Abstract
Complement system activation is associated with immunoglobulin A nephropathy (IgAN) activity and progression. The aim of the present study was to investigate the importance of urinary mannose-binding lectin (MBL), at the time of renal biopsy, for evaluating disease severity and predicting the progression of IgAN. A total of 162 patients with biopsy-proven IgAN were enrolled and 50 healthy individuals were selected as normal controls. Urinary MBL was measured by sandwich enzyme-linked immunosorbent assay (ELISA) and normalized for urinary creatinine concentration. Urinary MBL was significantly higher in IgAN patients than that in normal controls, and elevated as histopathological phenotypes upgraded. Urinary MBL was correlated significantly with the well-known clinical predictors for the prognosis of IgAN; that is, renal function (represented by serum creatinine and estimated glomerular filtration rate), proteinuria and arterial hypertension. Urinary MBL was demonstrated to be correlated with the histopathological parameters which have independent value in predicting renal outcome of IgAN according to the Oxford classification; that is, mesangial hypercellularity, segmental glomerulosclerosis, endocapillary hypercellularity and tubular atrophy/interstitial fibrosis. More importantly, non-remission patients at the end of follow-up had significantly higher levels of urinary MBL compared with patients in remission. In conclusion, urinary MBL can be a reliable non-invasive biomarker for evaluating disease severity and predicting the prognosis of IgAN. This is the first report on this issue. However, our conclusions should be verified further in large-scale studies with long-term follow-up.
Keywords: biomarker, disease severity, IgA nephropathy, MBL, prognosis
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis worldwide, with a prevalence of 20–45% in primary glomerular disease [1]. It is characterized primarily by mesangial deposition of IgA [2,3]. The clinical spectrum of IgAN covers a wide range of features, from minor urinary abnormalities to rapidly progressive renal failure. About 20–40% patients with IgAN could progress to end-stage renal disease (ESRD) within 20 years of diagnosis [4]. Thus, the evaluation of disease severity and progression is crucial in IgAN patients. Renal histological lesions, renal function, the presence of arterial hypertension and proteinuria at the time of renal biopsy are all well-known predictors in IgAN [5–7], and recently the investigation of novel biomarkers based on the pathogenesis of IgAN has been a research focus [8–10].
In IgAN, the deposits of IgA are associated frequently with complement components, and complement system activation is associated with IgAN activity and progression [11,12]. The complement system can be activated via three pathways: the classical pathway, the alternative pathway and the lectin pathway. Studies in vitro have indicated that the classical pathway is unlikely to contribute to IgAN, whereas substantial evidence has proved that the alternative pathway and the lectin pathway are involved in IgAN [13–15]. Mannose-binding lectin (MBL) is a member of the collectin family of proteins with a C-terminal lectin domain and a collagenous backbone [16]. Its collagen-like regions can react with the MBL-associated serin proteases mannose-associated serine protease (MASP)-1 and MASP-2, and thus activate the lectin pathway of complement. Furthermore, MBL deposition co-localized with IgA deposits has been identified as a marker for lectin pathway activation in a significant number of IgAN patients, and the presence of MBL may be associated with more severe glomerular injury of IgAN [15,17].
In recent years, urine has been an attractive sample because its collection is easy and non-invasive, and in addition a urine protein profile directly reflects glomerular disease, including IgA nephropathy [18]. Significantly elevated levels of urinary MBL have been documented in patients with contrast-induced nephropathy [19], but the significance of urinary MBL in IgAN has not yet been reported.
The aim of our study was to investigate the value of urinary MBL, at the time of renal biopsy, in evaluating the disease severity and predicting the progression of renal disease in a cohort of IgAN patients.
Materials and methods
Subjects
One hundred and sixty-two patients (92 men) who underwent renal biopsy in our department were enrolled consecutively and were diagnosed as primary IgAN from December 2008 to July 2010. The day of renal biopsy was regarded as the start-day of the study. The planned follow-up was to December 2011. Patients with secondary IgAN were excluded, such as those secondary to systemic lupus erythematosus, Henoch–Schönlein purpura and liver disease. No corticosteroids and immunosuppressants were applied before the beginning of the study. Fifty healthy age- and sex-matched individuals were selected as normal controls.
