Abstract
It has been reported that interferon (IFN)-γ-secreting T cells reactive to gluten can be detected in the peripheral blood of individuals with treated coeliac disease (CD) after a short consumption of wheat-containing food. By contrast, very little is known about the reproducibility of this in-vivo procedure in the same patient cohort which underwent two, or more, gluten consumptions. Fourteen coeliac patients in remission consumed wheat bread for 3 days; 13 underwent a second gluten challenge after a wash-out of 3–10 months on a strict gluten-free diet. Immune reactivity to gluten was analysed in peripheral blood by detecting IFN-γ before and 6 days after commencing a gluten diet. Gliadin-specific IFN-γ-secreting CD4+ T cells increased significantly on day 6 of the first challenge. These cells resulted as prevalently human leucocyte antigen (HLA)-DQ restricted and with a phenotype of gut homing, as suggested by the expression of β7-integrin. Similarly, reactiveness to gliadin was observed after the second wheat consumption, although with an individual variability of responses at each challenge. Our findings confirmed that the short wheat challenge is a non-invasive approach to investigate the gluten-related immune response in peripheral blood of subjects intolerant to gluten. Furthermore, we demonstrated that the in-vivo procedure can be reproduced in the same subject cohort after a gluten wash-out of at least 3 months. Our study has important implications for the application of this procedure to clinical practice.
Keywords: ELISPOT, in-vivo challenge, interferon-γ, peripheral blood, wheat gluten
Introduction
Coeliac disease (CD) is a chronic enteropathy due to an abnormal immune reaction to gluten, the storage proteins of wheat, barley and rye [1]. Gluten peptides escaping proteolysis from gastrointestinal enzymes activate proinflammatory T cells that play a central role in the induction of mucosal atrophy in coeliac patients [1]. Great progress in understanding CD pathogenesis has come from the use of gluten-specific T cell clones and T cell lines raised from intestinal biopsies [2,3]. This approach, although very successful in defining immune reactivity to gluten, required prolonged in-vitro cell culturing and the use of growth factors, with the risk of alterations of cell phenotype and function. Anderson and co-workers established an innovative approach that allows the detection of gluten-specific T cells in the peripheral blood of CD patients after a short period of gluten-containing food consumption [4,5]. Basically, gluten-sensitized CD4+ T cells, normally scarcely detectable in the blood of coeliac patients, circulate transiently in the peripheral blood after 3 days of wheat challenge, and can be detected by a sensitive interferon (IFN)-γ enzyme-linked inmmunospot (ELISPOT) assay. Using this in-vivo procedure, the authors further screened large libraries of prolamin peptides and assessed the hierarchy and immunodominance of gluten T cell epitopes [6]. More recently, T cells reactive to DQ2-α-I and DQ2-α-II epitopes were monitored in the peripheral blood of bread-challenged coeliacs with specific DQ2-tetramer constructs [7,8]. Of note, both Australian and Norwegian studies enrolled adult coeliac volunteers, with an average age of 43 years. To our knowledge, no information is available on the responsiveness to short gluten challenge in very young coeliac patients. Furthermore, very little is known about the in-vivo challenge reproducibility in the same subject cohort, with the exception of a few cases of coeliacs who underwent two separate gluten consumptions described in the above-mentioned studies [7,8].
In the present study we have validated the in-vivo short gluten challenge in a cohort of 14 young CD patients of Italian origin. In particular, we analysed the peripheral blood response against whole gliadin and the immunodominant 33-mer peptide (α-gliadin 57–89). We also assessed the feasibility of exposing the patient cohort to a second in-vivo challenge after a period of 3–10 months of wash-out, in order to estimate the reproducibility of the procedure in the same study population and the intra-individual variations.
If replicated successfully in other studies, the short wheat challenge could represent a strategic tool to evaluate non-invasively the individual's response to gluten, and could be applied to intervention studies. In fact, the evaluation of small bowel mucosa damage after long-term wheat challenge has been used since the 1950s to assess cereal toxicity or to define the toxic peptides [9–11]. Such studies required repeated endoscopies, before and after treatment, which are always not well accepted by participants. To detect a dysregulated response to gluten, other functional markers, such as faecal fat and xylose malabsorption, resulted in low specificity and sensitivity [12–15]. Furthermore, recent studies have indicated that a short gluten challenge could be used to support diagnosis in doubtful cases of CD [16–18].
