cells expressing this particular Vβ region displayed variable or restricted CDR3 sequences, as an indicator of the nature of the stimulus leading to the activation of these T cells in vivo. Additionally, we aimed to evaluate phenotypic characteristics of these cells that might be associated with pathology. CDR3 junctional region sequencing of Vβ5·1 expressing CD4+ T cells revealed the occurrence of a highly homologous CDR3 region with conserved TCR Jβ region usage among patients with cardiac, but not indeterminate, Chagas disease. Moreover, correlation analysis indicated that the frequency of CD4+Vβ5·1+ cells is associated with granzyme A expression, suggesting that these cells might display cytotoxic function. Together these results provide new insight into T cell recognition of antigens involved in Chagas disease and suggest that these cells may be implicated in the pathogenesis of chagasic cardiomyopathy.
Keywords: Chagas disease, TCR, T lymphocytes
Introduction
T cells recognize peptide antigens bound to major histocompatibility complex (MHC) classes I or II molecules via a surface T cell receptor (TCR) composed of a disulphide-linked α- and β-chain [1]. The αβ TCR repertoire is generated through the somatic rearrangement of variable (V), diversity (D) and junctional (J) region gene segments for the β chain and V and J regions of the α chain. During this rearrangement, the complementary determining region 3 (CDR3) of the β chain is formed through the removal and/or addition of nucleotides at the V–D and D–J junctions. This gives rise to highly diverse Vα and Vβ CDR3 that are involved in the recognition of MHC-bound peptide and can be used as molecular markers or clonotypes for the identification of disease-specific T cells. When all the mechanisms for generation of diversity are taken into consideration, the potential size of the TCR repertoire is estimated to be greater than 108 possible TCR molecules [2]. Thus, in a given population of T cells, there is little chance that any two expanded T cell clones will express nearly identical TCRs unless they are selected specifically to express related TCRs because they respond to the same MHC–peptide complex.
T cells have been shown to play an essential role in the immune response to parasites. Chagas disease is an endemic parasitic disease affecting approximately 18 million people in Latin America [3]. The disease is caused by infection with Trypanosoma cruzi and is transmitted predominantly by contact with contaminated vector faeces. However, transmission by transfusion of blood or blood derivatives and by transplantation has brought the disease to new areas where it does not naturally occur, such as the United States [4]. The majority of patients who survive the acute phase of the disease remain asymptomatic for many years, and are classified as having the indeterminate clinical form [5,6]. However, approximately 30% of infected individuals develop the cardiac clinical form, which is characterized by an inflammatory cardiomyopathy which can lead to heart failure and death [6,7]. There is a consensus that CD4+ T cells play key roles in the clinical evolution of Chagas disease, possibly orchestrating the activation or modulation of the pathogenic response [8] which may define the fate of infection; however, the molecular mechanisms involved in the activation of these cells are not clear.
Preferential usage of specific TCR Vβ regions by T cells has been reported in experimental infection with T. cruzi, as well as in human disease [9–12]. We have shown previously an increased frequency of Vβ5-expressing CD4+ T cells in the peripheral blood of chagasic patients [13], and that this preferential expansion occurred in both CD28+ and CD28- subsets of CD4+ T cells [14]. Stimulation with T. cruzi homogenate led to a further expansion of these cells [13]. Preferential Vα usage was also observed in T cells derived from cardiac lesions [15]. These data suggest that a dominant antigenic epitope may be responsible for eliciting T cell responses in human Chagas disease. However, whether T cells expressing the Vβ5 region display variable or restricted CDR3 regions, and what the function of these cells is in Chagas disease, are unanswered questions.
In the current study, we performed nucleotide sequencing of the CDR3 region of Vβ5+CD4+ T cells from individuals with Chagas disease, as well as non-infected controls. Our data demonstrated that Vβ5·1+CD4+ T cell clones from individual cardiac chagasic patients had a highly conserved CDR3 region sequence that was strikingly absent from indeterminate and non-infected individuals. Additionally, this same CDR3 region motif was highly homologous among different cardiac patients, which suggests strongly that the Vβ5·1+CD4+ T cell clones expressing this CDR3 motif were selected and expanded by a common antigenic peptide in individuals with cardiomyopathy. Moreover, Vβ5·1+CD4+ T cells from chagasic patients are associated with high expression of granzyme A. These results provide additional insight into the involvement of specific CD4+ T cells, activated through the recognition of a dominant peptide, whose activities could cause tissue damage leading to Chagas disease cardiomyopathy.
Materials and methods
Study population and human leucocyte antigen (HLA) typing
Chronic chagasic patients analysed in this study were from endemic areas within the State of Minas Gerais, Brazil. Detailed evaluations, including physical examinations, electrocardiogram, chest radiograph and echocardiogram, were performed in each patient in order to define and classify patients in indeterminate (I; n = 14) or cardiac (C; n = 14) clinical forms, according to criteria described by Rocha et al. [7]. Oesophagogram and barium enema were also performed to exclude digestive disease. Patients with diabetes mellitus, thyroid dysfunction, renal insufficiency, chronic obstructive pulmonary disease and rheumatic disease were excluded from the analysis. Indeterminate patients (age range 26–53 years) had positive serology for T. cruzi, normal electrocardiogram and normal cardiac and digestive radiological evaluation. Cardiac patients (age range 32–75 years) had positive serology for T. cruzi and displayed several alterations in the electrocardiogram, such as right or left bundle branch block, and dilated left ventricle, as shown by echocardiography (left ventricular diastolic diameter ≥ 55 mm). Indeterminate and cardiac chagasic patients were subjected to monitoring of their heart function as part of the clinical evaluation. One patient (referred to as I1 in the paper), diagnosed initially as indeterminate at the time of blood collection, was reclassified later as displaying mild cardiomyopathy, according to Rocha et al. [7]. The disease status of the other patients did not change during the course of the study. The results were compared with those obtained from non-chagasic control subjects (C; n = 5, age range 26–38 years) who had negative serology to T. cruzi and the absence of cardiac disease. Informed consent was obtained from all patients, and the Ethical Committee of Federal University of Minas Gerais approved all procedures.