The severity of renal histological lesions in IgAN patients was graded according to Lee's classifications [20]. The 162 patients included were divided further into three groups according to the degrees of renal damage; 49 patients fulfilling the histopathological criteria of Lee-I or II were defined as mild renal lesions (group 1), 63 patients with Lee-III as moderate renal lesions (group 2) and 50 patients with Lee-IV or V as severe renal lesions (group 3).
The clinical parameters of IgAN patients such as routine urinalysis, serum creatinine (SCr) and 24-h urinary protein excretion were obtained immediately before renal biopsy. The estimated glomerular filtration rate (eGFR) was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) formula [21], and the result was reduced by 25·8% for women. Patients were considered hypertensive if their arterial blood pressure was 140/90 mmHg or more, or if levels less than 140/90 mmHg were reached with anti-hypertensive medications. This study was performed according to recommendations outlined in the Declaration of Helsinki Principles (IV Adaptation). Sampling and renal biopsy were performed after signed informed consent.
Evaluation of renal histopathological lesion
Adequate tissue was obtained for diagnostics (at least eight glomeruli in light microscopy sections and complete immunohistology and electronmicroscopy examination). Sections were stained routinely for haematoxylin and eosin, periodic-acid Schiff and/or periodic-acid-silver methenamine for histopathology. Two pathologists who were blinded to patients' data evaluated the slides separately.
Besides the overall evaluation with Lee's classification, we further evaluated four variables of renal histopathological lesion recommended by the Oxford classification, which have independent value in predicting renal outcome [22]. Mesangial proliferation was scored as M0 if no more than half the glomeruli have more than three cells in a mesangial area, and M1 for more than half the glomeruli. Segmental glomerulosclerosis and endocapillary hypercellularity were categorized as either absent or present (i.e. S0 or S1, and E0 or E1). Tubular atrophy/interstitial fibrosis was classified according to the percentage of cortical area involved by the tubular atrophy or interstitial fibrosis; that is: ≤ 25% as T0, 26–50% as T1, and >50% as T2. It should be specified that all the included patients in the present study, even those cases with sclerosis, had active histological changes.
Treatment protocol
Patients with haematuria, proteinuria of less than 1 g/24 h and normal renal function received non-immunosuppressive therapy, including angiotensin converting enzyme inhibitors (ACEI) and/or angiotensin II receptor blockers (ARB), fish oil, statins and anti-platelets. Patients with proteinuria of 1·0 g/24 h or more and histological manifestations of cellular/fibrocellular crescents, moderate to severe mesangial proliferation and/or interstitial cell infiltration were treated with immunosuppressive therapy [23–25]; that is, corticosteroids alone or combined with other immunosuppressive drugs (e.g. cytostatics, cyclosporin A and mycophenolate mofetil). Non-immunosuppressive therapy could also be used in the latter group of patients as needed.
Outcome definitions
To evaluate further the value of urinary MBL for predicting progression in IgAN, we divided IgAN patients into remission group and non-remission group according to renal function and urinary protein excretion at the end of follow-up. Renal remission was defined as stable or improved renal function (i.e. no ‘progressive renal impairment’) with reduction of proteinuria by > 50%, proteinuria less than 3 g/24 h and serum albumin >30 g/l. Progressive renal impairment was defined as a reduction in eGFR ≥ 5 ml/min/1·73 m2/year or renal replacement therapy received during the follow-up period.
Urinary MBL measurement by enzyme-linked immunosorbent assay (ELISA)
On the day of renal biopsy, morning urine samples of IgAN patients were collected to measure urinary MBL and creatinine excretion. The urine samples were centrifuged at 769 g for 10 min to remove cellular components and the supernatant was frozen at −80°C until tested. Spot morning urine samples from healthy individuals were also collected and prepared in the same manner as for patients.
