Abstract
Neonates are born with quantitative and qualitative defects in both adaptive and innate immune responses. The immune system is regulated by several mechanisms, including the signalling of inhibitory receptors. Increased expression of inhibitory receptors may result in a higher threshold for activation and suppressed function of neonatal cells. The aim of this study was to determine whether the expression of seven inhibitory receptors is increased on neonatal immune cells compared to adult immune cells. In a healthy birth cohort, we examined the expression of seven inhibitory immune receptors on neonatal neutrophils, monocytes, natural killer (NK) cells, CD4+ and CD8+ T cells. The expression of leucocyte-associated immunoglobulin (Ig)-like receptor-1 (LAIR-1), signal inhibitory receptor on leucocytes-1 (SIRL-1), CD31, signal-regulatory protein alpha (SIRPα), Siglec-9, CD200R, immune receptor expressed on myeloid cells-1 (IREM-1) and the membrane-bound ligand CD200 was studied by flow cytometry on leucocytes in cord blood (n = 14), neonatal venous blood (n = 24) and adult venous blood (n = 22). Expression of LAIR-1, CD31 and CD200 was increased consistently across all neonatal T cell subsets. Neonatal monocytes exhibited decreased expression of LAIR-1 and IREM-1 compared to adults. Furthermore, cord blood and neonatal venous blood samples contained a distinct LAIR-1-positive neutrophil population, which was not detected in adult blood. We demonstrated distinct expression of inhibitory receptors on neonatal peripheral blood immune cells in a healthy birth cohort. This is the first evidence that inhibitory receptors play a role in regulation of the neonatal immune system. Consistently increased inhibitory receptor expression on T cells may be an important mechanism in preventing the development of allergy and autoimmunity.
Keywords: immune regulation, macrophages/monocytes, neonatal, neutrophils, T-cells
Introduction
Neonates are susceptible to severe bacterial and viral infections [1]. Susceptibility to infection appears to be due to immature innate and adaptive immune responses [2–4]. Monocytes and antigen-presenting cells (APCs) show a reduction in T helper type 1 (Th1) cell-polarizing proinflammatory cytokine secretion [5], being impaired in the production of tumour necrosis factor (TNF)-α[6–8] and type 1 interferons (IFNs) [9,10]. Neonatal neutrophils are functionally impaired, showing defects in chemotaxis, adhesion, transmigration and anti-microbial activity [7,11]. Neonatal lymphocytes are largely naive and dampened in their ability to respond to antigen stimulation [2]. The diminished proinflammatory function of the neonatal immune system may be crucial to protect them from dangerous, hyper-reactive immune responses during the transition from the sterile environment in utero to the antigen-rich world outside [12,13]. However, it leaves neonates more vulnerable to bacterial and viral infections and to the development of severe sepsis [11].
Both innate and adaptive immune responses are regulated by many factors, one of which is the signalling of inhibitory immune receptors [14–16]. Inhibitory immune receptors, most of which contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs), can antagonize cell-activating signals [17], thereby raising the threshold for immune cell activation [18]. The capacity of inhibitory immune receptors to prevent activation of cells depends on the relative strength of the activating and inhibitory signal given to the cell. This is influenced by the density of the receptor on the cell surface, as well as by the availability of the ligand.
To examine the expression of inhibitory immune receptors in neonates, we initially selected a panel of inhibitory receptors known to be expressed on innate myeloid cells. Several of these are also expressed on multiple lymphoid cell types. Activation of the ITIM-based inhibitory receptor leucocyte-associated immunoglobulin (Ig)-like receptor-1 (LAIR-1) by its natural ligand, collagen or by cross-linking antibodies has been shown to inhibit immune cell function in vivo[19–21]. Signal inhibitory receptor on leucocytes-1 (SIRL-1), another ITIM-based inhibitory receptor, is expressed at high levels on monocytes and neutrophils, and it has been demonstrated that higher SIRl-1 expression on monocytes correlates with a lower production of proinflammatory cytokines [22]. CD31, expressed at high levels on myeloid cells, is known to inhibit proinflammatory cytokine production in macrophages and dampen the immune response following endotoxin exposure [23]. Signal-regulatory protein alpha (SIRPα) is also expressed in high levels on myeloid cells, and is an important regulator of monocyte and neutrophil phagocytosis and endothelial transmigration [24,25]. Siglec-9 is a prominent inhibitory receptor on neutrophils and has been demonstrated to dampen neutrophil activation and bactericidal abilities [26]. Interactions between CD200R, found on both myeloid cells and T and B lymphocytes, and its membrane-bound ligand CD200, expressed on endothelial cells, neurones and lymphocytes, are important in controlling immune-cell infiltration and response in the lungs during infection [27,28]. Immune receptor expressed on myeloid cells-1 (IREM-1) negatively regulates the activity of myeloid cells and has been identified recently as important in the prevention of myeloid cell tissue infiltration [29,30].