HLA typing was performed by standard molecular techniques at ClinImmune Laboratories at the University of Colorado Denver (UCD) (Aurora, CO, USA).
Preparation of peripheral blood and flow cytometric analysis of TCR Vβ expression
Peripheral blood mononuclear cells (PBMC) from chagasic patients or non-infected control individuals were isolated from heparinized blood by Ficoll-Hypaque density gradient separation [14] and frozen in a solution of 20% dimethylsulphoxide (DMSO) plus 10% normal human sera in RPMI-1640, as described by Yssel et al. [16]. Frozen PBMC were shipped by mail from Brazil to UCD (CO, USA), where the samples were thawed and analysed for TCR Vβ region expression using monoclonal antibodies (mAbs) directed against 15 different TCR Vβ receptors and CDR3 sequencing, as described previously [17,18]. Most blood samples were stained using biotinylated mAbs directed against Vβ3·1, Vβ5, Vβ5·1, Vβ5·2, Vβ6·7, Vβ7, Vβ8·1, Vβ9, Vβ12, Vβ13·1, Vβ13·2, Vβ14, Vβ17, Vβ20, Vβ22 and Vβ23. Streptavidin–phycoerythrin (Fisher Biotech, Pittsburgh, PA, USA) was used as a second-step reagent for TCR staining, and cells were co-stained with anti-CD4-peridinin chlorophyll (PerCP) (BD Biosciences, San Jose, CA, USA). The lymphocyte population was identified using forward and 90° light-scatter patterns, and fluorescence intensity was analysed using a fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson), as described previously [18–20]. A minimum of 20 000 cells were gated for TCR Vβ expression analysis.
Intracellular immunostaining
Intracellular expression of granzyme A was assessed in cells submitted to 16 h in-vitro culture without antigenic stimulation. Cells were collected and labelled using anti-CD4-fluorescein isothiocyanate (FITC) monoclonal antibodies (BD Biosciences). After fixation, cells were permeabilized, washed and stained with phycoerythrin (PE)-labelled monoclonal antibodies anti-granzyme A (BD Biosciences), washed and stored until acquisition on FACScan. Isotype controls were included for all samples. A minimum of 20 000 events were collected per sample, and analysed using FloJo software (Tri Star Inc, Ashland, OR, USA). Data from CD4+ granzyme A+ cells were used to perform correlation analysis with the frequency of T cells expressing each of the analysed Vβ regions.
Analysis of expressed TCRB junctional regions in peripheral blood CD4+ T cells
Frozen PBMC from chagasic patients and non-chagasic individuals, obtained as described above, were thawed and stained with anti-CD4 (PE) (BD Biosciences) followed by cell sorting using a MoFlo Cell Sorter (Cytomation, Fort Collins, CO, USA). Analysis of the expressed TCRB junctional region was performed as described previously [21]. Typically, 1–2 × 106 cells were obtained from the sorting of CD4+ T cells. Total RNA was isolated using an acid guanidinium–phenol chloroform method, and cDNA was synthesized using 2 µg RNA/20 µl reaction. cDNA synthesis reagents included reverse transcriptase (SuperScript RT; Gibco BRL, Grand Island, NY, USA), ribonuclease inhibitor (Promega, Madison, WI, USA), deoxynucleotide triphosphates (dNTPs) and random hexamers (both from Pharmacia Biotech, Inc., Piscataway, NJ, USA). Three microlitres of the cDNA reaction was added per each 50 µl polymerase chain reaction (PCR) reaction mixture. PCR amplification (AmpliTaq, Perkin Elmer, Branchburg, NJ, USA) was performed for 35 cycles, and both 5′ and 3′ oligonucleotide primers were present at a concentration of 0·6 µM. The sequences of the 5′TCRBV5·1 and 3′TCRBC primers were 5′-ATACTTCAGTGAGACACAGAGAAAC-3′ and 5′-TTCTGATGGCTCAAACAC-3′, respectively. PCR products were ligated into the pCR II TA cloning vector (Invitrogen, Carlsbad, CA, USA), and the ligation products were transformed into Epicurian Coli Xl-1 Blue supercompetent Escherichia coli cells (Stratagene, La Jolla, CA, USA). Twenty to 40 bacterial colonies containing inserts were selected randomly for nucleotide sequencing. Cycle sequencing was performed using M13 reverse (5′-CAGGAAACAGCTATGAC-3′) sequencing primer and an automated ABI 377 sequencer (Applied Biosystems, Perkin Elmer, Foster City, CA, USA).
Statistical analysis
We used the unpaired t-test to compare non-infected individuals and chagasic patients' data. Correlation analyses were investigated by two-tailed Spearman's correlation test. All analyses were performed using GraphPad Prism Software (La Jolla, CA, USA, USA). We considered statistically different results with P < 0·05.
Results
TCR expression by peripheral blood T cells of chagasic patients and non-chagasic individuals
We have shown previously that CD4+ T cells from chagasic patients display a preferential expression of Vβ5 compared to non-chagasic individuals [13,14].
In the present study, we extended the study of Vβ expression by CD4+ T cells from chagasic and non-chagasic individuals to the expression of 15 different Vβ regions (3·1, 5·1, 5·2, 6·7, 7, 8·1, 9, 12, 13·1, 13·2, 14, 17, 20, 22, 23) in order to determine whether this preferential expression would be persistent or if there were any other missed expansions.