ELISA kits designed for human MBL (USCN Life Science, Inc., Missouri City, TX, USA) were used for detecting urinary MBL. This is a sandwich ELISA kit specific for the human MBL in which the polystyrene 96-well microtitre plate was precoated with a monoclonal antibody specific for MBL. Standards or urine samples were then added to the appropriate microtitre plate wells with a biotin-conjugated polyclonal antibody preparation specific for MBL. Next, avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. A 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution was then added to each well. The enzyme–substrate reaction was terminated by the addition of a sulphuric acid solution and the colour change was measured at a wavelength of 450 nm wavelength with a Model 680 Microplate Reader (Bio-Rad Laboratories K.K., Tokyo, Japan). The concentration of MBL in the samples was then determined by comparing the optical density (OD) of the samples to the standard curve, and expressed in ng/ml. The minimum detectable dose of human MBL of the kit was 0·28 ng/ml, and the detection range was 0·94–60 ng/ml. The measurement of urinary MBL was normalized for urinary creatinine (uCr) concentration (expressed as ng/mg uCr), and the ratios of urinary MBL to uCr were used for analysis.
Statistical analysis
Continuous data were analysed descriptively by using mean ± standard deviation (s.d.). Categorical data were described as absolute frequencies and percentages. χ2 test, independent t-test, one-way analysis of variance (anova) and the Mann–Whitney U-test were used for comparison of all the clinical and histopathological data, and Spearman's correlation was applied for correlation analysis. All the analyses were performed with spss version 16·0 (SPSS, Inc., Chicago, IL, USA), and a two-tailed P-value ≤0·05 was considered statistically significant.
Results
Characteristics of IgAN patients
The clinical characteristics of IgAN patients among different groups are described in Table 1. No significant differences were detected in mean age, sex and rate of loss to follow-up among three groups. The levels of SCr presented a significant increase as the histopathological phenotypes aggravated, whereas the levels of eGFR went in the opposite direction. There were no significant differences between groups 1 and 2 in the ratio of patients with hypertension and urinary protein excretion, but the two parameters were significantly higher in group 3 than those in groups 1 and 2. The remission ratio was not significantly different between groups 1 and 2, but was significantly lower in group 3.
Table 1.
Clinical characteristics of IgAN patients
| P-value | ||||||
|---|---|---|---|---|---|---|
| Group 1 (n = 49) | Group 2 (n = 63) | Group 3 (n = 50) | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| Mean age (years) | 33·51 ± 11·07 | 36·41 ± 11·91 | 35·98 ± 12·59 | 0·461 | 0·661 | 0·997 |
| Male/female | 26/23 | 25/28 | 31/19 | 0·552 | 0·368 | 0·131 |
| Hypertension | 9/49 | 13/63 | 35/50 | 0·764 | <0·001 | <0·001 |
| SCr (µmol/l) | 66·69 ± 18·16 | 88·60 ± 46·65 | 253·34 ± 197·57 | 0·003 | <0·001 | <0·001 |
| eGFR (ml/min/1·73 m2) | 121·42 ± 26·42 | 93·78 ± 34·59 | 42·45 ± 31·11 | <0·001 | <0·001 | <0·001 |
| Proteinuria (g/24 h) | 1·71 ± 2·38 | 1·93 ± 2·04 | 4·14 ± 3·11 | 0·938 | <0·001 | <0·001 |
| Loss to follow-up | 3/49 | 6/63 | 8/50 | 0·511 | 0·118 | 0·299 |
| Remission | 39/46 | 39/57 | 7/42 | 0·054 | <0·001 | <0·001 |
IgAN: immunoglobulin A nephropathy; SCr: serum creatinine; eGFR: estimated glomerular filtration rate.
The histopathological parameters of IgAN patients are shown in Table 2. The patients' ratios of M0 : M1, S0 : S1, E0 : E1 and T0 : T1 : T2 showed a trend of gradual decrease with the aggravation of histopathological phenotypes.
Table 2.
Histopathological characteristics of IgAN patients
| P-value | ||||||
|---|---|---|---|---|---|---|
| Group 1 (n = 49) | Group 2 (n = 63) | Group 3 (n = 50) | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| M0/M1 | 47/2 | 50/13 | 25/25 | 0·011 | <0·001 | 0·001 |
| S0/S1 | 35/14 | 17/46 | 3/47 | <0·001 | <0·001 | 0·004 |
| E0/E1 | 40/9 | 39/24 | 20/30 | 0·023 | <0·001 | 0·021 |
| T0/T1/T2 | 48/1/0 | 27/29/7 | 2/20/28 | <0·001 | <0·001 | <0·001 |
According to the Oxford classification, four variables were evaluated; that is, mesangial proliferation (M), segmental glomerulosclerosis (S), endocapillary hypercellularity (E) and tubular atrophy/interstitial fibrosis (T). IgAN: immunoglobulin A nephropathy.