We hypothesized that the expression of inhibitory immune receptors on both neonatal innate and adaptive immune cells is increased compared to those of adults. In this study, we compared the expression pattern of these seven inhibitory immune receptors and one membrane-bound ligand on monocytes, neutrophils, natural killer cells, T cells and B cells isolated from cord blood, blood from neonates at the age of 1 month and adult blood. We demonstrated that the expression of inhibitory receptors on cord blood and on neonatal immune cells is distinct from adults.
Materials and methods
Study population
This was a cross-sectional study [31]. Cord blood samples were obtained from healthy term infants born in the University Medical Centre (UMC) Utrecht either by uncomplicated spontaneous vaginal delivery or primary caesarean section. Neonatal blood samples came from healthy infants aged between 3 and 8 weeks. Adult blood samples were donated by healthy volunteers. Written informed consent was obtained from all participants or their caregivers. The study protocol was approved by the Medical Ethical Committee of the UMC Utrecht.
Blood samples and flow cytometry
Blood samples were used as whole blood. Erythrocytes were lysed using fluorescence activated cell sorter (FACS) lysing solution (BD Biosciences, San Jose, CA, USA). All samples were processed and analysed within 6 h of the time that blood was drawn. Six-colour flow cytometry was used to analyse the eight inhibitory molecules on various cell subtypes. T cells were stained with CD3-eFluor450 (eBioscience, San Diego, CA, USA), CD4-phycoerythrin cyanin 7 (PECy7) (eBioscience), CD8-peridinin chlorophyll (PerCP)Cy5,5 (BioLegend, San Diego, CA, USA) and CD45RO-APC (BioLegend). B cells were defined as CD3-negative and stained with CD19-PerCP (BioLegend); for practical purposes, B cell subsets were not distinguished. Natural killer (NK) cells were defined as CD3-negative and stained with CD56-APC (BD Biosciences). Monocytes were stained with CD14-APCCy7 (BD Biosciences) and CD16-Pacific Blue (BD Biosciences). Granulocytes were stained with CD16-Pacific Blue (BD Biosciences), CD62L-PECy5 (BD Biosciences) and CD11b-APC (BD Biosciences). Inhibitory receptors were stained with LAIR-1-PE (BD Biosciences), SIRL-FITC (as described previously [22]), CD31-PE (BD Biosciences), SIRPα-PE (clone SE5A5; BioLegend, and clone 15–414; Acris Antibodies GmbH, Herford, Germany), Siglec-FITC (BD Biosciences), CD200R-PE (MorphoSys UK Ltd t/a AbD Serotec, Oxford, UK) and IREM-1-PE (BioLegend). CD200 was stained with CD200-FITC (MorphoSys UK Ltd t/a AbD Serotec). Mouse IgG1κ-PE (BD Biosciences), IgG1κ-fluorescein isothiocyanate (FITC) (BD Biosciences) and IgG2a-FITC (Zebra Bioscience BV, Enschede, The Netherlands) were used as isotype controls. Cells were incubated with antibody in the dark at room temperature for 30 min.
Cells were analysed using a LSRII flow cytometer (BD Biosciences) and at least 10 000 events were collected per specified gate. LAIR+ neutrophils were sorted from whole blood samples using a FACSAria cell sorter (BD Biosciences). Lymphocytes, monocytes and granulocytes were gated depending on forward- and side-scatter characteristics. Data were analysed using the software program FACSDiva (BD Biosciences).
A receptor was considered to be expressed on a certain cell type if the median mean fluorescence intensity (MFI) was twice that of isotype control. The percentage of positive expression was calculated based on isotype controls. During the 18-month research period, regular maintenance to the flow cytometer resulted in a small shift of MFI. Correction was performed based on the ratio between the medians of the two data sets. Combined results are reported only if patterns in both data sets were similar.