Patients and methods
Patients and in-vivo wheat challenge procedure
Fourteen DQ2-positive volunteers with CD (mean age 18·6, range 15–24 years) participated in the study (Table 1). All patients received the diagnosis of CD during their paediatric age (mean age 4·7, range 1–12 years), according to the European Society of Paediatric Gastroenterology and Nutrition (ESPGHAN) criteria [19], and were followed-up regularly at the Department of Pediatrics, University Federico II, Naples. Patients were informed about the aim of the study and gave their full consent. The study was approved by the Ethical Committee of Department of Pediatrics, University Federico II, Naples. The serum level of endomysium (EMA) and tissue transglutaminase (anti-tTG) antibodies [immunoglogbulin (Ig)A] was measured immediately before both gluten challenges started (day 0). EMA were detected by indirect immunofluorescence on frozen sections of human umbilical cord and anti-tTG using the enzyme-linked immunosorbent assay (ELISA) technique with a commercial kit (Eu tTg IgA; Eurospital, Trieste, Italy). Results were interpreted according to the manufacturer's instructions: negative <9 U/ml, weak positive in the range 9–16 U/ml, positive >16 U/ml. Patients ate 200 g of wheat bread or cookies daily for 3 days, corresponding to about 12 g of gluten per day (first challenge). After a wash-out of 3–10 months on a strict gluten-free diet, 13 of 14 coeliacs consumed wheat for an additional 3 days (second challenge). At the time of the first gluten challenge, 11 patients were seronegative for EMA or anti-tTG and three had low antibody titres. Two patients complained about abdominal pain on the first day of the challenge, but they did not stop the gluten intake. The remaining patients reported no symptoms.
Table 1.
Patients enrolled into the study
| 1st challenge | 2nd challenge | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Age/sex | Duration of GFD years | EMA† | Anti-tTG‡ | EMA† | Anti-tTG | Elapsed time (months) between 1st and 2nd challenge |
| Pt 1 | 21/F | 20 | Neg | 2·8 | Neg | 2·8 | 6 |
| Pt 2 | 15/F | 6 | Low Pos | 30·2 | Low Pos | 9·2 | 6 |
| Pt 3 | 15/F | 13 | Neg | 4·8 | Neg | 3·8 | 3 |
| Pt 4 | 18/M | 6 | Neg | 3·2 | Neg | 4·2 | 3 |
| Pt 5 | 16/F | 5 | Neg | 2 | Neg | 2·4 | 3 |
| Pt 6 | 17/M | 16 | Neg | 0·8 | Neg | 0·4 | 10 |
| Pt 7 | 18/M | 17 | Neg | 2 | Neg | 2 | 6 |
| Pt 8 | 19/F | 17 | Neg | n.d. | n.d. | n.d. | n.d. |
| Pt 9 | 17/F | 16 | Neg | 0·4 | Neg | 1·2 | 10 |
| Pt 10 | 20/M | 14 | Neg | 1·8 | Neg | 2·6 | 3 |
| Pt 11 | 19/F | 18 | Low Pos | 38 | Low Pos | 26 | 6 |
| Pt 12 | 22/F | 20 | Low Pos | 5·4 | Low Pos | 7·6 | 3 |
| Pt 13 | 24/F | 14 | Neg | 4 | Neg | 3·6 | 6 |
| Pt 14 | 20/F | 13 | Neg | 0·8 | Neg | 2 | 3 |
The serum endomysium (EMA) titres were evaluated before the wheat consumption (day 0) of both 1st and 2nd challenge.
Anti-tissue transglutaminase (anti-tTG) immunoglobulin (Ig)A sensitivity: negative <9 U/ml, weakly positive in the range 9–16 U/ml, positive >16 U/ml. GFD, gluten-free diet; pos, positive; neg, negative; F, female; M, male; n.d., not done.
Antigen preparation
A commercial wheat flour was used for baking the bread and cookies. Gliadin was extracted according to Wieser et al. [20] and digested enzymatically with pepsin and trypsin, as described previously [21]. The 33-mer (α-gliadin 57–89) peptide was synthesized by solid-phase automated flow, as described elsewhere [2]. Both PT-gliadin (indicated hereafter as gliadin) and peptides were deamidated with guinea pig tTG, as reported elsewhere [2].
Detection of IFN-γ-secreting cells
Venous blood (15–20 ml) was collected in a heparizined syringe before (day 0) and 6 days after (day 6) the gluten challenge. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density centrifugation. PBMCs were analysed immediately for antigen recognition by IFN-γ ELISPOT assay, as described previously [22]. Briefly, 4 × 105 PBMCs were seeded in 200 µl of complete medium X-Vivo15 supplemented with 5% heat-inactivated AB pooled human serum, 1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) and 1% L-glutamine (2 mM) (all provided by BioWhittaker, Verviers, Belgium) in duplicate in 96-well plates (Millipore, Bedford, MA, USA) coated with purified anti-human IFN-γ antibody (MabTech, Nacka Strand, Sweden). Gliadin, either deamidated or native, was tested at 50 µg/ml and 33-mer peptide at 30 µg/ml (7·7 µM). Cells were incubated for 36–40 h with biotinylated anti-human IFN-γ antibody (MabTech) followed by incubation with streptavidin horseradish peroxidase (HRP) (BD-Pharmingen, San Diego, CA, USA). Spot-forming cells (SFC) were counted by an immunospot analyser (A.EL.VIS, Hannover, Germany).