Urinary MBL in IgAN patients and normal controls
The levels of urinary MBL in IgAN patients were significantly higher than those in normal controls (4·65 ± 9·64 ng/mg uCr versus 0·50 ± 0·39 ng/mg uCr, P < 0·001) (Fig. 1). Furthermore, among three groups with different histopathological phenotypes, the levels of urinary MBL were significantly higher in group 3 than those in group 2 (10·25 ± 14·99 ng/mg uCr versus 3·1 ± 4·96 ng/mg uCr, P = 0·006), and similarly, they were also significantly higher in group 2 compared with those in group 1 (3·1 ± 4·96 ng/mg uCr versus 0·94 ± 1·05 ng/mg uCr, P = 0·004) (Fig. 2).
Fig. 1.
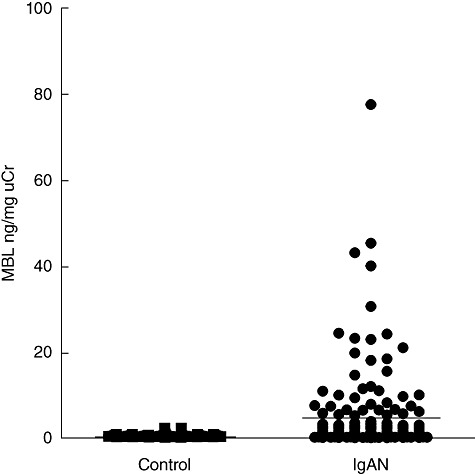
Comparison of the levels of urinary mannose-binding lectin (MBL) between immunoglobulin A nephropathy (IgAN) patients and normal controls. The horizontal solid lines indicate the median values for each group.
Fig. 2.
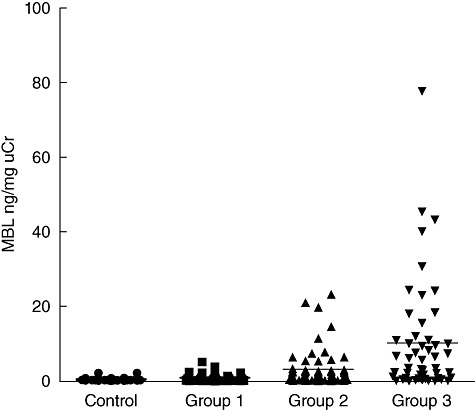
Comparison of the levels of urinary mannose-binding lectin (MBL) among different renal histopathological phenotypes. The horizontal solid lines indicate the median values for each group.
Correlations between urinary MBL and clinical parameters
In this cohort of IgAN patients, the levels of urinary MBL had no significant correlation with age (P = 0·398), and were not significantly different between male and female patients (4·06 ± 7·72 ng/mg uCr versus 5·44 ± 11·72 ng/mg uCr, P = 0·366). However, patients with hypertension had significantly higher levels of urinary MBL than those without hypertension (9·10 ± 14·45 ng/mg uCr versus 2·24 ± 3·85 ng/mg uCr, P = 0·001) (Fig. 3a).
Fig. 3.