Statistical analyses
All data were analysed using the spss program version 15·0. Statistical differences in mean MFIs or percentage expression between cord, neonatal and adult blood were determined by one-way analysis of variance (anova) with post-hoc analysis (Bonferroni test for multiple comparisons). Means in MFIs or percentage expression of inhibitory receptors of CD16+ and CD16– monocytes were compared using the paired Student's t-test.
Results
Concentrations of leucocyte subsets were analysed in 14 cord blood samples, 24 venous blood samples from 1-month-old neonates and 22 adult venous blood samples (Table 1). The concentrations of various T cell subsets varied, as expected, between neonates and adults [2]. Monocytes can be divided into the ‘classical’ CD14hiCD16– and ‘proinflammatory’ CD14+CD16+ subtypes [32,33]; there was no difference in the concentrations of these subpopulations between cord blood, 1-month-old neonates and adults [34,35]. Expression of LAIR-1, CD31 and Siglec-9 was found to be increased on CD14+CD16+ monocytes compared to the total monocyte population and the expression of SIRL-1 and SIRPα was decreased on CD14+CD16+ monocytes compared to total monocytes (Fig. S1). In contrast with previously reported findings, we found no difference in the expression of CD200R between these monocyte subtypes [36].
Table 1.
Relative cell counts of the lymphocyte and monocyte subsets in each of the study populations
| Cord blood | 1-month-old neonate | Adult | |
|---|---|---|---|
| Total patients | 14 | 24 | 22 |
| Lymphocytes | |||
| T cells | 69·2 | 72·3 | 74·5 |
| CD4+ | 74·2 (1·7)† | 73·6 (1·9)† | 65·4 (2·6) |
| CD45RO– | 60·6 (4·8)‡ | 79·7 (2·8)† | 46·7 (4·5) |
| CD45RO+ | 39·3 (4·8)‡ | 20·3 (2·8)† | 53·2 (4·5) |
| CD8+ | 25·8 (1·7)† | 26·4 (1·9)† | 34·6 (2·6) |
| CD45RO– | 70·6 (3·9) | 84·2 (2·7)† | 58·7 (4·9) |
| CD45RO+ | 29·3 (3·9) | 15·7 (2·7)† | 41·3 (4·9) |
| B cells | 16·2 (1·7) | 20·0 (1·9)† | 12·4 (1·3) |
| NK cells | 14·6 (2·2)‡ | 7·6 (1·0)† | 13·1 (1·3) |
| Monocytes | |||
| CD14hiCD16– | 88·2 (1·8) | 90·4 (1·0) | 88·3 (2·3) |
| CD14+CD16+ | 11·8 (1·8) | 9·6 (1·0) | 11·7 (2·3) |
Mean relative cell counts are shown with standard error of the mean in parentheses. P-values represent the outcome of one-way analysis of variance (anova).
Mean relative cell count differs from that of adult venous blood (P < 0·05, outcome of one-way anova).
Mean relative cell count differs from that of neonatal venous blood (P < 0·05, outcome of one-way anova). NK, natural killer.
Differences in the expression of inhibitory molecules between cord blood, 1-month-old neonates and adults were analysed in terms of both percentage expression and MFI. Distinct profiles of surface expression of inhibitory immune receptors were found. A complete overview of the findings of this study is shown in Table S1; these data are summarized in Table 2. We confirmed that the expression of Siglec-9 is increased on cord blood neutrophils compared to adults (MFI 296 versus 204, P < 0·01, Fig. 1a) [37]. Interestingly, the difference is no longer significant at the age of 1 month. While adult neutrophils do not express LAIR-1, cord blood and neonatal venous blood samples contained a small but distinct LAIR-1-positive neutrophil population (1·40% of total neutrophils, P < 0·01) (Fig. 1b). Because immature neutrophils have been described previously to express LAIR-1 [38], LAIR-1-expressing neutrophils were stained and sorted into two independent experiments. It was determined that 72–75% of the LAIR-1+ cells were mature polymorphonuclear neutrophils (PMNs) (data not shown). In contrast to the enhanced expression of Siglec-9 and LAIR-1 on cord blood neutrophils, expression of IREM-1 was down-regulated on neonatal monocytes. IREM-1 was expressed on only 27·0% of cord blood monocytes, while it was expressed on 67·9% of neonatal and 65·6% of adult monocytes (P < 0·001). Interestingly, this difference is smaller within the CD14+CD16+ monocyte population (Fig. 1c).
Table 2.