In the experiments with blocking monoclonal antibodies (mAbs), PBMCs were incubated with anti-DQ (10 µg/ml, clone SPV-L3; Biodesign International, Saco, ME, USA) at 37°C for 15 min, before the addition of deamidated gliadin. In depletion experiments of β7-integrin or CD4-positive cells, PBMCs were first incubated with phycoerythrin (PE)-conjugated β7-integrin or CD4 mAbs, and thereafter separated using anti-PE-conjugated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. In the functional experiments, total PBMCs, CD4-negative and β7-integrin-negative fractions were plated at 4 × 105 cells/well, while both β7-integrin-positive and CD4-positive cells were plated at 1 × 105 cells/well in the presence of 1 × 105 DQ2-positive Epstein–Barr virus B cells (EBV) as antigen-presenting cells (APC).
Statistical analysis and responsiveness criteria
All experiments were performed in duplicate. All variables at days 0 and 6 did not show normal distribution, estimated by skewness and kurtosis; hence, a non-parametric paired-sample Wilcoxon rank-sum test was used to compare day 6 versus day 0. Data (mean ± standard deviation of duplicates, or median and interquartile range 25–75) are expressed as total IFN-γ-SFC/4 × 105 PBMCs, or as net IFN-γ-SFC/4 × 105 (SFC detected in the presence of gliadin/peptides subtracted the SFC detected with medium alone), as indicated. Intra-assay variability was determined by stimulating with medium alone, or with deamidated gliadin, over six replicates of PBMCs from two separate individuals on day 6 of the first challenge. The intra-assay variation coefficient of IFN-γ-SFC/4 × 105 cells was 15·4%. Patients were considered responsive to oral gluten challenge when they showed an increase in SFC in response to gliadin and/or 33-mer peptide by three times the value observed before the gluten challenge started (fold increase ≥3), and a difference (ΔSFC) of at least 10 SFC/well between days 6 and 0.
Results
Peripheral blood response upon the first short wheat challenge
Fourteen DQ2-positive patients, aged between 15 and 24 years, participated in the study (Table 1). Two patients reported significant clinical symptoms during, and soon after, the 3 days' consumption of bread. Of note, these two symptomatic patients had low EMA/anti-tTG titres at the time the challenge began.
Peripheral blood mononuclear cells were tested for reactivity to either deamidated gliadin or 33-mer peptide (corresponding to the immunodominant α-gliadin 57–89) by detecting the IFN-γ-secreting cells at days 0 and 6 of the wheat challenge. In response to gliadin stimulation, the IFN-γ-SFC increased significantly in peripheral blood at day 6: median and interquartile range (25–75th centiles) of net IFN-γ-SFC/4 × 105 cells were 15·3 (7·0–39·5) and 66·5 (31·3–162·2) at days 0 and 6, respectively (P = 0·004) (Fig. 1a). Similarly, the in-vivo gluten challenge induced a significant increase of IFN-γ-positive cells in response to 33-mer: 0·3 (0·01–2·2) and 42·9 (0·5–127·1), respectively, at days 0 and 6 (P = 0·01) (Fig. 1b).
Fig. 1.
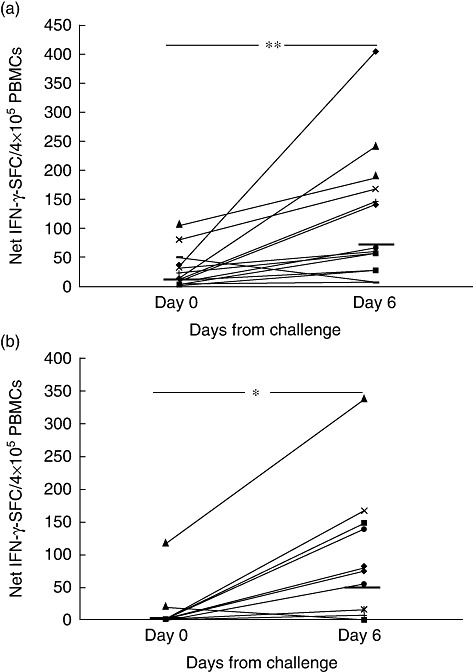
Gliadin-specific interferon (IFN)-γ production is elicited in peripheral blood upon a short (3 days) oral wheat challenge. Peripheral blood mononuclear cells from young coeliac patients (n = 14) were analysed for IFN-γ enzyme-linked immunospot (ELISPOT) assay before (day 0) and after (day 6) 3 days of wheat consumption. IFN-γ spot-forming cells (SFC) were assessed in response to in-vitro stimulation with (a) deamidated gliadin (50 µg/ml) and (b) deamidated 33-mer peptide (α-gliadin 57–89) (30 µg/ml/7·7 µM). Dashes indicate the median values of net IFN-γ-responses among patients. *P = 0·01; **P = 0·004 by non-parametric Wilcoxon's rank sum test.