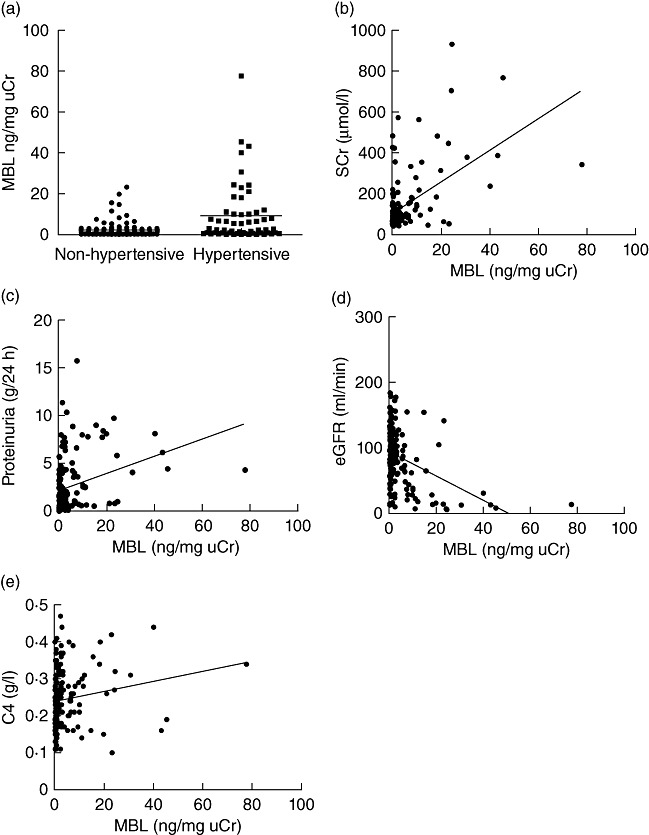
Correlations between urinary mannose-binding lectin (MBL) and clinical parameters in immunoglobulin A nephropathy (IgAN) patients. The horizontal solid lines indicate the median values for each group.
Urinary MBL was correlated positively with the levels of SCr and proteinuria, but negatively with eGFR (r = 0·541, P < 0·001; r = 0·322, P < 0·001; r = −0·405, P < 0·001) (Fig. 3b–d). There was no significant correlation between urinary MBL and haematuria (expressed by RBC counts per high-power field) (P = 0·146). Furthermore, the levels of urinary MBL had no correlation with the levels of serum IgA and C3 (P = 0·502, 0·314), but had a positive correlation with serum C4 (r = 0·171, P = 0·03) (Fig. 3e).
Correlations between urinary MBL and histopathological parameters
For mesangial proliferation, the levels of urinary MBL were significantly higher in patients with M1 than those in patients with M0 (12·57 ± 15·36 ng/mg uCr versus 2·06 ± 4·50 ng/mg uCr, P < 0·001) (Fig. 4a); for segmental glomerulosclerosis, patients scored as S1 had significantly higher levels of urinary MBL than those scored as S0 (5·78 ± 11·27 ng/mg uCr versus 2·47 ± 4·52 ng/mg uCr, P = 0·009) (Fig. 4b); for endocapillary hypercellularity, patients scored as E1 had also significantly higher levels of urinary MBL than those scored as E0 (7·59 ± 13·87 ng/mg uCr versus 2·78 ± 4·70 ng/mg uCr, P = 0·01) (Fig. 4c); and the levels of urinary MBL were elevated significantly with the severity of tubular atrophy or interstitial fibrosis upgraded; that is, they were significantly higher in patients scored as T2 than in those scored as T1 (14·23 ± 16·65 ng/mg uCr versus 3·44 ± 4·53 ng/mg uCr, P = 0·002) and T1 than T0 (3·44 ± 4·53 ng/mg uCr versus 1·09 ± 1·92 ng/mg uCr, P = 0·003) (Fig. 4d).
Fig. 4.
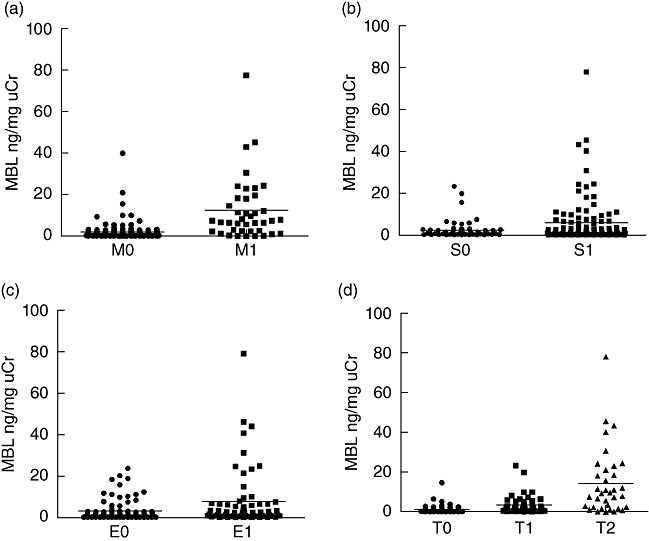
Correlations between urinary mannose-binding lectin (MBL) and four histopathological parameters in immunoglobulin A nephropathy (IgAN) patients. The horizontal solid lines indicate the median values for each group.