Peripheral blood cells were obtained from asymptomatic (indeterminate) and symptomatic (cardiac) patients and non-chagasic subjects. The percentage of CD4+ T cells expressing particular TCR Vβ regions was determined by immunofluorescence and FACS analysis. In the first analysis, we compared the TCR V region expression between non-chagasic and chagasic patients (indeterminate plus cardiac). Similar to other disorders, comparison of TCR V region expression in the blood of patients and control subjects demonstrated remarkable heterogeneity, with the levels of TCR Vβ expression being similar for most of the analysed Vβs (Fig. 1a). However, CD4+ T cells from chagasic patients displayed a significantly higher frequency of Vβ5·1 and Vβ5·2-expressing cells compared to non-chagasic individuals [Vβ5·1 mean ± standard deviation (s.d.): 8·7 ± 0·8% and 6·6 ± 0·08%, respectively, P = 0·045; Vβ5·2 mean ± s.d.: 1·8 ± 0·2 and 0·6 ± 0·07%, P < 0·0001, respectively]. In addition, we observed a significantly decreased expression of Vβ9 on CD4+ T cells from chagasic individuals compared to non-chagasic patients (1·3 ± 0·3% versus 3·2 ± 0·3%; P = 0·0013) (Fig. 1a).
Fig. 1.

T cell receptor (TCR) Vβ expression in CD4 T cells. (a) Comparison of Vβ regions expression between non-infected individuals (white bars, n = 5) and chagasic (indeterminate + cardiac) patients (black bars, n = 28). (b) Comparison of Vβ regions expression between non-infected individuals (white bars, n = 5) and indeterminate chagasic patients (black bars, n = 14). (c) Comparison of Vβ regions expression between non-infected individuals (white bars, n = 5) and cardiac chagasic patients (black bars, n = 14). Data are expressed as mean ± standard error percentage of CD4+ T cells expressing a particular TCR Vβ. Identical symbols indicate P < 0·05 between bars marked with the same symbol.
In order to verify whether the increased expression of Vβ5 was characteristic of indeterminate or cardiac groups, we compared TCR Vβ expression between non-chagasic individuals and the asymptomatic (Fig. 1b) or symptomatic (Fig. 1c) patients separately. The comparison of TCR V region expression between indeterminate patients and non-chagasic individuals (Fig. 1b) showed that CD4+ T cells from indeterminate patients displayed a significantly higher frequency of Vβ5·1 and Vβ5·2-expressing cells compared to non-chagasic individuals (Vβ5·1 mean ± s.d.: 9·3 ± 0·8% and 6·6 ± 0·08%, respectively, P = 0·042; Vβ5·2 mean ± s.d.: 2·1 ± 0·3, respectively, P = 0·0002). A significantly decreased expression of Vβ9 on CD4+ T cells from indeterminate patients compared to non-chagasic individuals was also observed (1·6 ± 0·5% versus 3·2 ± 0·3%, P = 0·01) (Fig. 1b). In cardiac patients, although the frequency of Vβ5·1-expressing cells was higher than that observed in the non-chagasic group (8. ± 1·4% versus 6·6 ± 0·08%, P = 0·2), this difference was not statistically significant (Fig. 1c). However, CD4+ T cells from cardiac patients displayed a significantly higher frequency of Vβ5·2-expressing cells compared to non-chagasic individuals (1·4 ± 0·3% versus 0·6 ± 0·07%, respectively, P = 0·01) (Fig. 1c). Vβ9-expressing CD4+ T cells from cardiac patients displayed a significantly lower frequency compared to non-chagasic individuals (0·9 ± 0·4% versus 3·2 ± 0·3%, respectively, P = 0·0007) (Fig. 1c).
Selection of individual chagasic patients for sequencing of CDR3 regions
Evaluating the total expression of Vβ5 by CD4+ T cells, we observed that indeterminate and cardiac patients, in association or in separated groups, presented a higher frequency of those cells than healthy individuals (Fig. 2a). These results corroborate our previous work, which identified an expansion of Vβ5-expressing CD4+ T cells from freshly isolated peripheral blood of chagasic patients [13]. In this work, using monoclonal antibodies directed against two different Vβ5 regions, Vβ5·1 and Vβ5·2, we determined the most prevalent Vβ5 chain utilized by chagasic individuals. It is important to note that, together, Vβ5·1 and Vβ5·2 are responsible for 85·4% of total Vβ5 expression by CD4+ T cells [22]. As seen in Figs 1 and 2b, Vβ5·1-expressing CD4+ T cells are between two and seven times more prevalent than Vβ5·2-expressing cells in chagasic and non-chagasic individuals.
Fig. 2.
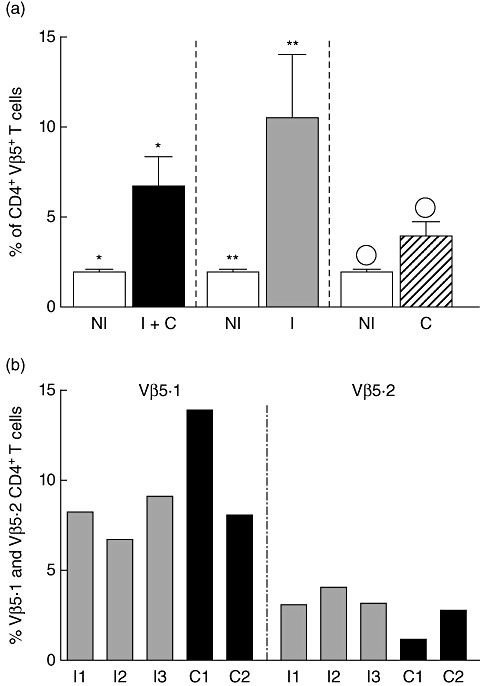
Expression of specific Vβ5 regions by CD4+ T cells from peripheral blood mononuclear cells of chagasic patients. (a) Comparison of total Vβ5 expression by CD4+ T cells between non-infected individuals (NI, white bars, n = 4) and grouped chagasic patients (indeterminate + cardiac, I + C, black bar, n = 17), indeterminate patients (I, grey bar, n = 7) or cardiac patients (c, dashed bar, n = 10). Data are expressed as mean ± standard error percentage of CD4+ T cells expressing Vβ5. Identical symbols indicate P < 0·05 between bars marked with the same symbol. (b) Grey and black bars represent indeterminate and cardiac individuals, respectively. Each bar from chagasic group shows the percent of CD4+ T cells expressing the given Vβ5 region individually. All values were obtained using Vβ5.1- or Vβ5.2-specific monoclonal antibodies with non-stimulated cells obtained from individuals whose Vβ5 region was sequenced, as described.