Summary of the study results
| LAIR-1 | SIRL-1 | CD31 | SIRPα | Siglec-9 | CD200 | CD200R | IREM-1 | |
|---|---|---|---|---|---|---|---|---|
| Cord blood | ||||||||
| Neutrophils | ↑ | = | = | = | ↑** | = | = | |
| Monocytes CD16– | = | = | = | = | = | = | ↓*** | |
| Monocytes CD16+ | = | = | = | = | = | = | ↓* | |
| NK cells | = | = | ||||||
| CD4+CD45RO– T cells | ↑* | ↑*** | ↑*** | |||||
| CD4+CD45RO+ T cells | ↑*** | ↑** | ↑*** | |||||
| CD8+CD45RO– T cells | ↑** | ↑*** | ||||||
| CD8-CD45RO– T cells | ↑*** | ↑*** | ↑*** | |||||
| B cells | ↑*** | ↑** | = | ↓** | ||||
| Neonatal venous blood | ||||||||
| Neutrophils | ↑** | = | = | = | = | = | = | |
| Monocytes CD16– | ↓* | = | = | = | = | = | = | |
| Monocytes CD16+ | = | = | = | = | = | = | = | |
| NK cells | = | ↓*** | ||||||
| CD4+CD45RO– T cells | ↑* | ↑* | = | |||||
| CD4+CD45RO+ T cells | ↑*** | = | ↑*** | |||||
| CD8+CD45RO– T cells | ↑** | ↑*** | ||||||
| CD8+CD45RO– T cells | ↑*** | ↑*** | ↑** | |||||
| B cells | ↑*** | = | ↓* | ↓*** |
P-values are the outcome of one-way analysis of variance (anova) with post-hoc Bonferroni correction (*P < 0·05; **P < 0·01; ***P < 0·001). Shows the expression of each inhibitory receptor per cell type as increased, decreased or similar compared to adult cells. Where no symbol is shown, a receptor was not expressed on that cell type. LAIR-1, leucocyte-associated immunoglobulin (Ig)-like receptor-1; SIRL-1, signal inhibitory receptor on leucocytes-1; SIRPα, signal-regulatory protein alpha; IREM-1, immune receptor expressed on myeloid cells-1; NK, natural killer.
Fig. 1.
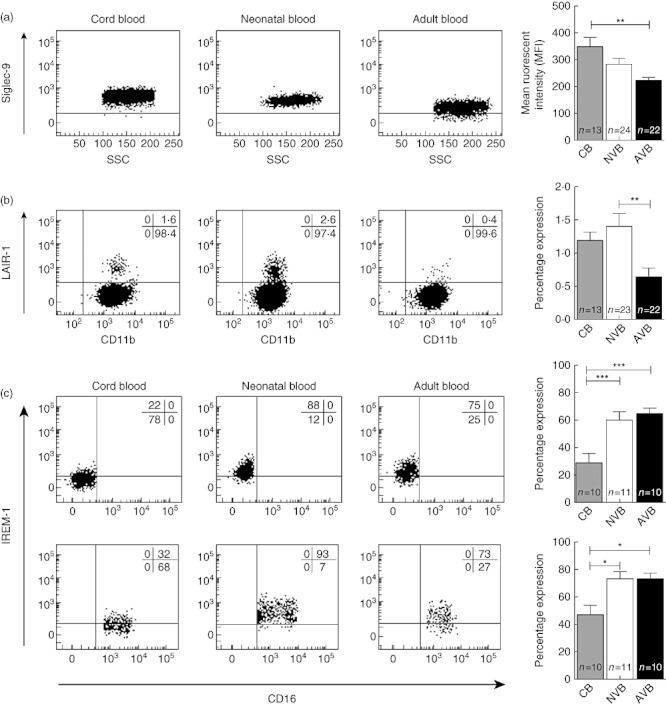
Neonatal neutrophils and monocytes express different levels of inhibitory receptors. (a) The expression of Siglec-9 is increased on cord blood neutrophils and this difference begins to disappear during the first weeks of life. Representative fluorescence activated cell sorter (FACS) dot-plots and a graphic representation of all data is shown. CB: cord blood; NVB: neonatal venous blood; AVB: adult venous blood. (b) Neonatal and cord blood samples contain a distinct leucocyte-associated immunoglobulin (Ig)-like receptor-1 (LAIR-1)-positive neutrophil population not seen in adult blood. The level of CD11b, a neutrophil activation marker, is the similar between LAIR-1+ and LAIR-1– neutrophils. Representative FACS dot-plots and a summary of all data is shown. (c) The expression of immune receptor expressed on myeloid cells-1 (IREM-1) is decreased on cord blood but not neonatal monocytes compared to adult monocytes. Interestingly, this difference is smaller within the CD14+CD16+ monocyte population. Representative FACS dot-plots and graphic representation of all the data are shown. P-values are the outcome of one-way analysis of variance (anova), with *P < 0·05; **P < 0·01 and ***P < 0·001.