Correlation between urinary MBL and renal remission
Of 162 IgAN patients, 17 were lost to follow-up. The mean time of follow-up was 28·6 ± 5·9 months. To clarify further the significance of urinary MBL for predicting the progression of IgAN we divided the 145 patients who completed the whole period of follow-up into remission and non-remission groups and compared the levels of urinary MBL between them, which showed significantly lower levels in the remission group compared with the non-remission group (1·07 ± 1·24 ng/mg uCr versus 9·71 ± 13·37 ng/mg uCr, P < 0·001) (Fig. 5a). Considering the effect of different treatment protocols on disease progression, the patients were stratified into non-immunosuppressive therapy group and immunosuppressive therapy group. In both subgroups, the patients who achieved remission had significantly lower levels of urinary MBL compared with non-remission patients (non-immunosuppressive therapy group: 1·02 ± 1·10 ng/mg uCr versus 7·14 ± 7·18 ng/mg uCr, P = 0·004, Fig. 5b; and immunosuppressive therapy group: 1·12 ± 1·35 ng/mg uCr versus 10·64 ± 14·96 ng/mg uCr, P < 0·001, Fig. 5c).
Fig. 5.
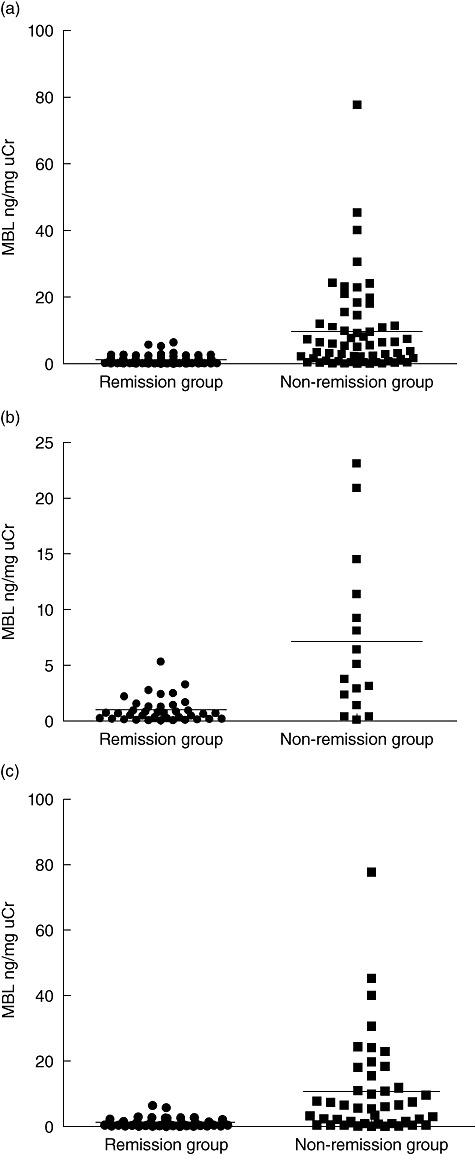
Comparison of urinary mannose-binding lectin (MBL) between remission and non-remission groups. (a) Comparison in all the patients who completed the whole period of follow-up. (b) Comparison in patients with non-immunosuppressive therapy. (c) Comparison in patients with immunosuppressive therapy. The horizontal solid lines indicate the median values for each group.
Discussion
Glomerular IgA deposition is associated with activation of the complement system, involving the alternative pathway and the lectin pathway of the complement. Although histological deposition of MBL, as a recognition molecule of the lectin pathway of the complement, had been demonstrated to be associated with more severe renal injury, no published study has examined urinary MBL in IgAN patients. To our knowledge, this study is the first to investigate the significance of urinary MBL as a non-invasive biomarker for estimating the severity of renal damage and predicting the progression of IgAN.