Given the higher frequency of Vβ5·1, and the expansion of these cells in chagasic patients, we performed junctional region nucleotide sequencing of the TCRB CDR3 of CD4+ T cell populations of chagasic patients who presented with an expression of CD4+ T cells expressing TCR Vβ5.1. In addition, we sequenced chagasic subjects who did not show evidence of TCR repertoire skewing for Vβ5·1, to verify whether similar or identical TCRs were present even in the absence of an expansion. Figure 2b shows the percentage of TCR Vβ5.1 and Vβ5·2 expression for all individuals whose CDR3 regions were sequenced for Vβ5·1. All the individuals chosen for sequencing presented an expression of Vβ5·1 region greater than the average expression of the control non-chagasic group (Fig. 2b).
Analysis of expressed TCRB genes in CD4+ T cells from patients with Chagas' disease
The presence of an expansion of T cells expressing a particular TCR Vβ region suggests that a population of cells has been activated in vivo. These cells could consist of either a polyclonal population of T cells such as those stimulated by superantigens (e.g. toxic shock syndrome toxin-1) or a restricted T cell population, consistent with the presence of a dominant peptide antigenic stimulation. Because identifying the complexity of the CDR3 region sequences from Vβ-expressing T cells allows us to make inferences as to the nature of the stimulus and possible involvement in pathology or protection in human Chagas' disease, we purified CD4+ T cells from individual chagasic patients and the non-chagasic group to perform junctional region nucleotide sequencing.
We sorted CD4+ T cells from the blood of individual patients from which RNA and cDNA were prepared. TCRBV5S1 fragments were amplified, cloned in bacteria and sequenced, as described previously [21]. The purity of the CD4+ T cells was >98%. Among the five patients included in the detailed TCRBV5S1 sequence analysis, three shared a dominantly expressed highly homologous CDR3 motif, including: BJ gene segment conservation, maintenance of CDR3 size (16 amino acids in length) and complete conservation of amino acid usage at positions 94, 95, 97 and 98 of the TCR β-chain, with the cysteine residue (C) of the β-chain representing position 90 (Table 1). This dramatic conservation was seen for 91% of the sequenced BV5S1 clones for C1, 13% for C2 and 45% for I1 (Table 1). Interestingly, C1 and C2 were cardiac patients and I1 was classified as indeterminate but progressed later to cardiac disease. The probability for one set of two repeated sequences to be found by chance alone (assuming > 5000 cells within a particular TCRBV subset from >100 000 CD4+ T cells sorted) was calculated to be P = 1·6 × 10−7[19]. Patients I2 and I3, who were classified as indeterminate and remain as such, did not show any dominantly expanded CDR3 that were homologous to the regions present in CD4+ Vβ5·1+ T cells from C1, C2 and I1. While other conserved expansions were apparent in the CD4+TCRBV5S1 subset, they were minor (sequences present at a frequency of ≥10%) (Table 1). Importantly, no clonal CD4+ T cell expansions were present in the blood of non-chagasic subjects (data not shown), which is consistent with our previously published findings [17].
Table 1.
Highly homologous CDR3 region in CD4+Vβ5·1+ cells among chagasic patient cells
| CDR3 deduced amino acid sequence | ||||||
|---|---|---|---|---|---|---|
| Patients | TCRBV5S1 | NDBN | BJ | BJ family | #/total | %identical |
| C1 | CASSL | DVGTA | YEQYFG | BJ2·7 | 2/21 | 10 |
| CASSL | DLGTA | YEQYFG | BJ2·7 | 17/21 | 81 | |
| C2 | CASS | VTGGAVY | EQFFG | BJ2·1 | 6/38 | 16 |
| CASSL | DQGTT | YEQYFG | BJ2·7 | 5/38 | 13 | |
| CASS | VTSGGI | YEQYFG | BJ2·7 | 10/38 | 26 | |
| I1 | CASSL | DGGSS | YEQYFG | BJ2·7 | 2/20 | 10 |
| CASSL | DSGTN | YEQYFG | BJ2·7 | 4/20 | 20 | |
| CASSL | DSGTS | YEQYFG | BJ2·7 | 5/20 | 25 | |
| CASS | PKGA | GANVLTFG | BJ2·6 | 2/20 | 10 | |
| CASS | SGLAGVSD | EQFFG | BJ2·1 | 2/20 | 10 | |
| CASS | YDRGG | ETQYFG | BJ2·5 | 2/20 | 10 | |
| I2 | CASSL | DQGPMFG | ETQYFG | BJ2·5 | 2/20 | 10 |
| CASS | FWQGV | ETQYFG | BJ2·5 | 2/20 | 10 | |
| CASS | PGTG | NTEAFG | BJ2·1 | 2/20 | 10 | |
| CASS | QD | STDTQYFG | BJ2·3 | 2/20 | 10 | |
| I3 | CASSL | GGGTRR | TQYFG | BJ2·5 | 4/26 | 15 |
Analysis of deduced Vβ5·1 CDR3 amino acid sequences expressed by CD4+ lymphocytes of chagasic patients. TCRBV5·1 sequences found in a frequency of 10% or more are shown. For each T cell clone, the entire TCRB junctional region is shown, extending from the 5′ end of the selected TCRBV family gene including the highly rearranged NBDN gene segment, and ending at the selected BJ gene segment. The cysteine (C) of the β-chain is designated as position 90. Homologous sequences are shown in bold type. These sequence data are available from GeneBank under accession numbers EF648281 to EF648296.