When we evaluated the responsiveness of each individual coeliac volunteer, according to an arbitrary criterion of responsiveness (see Methods for details), we observed that 10 of 14 (71%) patients responded to the bread challenge with an increased IFN-γ-SFC to gliadin and/or to 33-mer at day 6 (Table 2).
Table 2.
Immune response to gliadin and 33-mer after the in-vivo wheat challenges
| 1st challenge | 2nd challenge | |||||||
|---|---|---|---|---|---|---|---|---|
| Gliadin | 33-mer | Gliadin | 33-mer | |||||
| ΔSFC† | FI‡ | ΔSFC† | FI‡ | ΔSFC† | FI‡ | ΔSFC† | FI‡ | |
| Pt 1 | 25 | 25 | 0 | 1 | 233 | 234 | 0 | 1 |
| Pt 2 | 368 | 11·5 | 81 | 81 | 69 | 1·4 | 164 | 5·1 |
| Pt 3 | 29 | 1,9 | 17 | 17 | 10 | 10 | 0 | 1 |
| Pt 4 | 0 | 0·14 | 0 | 0 | 56 | 1·3 | 0 | 0·7 |
| Pt 5 | 58 | 8·2 | 139 | 139 | 81 | 5,3 | 0 | 1 |
| Pt 6 | 56 | 56 | 148 | 148 | 0 | 0·9 | 11 | 12 |
| Pt 7 | 83 | 1·8 | 221 | 2,9 | 24 | 1·2 | 104 | 2·5 |
| Pt 8 | 130 | 15·4 | 77 | 77 | n.d. | n.d | n.d. | n.d. |
| Pt 9 | 225 | 16 | 0 | 0 | 0 | 1 | 14 | 14 |
| Pt 10 | 32 | 2·4 | 8 | 8 | 328 | 1·6 | 0 | 3 |
| Pt 11 | 16 | 2·6 | 22 | 22 | 343 | 8·6 | 7 | 7 |
| Pt 12 | 0 | 1 | 0 | 0 | 47 | 1·5 | 4 | 4 |
| Pt 13 | 131 | 10 | 79 | 79 | 0 | 0·4 | 0 | 0·8 |
| Pt 14 | 88 | 2·1 | 167 | 167 | 1 | 1·1 | 2 | 2 |
ΔSFC (spot-forming cells): net interferon (IFN)-γ SFC detected on day 6 minus net IFN-γ SFC detected on day 0.
FI (fold increase): net IFN-γ SFC in response to gliadin/peptide 33-mer observed on day 6 divided by the net IFN-γ SFC observed on day 0; n.d., not done.
As mentioned previously, some patients showed weak EMA/anti-tTG positivity (patients 2, 11 and 12, Table 1). Of note, two of these three patients responded to the challenge (Table 2), suggesting that the presence of CD-associated antibodies, at least at low titres, does not hamper responsiveness to the short oral wheat challenge.
Gliadin specificity and intestinal origin of IFN-γ-releasing cells in peripheral blood after the short wheat challenge
We investigated whether the IFN-γ responses elicited in peripheral blood by short wheat consumption were triggered specifically by gliadin and, more importantly, if they were mediated by mucosal activated T cells. Because it is well documented that the deamidation of gliadin peptides by tTG strongly increase the stimulation of CD4+ T cells in CD patients due to the stronger binding of negatively charged peptides to DQ2/DQ8 HLA molecules [2,3], we evaluated IFN-γ production against either native or deamidated gliadin in the ELISPOT assay, in order to assess antigen specificity. As shown in Fig. 2a, IFN-γ found at day 6 was elicited mainly by deamidated gliadin, as the native gliadin preparation induced approximately 20% of the response obtained with deamidated gliadin. In addition, the number of specific spots were reduced strongly upon blocking HLA-DQ molecules (Fig. 2b), and were abolished almost completely when we depleted the CD4-positive cells from the total PBMCs (Fig. 2c). Conversely, the enriched CD4-positive fractions, with a purity of 99% and 98·66% in patients 13 and 14, respectively, showed an increased IFN-γ response to gliadin in both patients.
Fig. 2.