In the present study, we first demonstrated that the levels of urinary MBL were significantly higher in IgAN patients than those in normal controls, and increased with the aggravation of histopathological phenotypes. Furthermore, we illustrated that urinary MBL was correlated with the well-known clinical prognostic factors for the development of ESRD in IgAN patients; that is, renal function (expressed by SCr and eGFR), proteinuria and arterial hypertension at the time of renal biopsy. Then, we showed that urinary MBL was correlated with the histopathological parameters which have independent value in predicting renal outcome of IgAN according to the Oxford classification; that is, mesangial hypercellularity, segmental glomerulosclerosis, endocapillary hypercellularity and tubular atrophy/interstitial fibrosis. More importantly, our follow-up data showed that non-remission patients had higher levels of urinary MBL than patients in remission. Above all, we conclude that the higher levels of urinary MBL are associated with more severe renal lesions and poorer renal prognosis of IgAN patients, and therefore urinary MBL can be a reliable non-invasive biomarker for evaluating the severity and predicting the progression of IgAN.
Previous studies have demonstrated that serum levels of MBL had no association with glomerular deposition of MBL or severity of renal lesions, while glomerular deposition of MBL was correlated with more severe renal disease in IgAN [15]. According to our study, urinary MBL was elevated with aggravation of renal histopathological lesions. These findings suggest that elevated MBL in urine may derive from MBL accumulation in renal tissue instead of the circulation. Accumulated MBL can trigger glomerular activation of the lectin pathway of complement and, consequently, the increased proinflammatory activation products of the complement system lead to cell injury and induction of the inflammation, which promotes disease progression [26]. With the progression of renal disease, the defect of glomerular filtration occurs which results further in the presence of abnormal material in urine, including MBL. However, our hypothesis needs to be proved further in future studies.
In the present study, we evaluated the renal histological lesions at two levels. First, we performed overall evaluation with Lee's classification and compared the levels of urinary MBL among three groups divided by different histopathological phenotypes, which indicated that the levels of urinary MBL were elevated with renal histopathological phenotypes upgraded. Furthermore, we evaluated the associations between urinary MBL and the specific four variables with predictive significance according to the Oxford classification, including glomerular and interstitial lesions, which suggested that urinary MBL was correlated with not only glomerular but also interstitial lesions. The combined application of two classifications for evaluating renal histopathological lesions may supply more comprehensive information and more powerful evidence.
We avoided selection bias by including all consecutive patients receiving a histological diagnosis of IgAN in our department. Normal controls were selected as sex- and age-matched to exclude bias from the effect of sex and age. Because it is well accepted that the protein : creatinine ratio in spot urine may represent 24-h proteinuria, we used the MBL : creatinine ratio to reflect total urinary MBL excretion to avoid the effect of urine volume [27,28].
However, there are some limitations in our study. First, as a single-centre study, its external validity may be limited. Secondly, the short-term follow-up period might weaken the strength of our conclusions. In fact, we are maintaining a regular follow-up of this cohort of IgAN patients, and the results could be published in the future.
In conclusion, our study demonstrates that urinary MBL can be a useful and reliable biomarker for evaluating the severity of renal injury and predicting the progression in IgAN. However, our conclusions should be verified further in large-scale studies with long-term follow-up.
Disclosure
The authors declare no competing financial interests.