The deduced amino acid sequences of the NDBN regions of these related CDR3s are shown inside the box in Fig. 3 and usually had an aspartic acid (D) at position 95, a glycine (G) at position 97 and a threonine (T) at position 98. Sets of CDR3s had the sequence CASSLDLGTA, CASSLDQGTT or CASSLDSGTN, usually with a neutral amino acid at positions 96 and 99, forming the following CDR3 motif, CASSLDXGTX, with an identical junctional region length. These findings suggest strongly that these T cell clones recognize the same or very closely related antigens. All the Vβ5·1-expressing CD4+ T cell clones shown in Fig. 3 utilized the same BJ gene segment, BJ2S7. Thus, these findings clearly demonstrate the expansion of a common motif among Vβ5·1-expressing CD4+ T cells between unrelated chagasic patients of the cardiac clinical form.
Fig. 3.

Dominant CDR3 motif expressed by Vβ5·1+ CD4+ T cells from chagasic patients. A group of related CDR3 is shown, revealing different nucleotide sequences that encode identical or homologous amino acid sequences. Bold letters indicate nucleotides that differ in each patient, yet lead to maintenance of amino acid usage. Box surrounds the NDβN regions contained within CDR3s of identical length. The cysteine (C) of the β-chain is designated as position 90 with the conserved aspartic acid (D) at position 95, glycine (G) at position 97 and a threonine (T) at position 98.
MHC class II molecular typing of Chagas patients
CD4+ T cells recognize antigen presented in the context of MHC class II molecules via a surface TCR. Given our striking findings of a TCRBV5S1 CDR3 motif in CD4+ T cells, we investigated the HLA-DR and DQ alleles to determine if there was a bias in MHC class II molecules among chagasic individuals that could help to explain the existence of a dominantly expressed CDR3 region among CD4+ Vβ5·1+ T cells.
Table 2 compares the HLA-DR and -DQ alleles expressed by three patients with indeterminate disease and 11 cardiac chagasic subjects, presenting with a dilated cardiomyopathy. While no particular predominance of a given allele was seen within indeterminate patients, six of 11 cardiac patients expressed a DRB1*13 allele and seven of 11 cardiac patients displayed a shared epitope of the following amino acids at positions 70–74 of the β-chain, aspartic acid (D), glutamic acid (E), arginine (R), alanine (A) and alanine (A), compared to none of three patients with the indeterminate form of Chagas disease. These findings raise the possibility that these amino acids may be involved in the binding and presentation of Chagas-related peptides to CD4+ T cells.
Table 2.
Class II humal leucocyte antigen (HLA) types of chagasic patients with different clinical forms
| HLA-DRB1 | HLA-DQB1 | ||||
|---|---|---|---|---|---|
| Patient | Clinical form | Allele 1 | Allele 2 | Allele 1 | Allele 2 |
| I-3 | I† | *0405 | *1502 | *0302 | *0601 |
| I-4 | *0102 | *0802 | *0402 | *0501 | |
| I-32 | *0407 | *1402 | *0301 | *0302 | |
| C-3 | C | *0102 | *0701 | *0202 | *0501 |
| C-4 | *0103 | *0405 | *0302 | *0501 | |
| C-5 | *0804 | –‡ | *0301 | *0402 | |
| C-14 | *0301 | *1301 | *0201 | *0603 | |
| C-15 | *0301 | *1301 | *0201 | *0603 | |
| C-16 | *0402 | *1301 | *0302 | *0603 | |
| C-19 | *0403 | *0404 | *0302 | –‡ | |
| C-20 | *0701 | *0804 | *0202 | *0402 | |
| C-44 | *0701 | *1302 | *0202 | *0501 | |
| C-47 | *1302 | –‡ | *0604 | *0609 | |
| C-66 | *0101 | *1302 | *0501 | *0609 | |
Bold type: alleles related to the DERAA motif.
Forms of Chagas disease: I, indeterminate; C, cardiac (with dilated cardiomyopathy).
Indicates that subject is homozygous at the particular locus.
Vβ5·1 expressing CD4+ T cells from chagasic patients are related to cytotoxic activities
Given that Vβ5·1 expressing CD4+ T cells are over-represented in cardiac chagasic patients, we sought to verify whether they expressed phenotypic characteristics related to cytotoxic functions. Thus, we performed a correlation analysis between the frequency of CD4+granzyme A+ T cells from chagasic patients and non-chagasic individuals and the frequency of CD4+Vβ5·1+ cells. We also performed a correlation analysis between the frequencies of the other Vβ-expressing T cells evaluated in this work. Figure 4 shows the correlation analysis between the frequencies of CD4+granzyme A+ cells and CD4+Vβ5·1+, CD4+Vβ5·2+ and CD4+Vβ9+ cells, as the frequency of these subpopulations changed in the patient groups in relation to non-chagasic individuals (Fig. 1). We observed a significant correlation between the frequency of the CD4+Vβ5·1+ population and CD4+granzyme A+ T cells from cardiac patients (Fig. 4c, P = 0·04) but not from non-chagasic individuals (Fig. 4a, P = 0·2) or indeterminate chagasic patients (Fig. 4c, P = 0·4). No significant differences were observed for the correlations between the frequencies of CD4+Vβ5·2+ and CD4+granzyme A+ T cells (Fig. 4d–f) nor CD4+Vβ9+ and CD4+granzyme A+ T cells (Fig. 4g–i). Moreover, no statistically significant correlation was found between the frequency of CD4+granzyme A+ cells and the other Vβ-expressing populations, for which the frequency did not change (Vβ3·1, 6·7, 7, 8·1, 12, 13·1, 13·2, 14, 17, 20, 22 and 23) (data not shown).
Fig. 4.