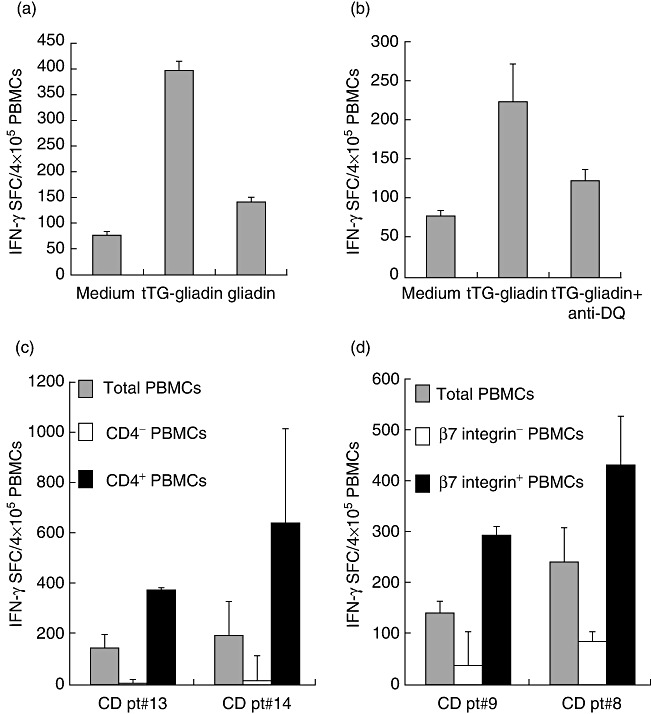
Interferon (IFN)-γ responses raised in peripheral blood after the brief wheat challenge are gliadin-specific and mediated by DQ-restricted, CD4+ T cells of intestinal origin. IFN-γ enzyme-linked immunospot (ELISPOT) responses were assessed in peripheral blood mononuclear cells (PBMCs) of coeliac disease (CD) subjects on day 6 of the first wheat challenge in the presence of native or deamidated gliadin (a). IFN-γ ELISPOT response to deamidated (tTG) gliadin were assessed in PBMCs in the presence or absence of anti-DQ antibodies (10 µg/ml) (b). IFN-γ responses were monitored in whole PBMCs or in CD4– (c) and β7-integrin-depleted cells (d). Mean values of duplicate wells (± standard deviation) are reported. One of three independent experiments is shown in each panel.
Finally, a crucial question raised when investigating peripheral blood immune responses against dietary antigens is whether the circulating T cells are primed or recalled in the gut upon the antigen oral exposure. We addressed the intestinal origin of the observed response to gliadin by separating the cell fraction expressing the β7-integrin, a marker of gut-homing/commitment, from the PBMCs. Similarly to CD4-positive cells, depletion of the β7-integrin-expressing cells resulted in a drastic reduction of the IFN-γ-SFC in response to gliadin (75 and 66% inhibition compared to the response of total PBMCs) (Fig. 2d), while the β7-integrin-enriched cell fractions, with a purity of 91·56 and 95·15% in patients 8 and 9, respectively, showed an increased number of spots compared to those observed in whole PBMCs.
Consistency of peripheral blood response to gliadin in patients challenged twice
Next we investigated the consistency of the response to gluten challenge in our cohort of coeliac volunteers who underwent two separate wheat consumptions performed with the same procedure. After a wash-out of 3–10 months on a strict gluten-free diet, 13 of 14 subjects consumed wheat for 3 days (Table 1). Similarly to the first challenge, a statistically significant increase of IFN-γ-SFC in response to gliadin was observed after the second challenge: median and interquartile range (25–75th centiles) of net IFN-γ-SFC/4 × 105 cells were: 37·6 (1·6–150·9) and 152·3 (24·1–292·8) in response to gliadin, respectively, at days 0 and 6 (P < 0·04) (Fig. 3a); and 0·01 (0·01–6·7) and 4·3 (0·01–17·3) in response to 33-mer, respectively, at days 0 and 6 (P < 0·04) (Fig. 3b).
Fig. 3.
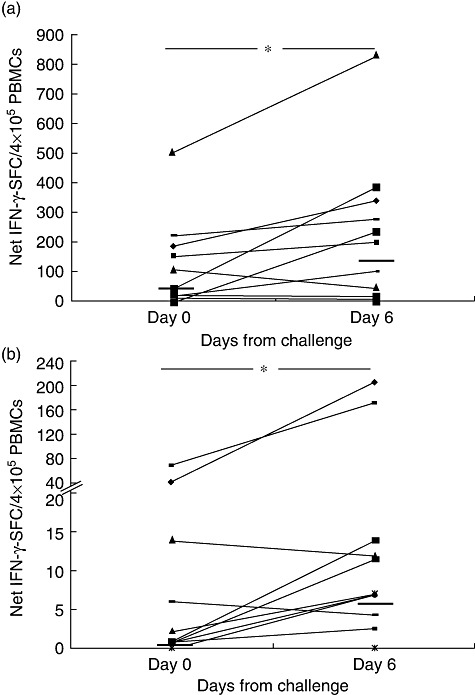
Consistency of interferon (IFN)-γ responses in coeliac patients underwent two separate wheat challenges. Responsiveness to gliadin in 13 coeliac patients underwent a second short wheat consumption after 3–10 months of a strict gluten-free diet (wash-out). Peripheral blood mononuclear cells (PBMCs) were assayed by enzyme-linked immunospot (ELISPOT) for recognition of deamidated gliadin (a) or 33-mer (b) on day 0 and day 6 of oral challenge. Results are shown as in Fig. 1. Dashes indicate the median values of IFN-γ responses among patients (*P < 0·04).