References
- 1.Schena FP, Coppo R. IgA nephropathies. In: Davison AM, Cameron JS, Grunfeld JP, et al., editors. Oxford textbook of clinical nephrology. 3rd edn. New York: Oxford University Press; 2004. pp. 470–501. [Google Scholar]
- 2.Donadio JV, Grande JP. IgA nephropathy [see comment] N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 3.Tomino Y, Sakai H, Miura M, Endoh M, Nomoto Y. Detection of polymeric IgA in glomeruli from patients with IgA nephropathy. Clin Exp Immunol. 1982;49:419–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Emancipator SN. IgAN and Henoch–Schönlein syndrome. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall's pathology of the kidney. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 479–539. [Google Scholar]
- 5.D'Amico G, Minetti L, Ponticelli G, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–78. [PubMed] [Google Scholar]
- 6.Alamartine E, Sabatier JC, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–9. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 7.Katafuchi R, Oh Y, Hori K, et al. An important role of glomerular segmental lesions on progression of IgA nephropathy: a multivariate analysis. Clin Nephrol. 1994;41:191–8. [PubMed] [Google Scholar]
- 8.Tan Y, Zhang JJ, Liu G, Zhang H, Zhao MH. The level of urinary secretory immunoglobulin A (sIgA) of patients with IgA nephropathy is elevated and associated with pathological phenotypes. Clin Exp Immunol. 2009;156:111–16. doi: 10.1111/j.1365-2249.2008.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohi K, Iwano M, Muraguchi A, et al. The prognostic significance of urinary interleukin 6 in IgA nephropathy. Clin Nephrol. 1991;35:1–5. [PubMed] [Google Scholar]
- 10.Torres DD, Rossini M, Manno C, et al. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008;73:327–33. doi: 10.1038/sj.ki.5002621. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt RJ, Kanayama Y, Julian BA, et al. Complement activation in IgA nephropathy. Kidney Int. 1987;31:1019–23. doi: 10.1038/ki.1987.101. [DOI] [PubMed] [Google Scholar]
- 12.Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R. Innate immunity and IgA nephropathy. J Nephrol. 2010;23:626–32. [PubMed] [Google Scholar]
- 13.Miyazaki R, Kuroda M, Akiyama T, Otani I, Tofuku Y, Takeda R. Glomerular deposition and serum levels of complement control proteins in patients with IgA nephropathy. Clin Nephrol. 1984;21:335–40. [PubMed] [Google Scholar]
- 14.Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 15.Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–34. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 16.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectin of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, Shikata K, Wada J, et al. Deposition of mannan binding protein and mannan binding protein-mediated complement activation in the glomeruli of patients with IgA nephropathy. Nephron. 1998;80:408–13. doi: 10.1159/000045212. [DOI] [PubMed] [Google Scholar]
- 18.Julian BA, Wittke S, Haubitz M, et al. Urinary biomarkers of IgA nephropathy and other IgA-associated renal diseases. World J Urol. 2007;25:467–76. doi: 10.1007/s00345-007-0192-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Ni Z, Xie Z, et al. Analysis of the urine proteome of human contrast-induced kidney injury using two-dimensional fluorescence differential gel electrophoresis/matrix-assisted laser desorption time-of-flight mass spectrometry/liquid chromatography mass spectrometry. Am J Nephrol. 2010;31:45–52. doi: 10.1159/000255439. [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Rao VM, Franklin WA, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol. 1982;13:314–22. doi: 10.1016/s0046-8177(82)80221-9. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran DC, Coppo R, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H. IgA nephropathy. In: Wang HY, Li XM, Zhao MH, et al., editors. Nephrology. 3rd edn. Beijing: People's Medical Publishing House; 2008. pp. 993–1016. [Google Scholar]
- 24.Tomiyoshi Y, Sakemi T, Ikeda Y, Ohtsuka Y, Nakamura M, Fujisaki T. Cellular crescents and segmental glomerular necrosis in IgA nephropathy are indicative of the beneficial effects of corticosteroid therapy. Intern Med. 2001;40:862–6. doi: 10.2169/internalmedicine.40.862. [DOI] [PubMed] [Google Scholar]
- 25.Moriyama T, Honda K, Nitta K, Yumura W, Nihei H. The effectiveness of steroid therapy for patients with advanced IgA nephropathy and impaired renal function. Clin Exp Nephrol. 2004;8:237–42. doi: 10.1007/s10157-004-0298-7. [DOI] [PubMed] [Google Scholar]
- 26.Roos A, Daha MR, van Pelt J, Berger SP. Mannose-binding lectin and the kidney. Nephrol Dial Transplant. 2007;22:3370–7. doi: 10.1093/ndt/gfm524. [DOI] [PubMed] [Google Scholar]
- 27.Cote AM, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein: creatinine ratio for proteinuria in hypertensive pregnant women: systematic review [see comment] BMJ. 2008;336:1003–6. doi: 10.1136/bmj.39532.543947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169–80. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]