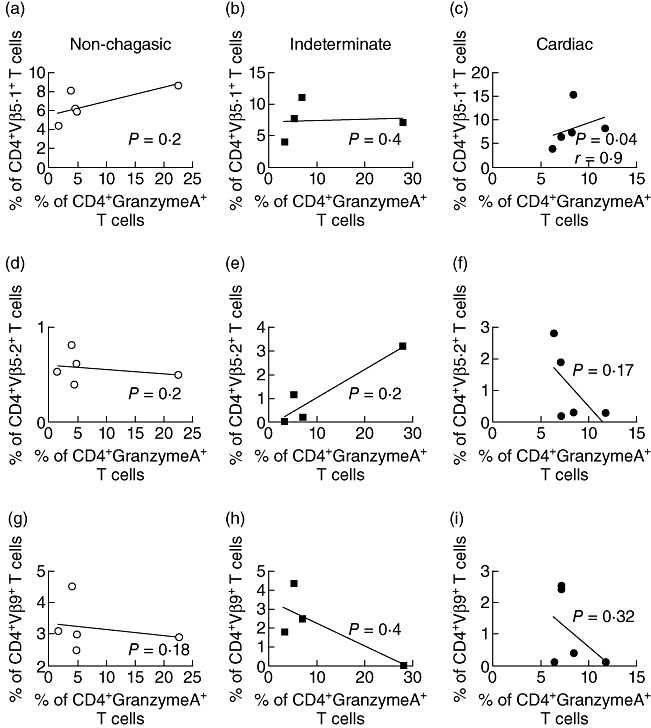
Intracellular expression of granzyme A by CD4+ expressing particular Vβ regions. Correlation between percentage of CD4+Vβ5.1+ T cells and granzyme A expressing CD4+ T cells from non-infected individuals (a), indeterminate (b) and cardiac chagasic patients (c). Correlation between percentage of CD4+Vβ5.2+ T cells and granzyme A expressing CD4+ T cells from non-infected individuals (d), indeterminate (e) and cardiac chagasic patients (f). Correlation between percentage of CD4+Vβ9+ T cells and granzyme A expressing CD4+ T cells from non-infected individuals (g), indeterminate (h) and cardiac chagasic patients (i). Linear correlation was tested by Spearman's correlation test, with P-values indicated in each figure.
Discussion
It has been estimated that approximately 20 million individuals in Latin America are infected with T. cruzi, resulting in Chagas disease [3]. The chronic phase of Chagas disease can progress towards different clinical forms, including indeterminate and cardiac (subjects of our study), as well as digestive or cardiodigestive. The cardiac lesions are responsible for the largest number of deaths among chagasic patients [23]. Morbidity in Chagas disease is related to parasite factors, as well as the host's immune response, particularly involving activated T cells [24–26]. In order to determine the stimuli responsible for T cell activation in human Chagas disease, it has been shown that T cells from chagasic patients recognize parasite-derived antigens as well as autologous antigens [24,27,28]. However, the molecular mechanisms responsible for T cell activation are not completely understood.
The data presented here provide new insight into CD4+ T cell antigenic recognition in Chagas disease. Through the use of specific TCR Vβ CDR3 sequencing, we aimed to identify the nature of skewed TCR responses identified by us in earlier studies [13,14]. In the first study, it was observed that CD4+Vβ5+ T cells were increased in chronic patients with cardiac alterations, but not indeterminate clinical form [13]. In the second study, in order to characterize the CD28- T cell population more clearly, which is increased in chagasic patients [25], the expression of TCRVβ in CD28+ and CD28- T cells was evaluated from polarized indeterminate and cardiac patients. Flow cytometric analysis demonstrated a predominance of Vβ5 expression in the CD4+CD28+ and CD4+CD28- populations in both groups (indeterminate and cardiac) [14]. In both previous studies, we compared Vβ5 expression with a limited panel of four other Vβ regions (2, 3·1, 8·1 and 17). The age ranges of patients analysed in our previous studies were 32–54 years [13] and 26–62 years [14], which are not statistically different from the current study (26–75 years). Therefore, we feel that this should not influence the repertoire, comparing these three studies. In fact, when we compared the three studies with regard to the Vβ regions evaluated, the same differences were observed in all of them. These data suggest that the slight difference in age range (not statistically significant) did not influence repertoire in the groups analysed here. Sequence analysis of the TCRs expressed in the peripheral blood of chagasic patients showed the occurrence of highly conserved CDR3 regions in CD4+Vβ5·1+ T cells from individuals with cardiac disease. This CDR3 motif was flexible in a few positions, but appeared to require an aspartic acid (D) at the 95th position, a glycine (G) at the 97th position and a threonine (T) at the 98th position of the β-chain. Interestingly, common TCRBJ usage was also conserved along with a tyrosine (Y) at the 100th position. Considering the enormous diversity of the TCR repertoire, related TCRs are predicted to occur extremely rarely (less than one in a million) by chance alone, and almost certainly represent selection by the same or similar antigen [21].
This highly conserved motif was present in three of the five individuals selected for TCRBV5S1 gene sequencing. This finding is particularly interesting, because two of these patients displayed dilated cardiomyopathy and the other subject, denoted as indeterminate at the blood collection time, developed mild clinical cardiac alterations detected in a subsequent echocardiogram. This patient was, thus, classified as cardiac (chronic chagasic cardiomyopathy level 1: CCC1), according to Rocha et al. [7]. Indeterminate patients, who remained asymptomatic, did not express similar CDR3 sequences. Furthermore, despite a database in our laboratory containing thousands of sequences derived from other patient groups and healthy individuals, we have not previously found this or a related CDR3 motif. Thus, our data suggest strongly that the cells expressing the conserved motif may be involved in the establishment of cardiac damage in human Chagas disease. Another interesting aspect is that, while C1 had a very high frequency of CD4+Vβ5·1+ T cells in relation to controls, C2 and I1, who displayed similar CDR3 regions, did not show such a high frequency of these cells. Thus, the conserved CDR3 region of CD4+Vβ5·1+ T cells was observed even in the absence of a large expansion. Although immunofluorescence staining has several advantages, such as being easily performed, relatively rapid and accurate for the determination of the size of a Vβ subset, it does not predict accurately the presence of large clones or sets of related clones in the peripheral blood of chagasic patients at the individual level. Similar findings have also been seen in other diseases, such as rheumatoid arthritis and chronic beryllium disease [21,29]. Previous studies by our group have shown a correlation between the expression of Vβ 5·1 and lesion development in human leishmaniasis [30]. This is an interesting observation because, although Chagas disease and leishmaniasis are two very distinct diseases, they share an inflammatory component and the parasites that cause these diseases belong to the same family (Trypanosomatidae).