Surprisingly, although these donors repeated the wheat challenge at least 3 months after the first one, and were on a strict gluten-free diet regimen, the IFN-γ-SFC elicited by gliadin at day 0 of the second challenge was increased if compared to the SFC obtained just before the first challenge (median 15·0, interquartile range 7·8–35), although the increase did not reach statistic significance (P < 0·078). Similarly, the responses observed at day 6 of the second challenge exceeded those elicited during the first challenge (median 61·0, interquartile range 25·6–166·0), although the difference was not statistically significant (P = 0·23). Conversely, the increment of reactivity to the 33-mer peptide was reduced after the second challenge when compared to the first challenge (median 22·0, interquartile range 0·33–139·67, P = 0·1).
When we evaluated individual reactiveness after the second challenge, seven of 13 (53%) subjects were responsive to gliadin and/or 33-mer (Table 2). Interestingly, these seven patients also had a positive response to the first challenge (Table 2). Conversely, patients 4, 7, 10 and 12 responded to neither the first nor the second challenges, while the remaining two patients (patients 13 and 14), who responded to neither gliadin nor 33-mer after the second challenge, had a substantial increase of IFN-γ-secreting cells at the first challenge. Next, we investigated whether the time elapsed between the two challenges might have influenced the individual responsiveness, but no correlation was observed with the increment of response to gliadin and to 33-mer (Pearson's correlation: r = −0·264, P > 0·3 and r = 0·312, P > 0·2, respectively). Overall, our findings indicated a concordance of responsiveness to the short wheat challenges (considered either as positive or negative responses) in 11 of 13 (85%) of the patients, and confirm that short gluten consumption is a valid and reproducible tool to monitor immune reactiveness to gluten.
Discussion
The detection in peripheral blood of gluten-reactive T cells that have been activated, or primed, in the gut-associated lymphoid tissue during gluten consumption might have important therapeutic and diagnostic implications in CD. In this context, the short-term oral wheat challenge, reported first by Anderson and co-workers, is a simple and safe method that allows analysis and quantification of gluten-reactive T cells raised in peripheral blood of coeliac patients after 3 days of wheat consumption [4–6]. In view of a potential translational practice of the short-term wheat challenge, it is crucial to replicate the in-vivo procedure in different patient cohorts, as well as to assess its reproducibility in the same individual. To our knowledge, this test was replicated by another research group in a Norwegian cohort of adult CD patients [7,8]. In the present study we validated this method in a cohort of 14 young CD patients recruited in the south of Italy, and estimated the level of its reproducibility by exposing the same individual twice to gluten consumption.
After the first in-vivo challenge we observed a significant increase of IFN-γ-secreting cells in response to gliadin 6 days after the wheat intake, confirming the data reported in both Australian and Norwegian adult coeliac patients [4,7,8,23]. Similarly, the magnitude of the IFN-γ responses was comparable to the values found in previous studies [4–7]. When we looked at individual responses we found that, upon wheat consumption, the frequency of IFN-γ-releasing cells to whole gliadin increased at least three times in eight of 14 (57%) subjects, barely within the average obtained in previous studies, that ranged from 40% [23] to 90% [5] of exposed coeliac patients. In agreement with these studies, the specific response to gluten elicited by the in-vivo challenge was mediated by CD4+ T cells and was DQ2-restricted. Furthermore, the IFN-γ-producing cells expressed beta-7 integrin, indicating a phenotype of gut-homing cells.