Besides their expression on myeloid cells, three of these receptors are also expressed on lymphoid cells. The expression of LAIR-1 was increased across all neonatal lymphocyte subsets (Fig. 2). We found a similar pattern of increased expression on neonatal lymphocytes for CD31 and CD200 (Table S1). In contrast, CD200R expression was decreased significantly on B cells from cord blood and neonates compared to adults (Table S1).
Fig. 2.
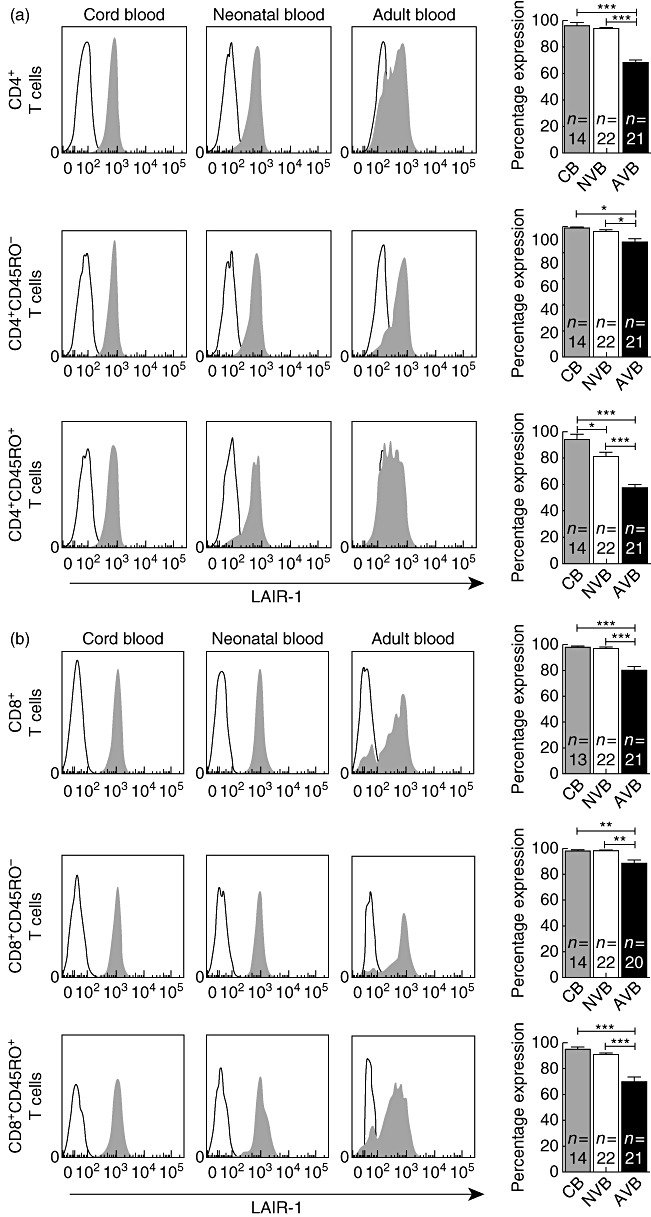
Leucocyte-associated immunoglobulin (Ig)-like receptor-1 (LAIR-1) expression is higher on neonatal T cells. (a) LAIR-1 expression is increased on both neonatal CD45RO– and CD45RO+ CD4+ T cells. Representative fluorescence activated cell sorter (FACS) histograms and graphic representation of all data are shown. CB, cord blood; NVB, neonatal venous blood; AVB, adult venous blood. (b) A similar increase in LAIR-1 expression is seen on all CD8+ T cell subsets. Representative FACS histograms and data summaries are shown. P-values are the outcome of one-way analysis of variance (anova), with *P < 0·05; **P < 0·01 and ***P < 0·001.