We performed MHC class II haplotyping of a group of chagasic patients as the presence of a highly dominant CD4+ TCR CDR3 would probably depend not only on a common peptide, but also on a similar MHC II-presenting molecule. Due to limited material, we could not perform these analyses in all patients whose TCR were sequenced. However, because these data were obtained from patients with similar clinical status, it still provides useful information. While HLA-DQ alleles varied among the groups, the dilated cardiomyopathy patients showed a dramatic presence of at least one HLA-DR allele expressing the ‘DERAA’ epitope at amino acids positions 70–74 of the β-chain (*0103, *402, *1301 or *1302) [31]. The presence of this ‘shared epitope’ was seen in seven of 11 (64%) cardiac patients, but none of the indeterminate individuals had any of the epitopes. While these findings are encouraging, there is a possibility of a type I statistical error when analysing 11 patients, warranting the performance of a larger study investigating genetic susceptibility of Chagas disease. Moreover, in addition to increasing the number of cardiac patients, i.e. those who showed the association, it is also important to analyse a higher number of indeterminate patients, as they represent a control of infected individuals but with no cardiac disease. While this HLA-DR epitope has been associated with protection in rheumatoid arthritis [32], our results suggest that the HLA class II gene, DRB1*13, in association with the observed CDR3 motif, may be implicated in the recognition of an antigen that could trigger (or amplify) heart damage in Chagas disease. In fact, recent studies have shown an association between DRB1*13 and susceptibility to Whipple's disease [33]. Conversely, it is possible that these cells can be related to the control of the response and would only appear (or expand) after the establishment of the heart inflammation. Further studies to determine the nature of the antigen and the presenting molecule responsible for the selection of the dominant motif might aid in possible alternative immunotherapy in human Chagas disease.
Testing the possibility that Vβ5·1 expressing CD4+ T cells are associated with heart damage, we performed correlation analyses of this cellular subpopulation and granzyme A expression. Granzyme A induces target cell apoptosis and is one of the most abundant cytolytic enzymes produced by T cells in order to eliminate target cells [34]. Interestingly, a positive correlation between the percentages of CD4+Vβ5·1+ T cells and granzyme A expressing CD4+ T cells from cardiac chagasic patients but not from indeterminate chagasic patients or non-chagasic individuals was observed. Interestingly, no correlation was found between the frequency of the other populations that displayed different frequencies between patients and non-chagasic individuals (CD4+Vβ5·2+ or CD4+Vβ9+) T cells and CD4+granzyme A+ cells. In fact, no correlation was observed between the frequency of CD4+granzyme A+ cells and any other analysed Vβ-expressing population, except for the CD4+Vβ5·1+. Considering that the expanded population might be recruited to inflammatory site, there is a possibility that these cells contribute to tissue damage through this lytic enzyme release. It is important to emphasize that, while Chagas disease has tissue-specific pathology, it has systemic effects, as several immunological criteria are altered in the blood of patients [6]. In fact, a correspondence between findings in tissue versus blood has been reported consistently in Chagas disease [25,35–37]. Thus, the expansion of Vβ5·1+ CD4+ T cells in the peripheral blood does not rule out their role at lesion sites, which merits further investigation.
In summary, we have demonstrated, for the first time, the presence of a highly homologous CDR3 region in human CD4+ T cells expressing TCRBV5S1. Most importantly, we found a conserved CDR3 motif displayed by CD4+ T cells that could work in association with DR*13 only in patients with chagasic cardiomyopathy. Moreover, we found an association of these cells with the expression of the cytolytic molecule granzyme A. Taken together, these data provide the first step towards the discovery of an antigenic peptide responsible for this skewed T cell activation and could potentially be helpful in designing immunotherapy to prevent pathology in human Chagas disease.
Acknowledgments
We thank Allison Martin and Fenneke Joslin for technical assistance. We also thank Karen Helm and Michael Ashton for expert cell sorting support. This work was supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR), NIH/NIAID – AI066044-03, INCT-DT; W.O.D., K.J.G. and C.A.S.M. are CNPq fellows.
Disclosure
The authors have declared that no conflicts of interest exist.
References
- 1.Marrack P, Kappler J. The T cell receptor. Science. 1987;238:1073–9. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- 2.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human αβ T cell receptor diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Control of Chagas disease. WHO Technical Report Series. Geneva: WHO; 2002. [PubMed] [Google Scholar]
- 4.Kirchhoff LV, Paredes P, Lomeli-Guerrero A, et al. Transfusion-associated Chagas disease (American trypanosomiasis) in Mexico: implications for transfusion medicine in the United States. Transfusion. 2006;46:298–304. doi: 10.1111/j.1537-2995.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- 5.Macedo V. Indeterminate form of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94:311–6. doi: 10.1590/s0074-02761999000700059. [DOI] [PubMed] [Google Scholar]
- 6.Dutra WO, Rocha MO, Teixeira MM. The clinical immunology of human Chagas disease. Trends Parasitol. 2005;21:581–7. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Rocha MOC, Ribeiro AL, Teixeira MM. Clinical management of chronic chagas cardiomyopathy. Front Biosci. 2003;8:44–54. doi: 10.2741/926. [DOI] [PubMed] [Google Scholar]