Short-term gluten consumption also induced a significant increase of T cells reacting to the immunodominant 33-mer peptide, although contrasting findings were reported on the frequency of responder patients [2,3]. Anderson and co-workers reported that the great majority of coeliacs reacted to 33-mer (or to truncated peptide, α-gliadin (57–73) [5,6], while in a more recent study reactivity was observed in only six of 10 patients [23]. Our results are in agreement with this latter finding, as we found an evident increase of IFN-γ responses induced by immunodominant gliadin peptide in 8 of 14 patients at first challenge. Unexpectedly, upon the second challenge the number of reacting subjects was far fewer (three of 13 subjects challenged). In this regard, we found that approximately 50% of intestinal T cell lines generated from south Italian CD patients who were assayed in vitro reacted to 33-mer, suggesting that only a subgroup of our coeliac donors seems to display a response to this epitope [2]. These data are not surprising because, despite its strong immunogenicity, 33-mer is one of several gliadin-derived T cell epitopes active in coeliac patients [2,6], and this could explain the increased magnitude of IFN-γ-positive cells found in response to whole gliadin digest. In contrast to previous studies, in which the immune reactivity to gluten was very low, or totally absent, before wheat consumption at day 0, we also found substantial IFN-γ production instead. It cannot be excluded that in some CD patients, gliadin-reactive T cells circulate in the peripheral blood as resting memory cells, and their detection does not require in-vivo gluten stimulation. Corroborating this hypothesis, a marked proliferation triggered by gliadin was reported in the peripheral blood of treated CD patients in the absence of gluten oral load, and accounted for predominantly by memory CD4+ T cells [24–26]. In addition, CD8+ T lymphocytes reactive to a gliadin peptide and restricted by the HLA class I A2 molecule can be detected by the sensitive IFN-γ-ELISPOT assay in the peripheral blood of both treated and untreated CD patients who did not undergo an in-vivo wheat gluten challenge [22]. Although our coeliac volunteers declared strict adherence to a gluten-free diet, we cannot exclude that for some of them an accidental gluten introduction might have occurred. It can be envisaged that occasional exposure to gluten could, in some cases, produce an increased frequency of gluten-reactive T cells detectable in the blood, associated presumably with the production of anti-tTG antibodies. However, although we found slight EMA/anti-tTG-positive titres in three patients, they showed no evident differences in their response to gluten challenge compared to the EMA-negative subjects.
In this study we compared the peripheral responses of 13 volunteers who underwent two separate wheat consumptions, separated by 3–10 months of a strict gluten-free diet. We found that the IFN-γ responses increased significantly in peripheral blood sampled 6 days after the second challenge and, unexpectedly, cells reactive to whole gliadin were often more frequent than those observed in the first challenge, due most probably to the increased frequency of memory T cells activated upon the first gluten exposure. However, the relatively small size of the patient cohort did not allow us to observe a statistically significant difference in the frequency of responsive cells at day 0 between the first and second challenges. Furthermore, there was no significant correlation between the specific PBMC responses to gluten and the time elapsed between the two wheat challenges. Overall, our findings suggest that a wash-out of at least 3 months is sufficient time to raise gluten-specific cells in the blood. Further studies are required to assess the memory phenotype and life turnover of circulating T cells raised during the gluten in-vivo exposure. To our knowledge, reproducibility of the short gluten challenge in the same study cohort has been poorly investigated. Importantly, we observed consistent responsiveness to the two short wheat challenges, either in terms of positive or negative responses, in 11 of 13 (85%) the patients. Raki et al. [7] reported a reduction of DQ2-α-I tetramer-positive T cells in the only patient subjected to a repeated challenge, suggesting recruitment of specific T cells in the gut after the first activation. Anderson et al. also observed an overall reduction of responses to the 17-mer peptide after the second challenge in seven subjects who were challenged twice at 3–12 months [5].
Unfortunately, it was not possible to recruit non-coeliac DQ2-positive control individuals who had not been exposed previously to gluten for 2–3 years, although these would have been the ideal controls in our study population.
Additional studies involving a larger number of patients are required to ascertain the specificity and sensitivity of the in-vivo gluten challenge, in order to assess its potential suitability as a diagnostic tool, as investigated in a recent study [16]. To this purpose, it would be interesting to monitor the reactiveness of small children at the early stage of CD, or in those with ‘potential’ CD, as well as in first-degree relatives with the highest risk of developing the disease [17].
In conclusion, in the present study we replicated successfully the in-vivo gluten challenge approach in a cohort of 14 adolescent Italian CD patients. The short-term wheat challenge proved to be a reproducible tool to monitor the immune response to gluten. Assay replication, as well as reproducibility, represent crucial prerequisites in view of a potential application of the short-term oral challenge in a clinical setting. The design of clinical trials aimed to evaluate novel therapeutic drugs, or the safety of alternative cereals, could benefit greatly by this non-invasive short-term procedure.
Acknowledgments
The technical assistance of Dr Patrizia Iardino of Department of Laboratory Medicine, Second University of Naples (SUN) for anti-tTG determinations is greatly acknowledged. We are extremely grateful to Dr Robert Anderson for constructive and helpful discussion. This study was supported partially by a research grant from the Italian Celiac Association (AIC).