- 8.Dutra WO, Colley DG, Pinto-Dias JC, et al. Self and nonself stimulatory molecules induce preferential expansion of CD5+ B cells or activated T cells of chagasic patients, respectively. Scand J Immunol. 2000;51:91–7. doi: 10.1046/j.1365-3083.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardoni RL, Antunez MI, Orn A, Gronvik KO. T cell receptor V beta repertoire in the thymus and spleen of mice infected with Trypanosoma cruzi. Cell Immunol. 1996;169:238–45. doi: 10.1006/cimm.1996.0114. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro da Silva A, Lima EC, Vicentelli MH, Minoprio P. V beta 6-bearing T cells are involved in resistance to Trypanosoma cruzi infection in XID mice. Int Immunol. 1996;8:1213–9. doi: 10.1093/intimm/8.8.1213. [DOI] [PubMed] [Google Scholar]
- 11.Leite-de-Moraes MC, Coutinho A, Hontebeyrie-Joskowicz M, Minoprio P, Eisen H, Bandeira A. Skewed V beta TCR repertoire of CD8+ T cells in murine Trypanosoma cruzi infection. Int Immunol. 1994;6:387–92. doi: 10.1093/intimm/6.3.387. [DOI] [PubMed] [Google Scholar]
- 12.Sunnemark D, Andersson R, Harris RA, Jeddi-Tehrani M, Orn A. Enhanced prevalence of T cells expressing TCRBV8S2 and TCRBV8S3 in hearts of chronically Trypanosoma cruzi-infected mice. Immunol Lett. 1998;60:171–7. doi: 10.1016/s0165-2478(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 13.Costa RP, Gollob KJ, Fonseca LL, et al. T-cell repertoire analysis in acute and chronic human Chagas' disease: differential frequencies of Vbeta5 expressing T cells. Scand J Immunol. 2000;51:511–9. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 14.Menezes CA, Rocha MO, Souza PE, Chaves AC, Gollob KJ, Dutra WO. Phenotypic and functional characteristics of CD28+ and CD28 cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–38. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha-Neto E, Moliterno R, Coelho V, et al. Restricted heterogeneity of T cell receptor variable alpha chain transcripts in hearts of Chagas disease cardiomyopathy patients. Parasite Immunol. 1994;16:171–9. doi: 10.1111/j.1365-3024.1994.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 16.Yssel H, de Vries JE, Koken M, Van Blitterswijk W, Spits H. Serum-free medium for the generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–27. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot AP, Kotzin BL, Comment CE, Newman LS. Expansions of T-cell subsets expressing particular T-cell receptor variable regions in chronic beryllium disease. Am J Respir Cell Mol Biol. 1998;18:581–9. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot AP, Maier LA, Canavera SJ, et al. Beryllium skin patch testing to analyze T cell stimulation and granulomatous inflammation in the lung. J Immunol. 2002;168:3627–34. doi: 10.4049/jimmunol.168.7.3627. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot AP, Palmer BE, Sullivan AK, et al. Frequency of beryllium-specific, central memory CD4+ T cells in blood determines proliferative response. J Clin Invest. 2005;115:2886–93. doi: 10.1172/JCI24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot AP, Gharavi L, Bennett SR, Canavera SJ, Newman LS, Kotzin BL. CD28 costimulation independence of target organ versus circulating memory antigen-specific CD4+ T cells. J Clin Invest. 2003;112:776–84. doi: 10.1172/JCI18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J Immunol. 1999;163:1019–26. [PubMed] [Google Scholar]
- 22.Lima M, Almeida J, Santos AH, et al. Immunophenotypic analysis of the TCR-Vbeta repertoire in 98 persistent expansions of CD3(+)/TCR-alphabeta(+) large granular lymphocytes: utility in assessing clonality and insights into the pathogenesis of the disease. Am J Pathol. 2001;159:1861–8. doi: 10.1016/s0002-9440(10)63032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–23. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 24.Cunha-Neto E, Bilate AM, Hyland KV, Fonseca SG, Kalil J, Engman DM. Induction of cardiac autoimmunity in Chagas heart disease: a case for molecular mimicry. Autoimmunity. 2006;39:41–54. doi: 10.1080/08916930500485002. [DOI] [PubMed] [Google Scholar]
- 25.Dutra WO, Martins-Filho OA, Cancado JR, et al. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira RC, Ianni BM, Abel LC, et al. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/‘indeterminate’ and Chagas disease cardiomyopathy patients. Mem Inst Oswaldo Cruz. 2003;98:407–11. doi: 10.1590/s0074-02762003000300021. [DOI] [PubMed] [Google Scholar]
- 27.Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J. Autoimmunity in Chagas disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas' cardiomyopathy patient. J Clin Invest. 1996;98:1709–12. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzinelli RT, Parra JF, Correa-Oliveira R, et al. Idiotypic/anti-idiotypic interactions in schistosomiasis and Chagas disease. Am J Trop Med Hyg. 1988;39:288–94. doi: 10.4269/ajtmh.1988.39.288. [DOI] [PubMed] [Google Scholar]
- 29.Striebich CC, Falta MT, Wang Y, Bill J, Kotzin BL. Selective accumulation of related CD4+ T cell clones in the synovial fluid of patients with rheumatoid arthritis. J Immunol. 1998;161:4428–36. [PubMed] [Google Scholar]
- 30.Keesen TS, Antonelli LR, Faria DR, et al. CD4(+) T cells defined by their Vβ T cell receptor expression are associated with immunoregulatory profiles and lesion size in human leishmaniasis. Clin Exp Immunol. 2011;165:338–51. doi: 10.1111/j.1365-2249.2011.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries RR, Huizinga TW, Toes RE. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J Autoimmun. 2005;25:21–5. doi: 10.1016/j.jaut.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Zanelli E, Gonzalez-Gay MA, David CS. Could HLA-DRB1 be the protective locus in rheumatoid arthritis? Immunol Today. 1995;16:274–8. doi: 10.1016/0167-5699(95)80181-2. [DOI] [PubMed] [Google Scholar]
- 33.Martinetti M, Biagi F, Badulli C, et al. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple's disease. Gastroenterology. 2009;136:2289–94. doi: 10.1053/j.gastro.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay PK, Betts MR, Price DA, et al. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis. 2008;21:287–92. doi: 10.1097/QCO.0b013e3282f88b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes JA, Bahia-Oliveira LM, Rocha MO, et al. Type 1 chemokine receptor expression in Chagas' disease correlates with morbidity in cardiac patients. Infect Immun. 2005;73:7960–6. doi: 10.1128/IAI.73.12.7960-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis DD, Jones EM, Jr, Lopes SER, Gazzinelli G, Colley DG, McCurley TL. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–44. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]