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–25. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 2.Camarca A, Anderson RP, Mamone G, et al. Intestinal T cell responses to gluten peptides are largely heterogeneous: implications for a peptide-based therapy in celiac disease. J Immunol. 2009;182:4158–66. doi: 10.4049/jimmunol.0803181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz-Hansen H, McAdam SN, Molberg Ø, et al. Celiac lesion T cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroenterology. 2002;123:803–09. doi: 10.1053/gast.2002.35381. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RP, Degano P, Godkin AJ, et al. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RP, van Heel DA, Tye-Din JA, et al. T cells in peripheral blood after gluten challenge in coeliac disease. Gut. 2005;54:1217–23. doi: 10.1136/gut.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tye-Din JA, Stewart JA, Dromey JA, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2:41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 7.Ráki M, Fallang LE, Brottveit M, et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc Natl Acad Sci USA. 2007;104:2831–36. doi: 10.1073/pnas.0608610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodd M, Ráki M, Tollefsen S, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010;3:594–01. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- 9.Ellis HJ, Ciclitira PJ. In vivo gluten challenge in celiac disease. Can J Gastroenterol. 2001;15:243–47. doi: 10.1155/2001/127241. [DOI] [PubMed] [Google Scholar]
- 10.Dewar DH, Amato M, Ellis HJ, et al. The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur J Gastroenterol Hepatol. 2006;18:483–91. doi: 10.1097/00042737-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Fraser JS, Engel W, Ellis HJ, et al. Coeliac disease: in vivo toxicity of the putative immunodominant epitope. Gut. 2003;52:1698–02. doi: 10.1136/gut.52.12.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciacci C, Maiuri L, Caporaso N, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr. 2007;26:799–05. doi: 10.1016/j.clnu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacodynamic effects of single doses of AT-1001 in celiac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 14.Carroccio A, Iacono G, Montalto G, et al. Immunologic and absorptive tests in celiac disease: can they replace intestinal biopsies? Scand J Gastroenterol. 1993;28:673–76. doi: 10.3109/00365529309098270. [DOI] [PubMed] [Google Scholar]
- 15.Tveito K, Brunborg C, Bratlie J, et al. Intestinal malabsorption of d-xylose: comparison of test modalities in patients with celiac disease. Scand J Gastroenterol. 2010;45:1289–94. doi: 10.3109/00365521.2010.503969. [DOI] [PubMed] [Google Scholar]
- 16.Brottveit M, Ráki M, Bergseng E, et al. Assessing possible celiac disease by an HLA-DQ2-gliadin tetramer test. Am J Gastroenterol. 2011;106:1318–24. doi: 10.1038/ajg.2011.23. [DOI] [PubMed] [Google Scholar]
- 17.Troncone R, Ivarsson A, Szajewska H, et al. Review article: future research on coeliac disease – a position report from the European multistakeholder platform on celiac disease (CDEUSSA) Aliment Pharmacol Ther. 2008;27:1030–43. doi: 10.1111/j.1365-2036.2008.03668.x. [DOI] [PubMed] [Google Scholar]
- 18.Tosco A, Salvati V, Auricchio R, et al. Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol. 2011;9:320–25. doi: 10.1016/j.cgh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Report of the Working Group of European Society of Paediatric Gastroenterology and Nutrition. Revised criteria for the diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieser H, Antes S, Seilmeier W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem. 1998;75:664–50. [Google Scholar]
- 21.Gianfrani C, Siciliano RA, Facchiano AM, et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology. 2007;133:780–89. doi: 10.1053/j.gastro.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Gianfrani C, Troncone R, Mugione P, et al. Coeliac disease association with CD8+ T cell responses: identification of a novel gliadin-derived HLA-A2 restricted epitope. J Immunol. 2003;170:2719–26. doi: 10.4049/jimmunol.170.5.2719. [DOI] [PubMed] [Google Scholar]
- 23.Tye-Din JA, Anderson RP, Ffrench RA, et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin Immunol. 2010;134:289–95. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Gjertsen HA, Sollid LM, Ek J, Thorsby E, Lundin KE. T cells from the peripheral blood of coeliac disease patients recognize gluten antigens when presented by HLA-DR, -DQ, or -DP molecules. Scand J Immunol. 1994;39:567–74. doi: 10.1111/j.1365-3083.1994.tb03414.x. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Horin S, Green PH, Bank I, Chess L, Goldstein I. Characterizing the circulating, gliadin-specific CD4+ memory T cells in patients with celiac disease: linkage between memory function, gut homing and Th1 polarization. J Leukoc Biol. 2006;79:676–85. doi: 10.1189/jlb.0705414. [DOI] [PubMed] [Google Scholar]
- 26.Jensen K, Sollid LM, Scott H, et al. Gliadin-specific T cell responses in peripheral blood of healthy individuals involve T cells restricted by the coeliac disease associated DQ2 heterodimer. Scand J Immunol. 1995;42:166–70. doi: 10.1111/j.1365-3083.1995.tb03640.x. [DOI] [PubMed] [Google Scholar]