Discussion
Neonates are more vulnerable to severe infections [38] as a result of immature innate and adaptive immune responses [2,7,11]. The immune system is regulated by a fine balance of activating and inhibitory receptor signalling [17,18,39,40]. Previous studies have demonstrated that inhibitory immune receptors are important regulators of innate immune responses to infection [41,42]. In this descriptive study, we demonstrated distinct neonatal expression of inhibitory receptors on both adaptive and innate immune cells. We confirmed previously published findings that the surface expression of Siglec-9 is increased on cord blood neutrophils [37], and extend this observation to the neonatal age. We showed that the expression of several other inhibitory immune receptors is also distinct on neonatal monocytes, neutrophils and natural killer cells. Additionally, we showed that across all T cell subsets, neonatal T cells express higher levels of inhibitory receptors than adults.
This study focused on innate immune cells because these cells are most important in protecting neonates during their first month of life. We chose seven inhibitory receptors for the current study. First, we selected well-characterized receptors that have been shown to be important in regulating innate immune cell function. Secondly, we also chose to study those receptors in which we have a long-standing interest [22,27,40]. Although the majority of inhibitory receptors on each innate immune cell type are expressed at similar levels in adults and neonates, we also observed some distinct differences. We found that IREM-1 was decreased on neonatal monocytes and that Siglec-9 and LAIR-1 were increased on neonatal neutrophils [37]. A previous study has demonstrated that Siglec-9 is increased on cord blood neutrophils, which is relevant, as the sialylated capsular polysaccharide of group B streptococcus uses Siglec-9 to dampen the bactericidal neutrophil response [26]. Group B streptococcus is the major pathogen during neonatal age, but not thereafter [43]. We were able to confirm increased Siglec-9 expression in neonates and show that this difference largely disappears during the first month of life. This is consistent with other observations that neonatal neutrophils obtain a mature phenotype during the first few weeks after birth [44]. Adult PMNs in peripheral blood do not express LAIR-1 [45]. We observed that cord blood and neonatal venous blood samples contain small populations of neutrophils expressing high levels of LAIR-1. Interestingly, these were not immature neutrophils, which are known to express LAIR-1 [45], but mature PMNs. More functional studies are required to understand fully the implications of the distinct neonatal expression profile of inhibitory receptors on innate immune cells.
In addition to the innate immune response, this study adds to our understanding of the function of the neonatal adaptive immune system. During the first weeks of life the adaptive immune system is immature; this is due partly to the fact that neonates have yet to develop large memory and effector lymphocyte populations, but even following adequate antigen stimulation neonatal T lymphocyte responses are often diminished in magnitude [2]. We confirmed our previous finding that LAIR-1 expression is increased on naive T cells compared with memory and effector cells [46], and demonstrate for the first time that, compared to adults, LAIR-1 expression is increased on both neonatal naive T cells as well as neonatal effector T cells. Interestingly, LAIR-1 expression is highest on cord blood T cells, showing that while not reaching the level on adult cells, LAIR-1 levels decrease during the first 4 weeks of life. We also found that the expression of the inhibitory receptors CD31 as well as the expression of CD200, the membrane-bound ligand of CD200R, is generally increased on all subsets of neonatal T cells. Therefore, we theorize that the consistent increase of inhibitory receptors on neonatal T cells plays a role in dampening the adaptive immune response in utero and during the first weeks of life, leaving neonates susceptible to infection with intracellular pathogens.
The potential strengths and weaknesses of this study require discussion. The major strength of this study is that we had access to unique samples, namely, venous blood from a population healthy 1-month-old neonates. Examining both immune cells from cord blood as well as from healthy neonates has two advantages. First, it eliminates the possibility that specific observations are restricted to cord blood, which has a low pH [47] and is drawn under stressful conditions. Secondly, it provides insight into the rapid changes that the normal immune system undergoes during this critical neonatal age. A weakness of this study is that multiple comparisons were performed. When comparing between our three populations, Bonferroni correction was performed to correct for multiple comparisons. No attempt was made to correct for the fact that we tested multiple receptors on multiple cell types. However, we note that of 79 statistical analyses performed, 45 were significant and many were highly significant (P < 0·001). Finally, we tested only a limited number of seven inhibitory receptors, while more than 40 different inhibitory receptors exist [14,48].
We conclude that neonatal innate and adaptive immune cells exhibit a pattern of inhibitory receptor expression that is distinct from adults. We also demonstrate that there is a consistent increase of the inhibitory molecules LAIR-1, CD31 and CD200 on both naive and memory T cells in neonates. This suggests a novel mechanism by which neonates regulate their immune response to novel food and aeroallergens during the first few weeks of life.
Acknowledgments
Geertje Westerlaken, Mirjam Belderbos, Linde Meyaard and Louis Bont participated in the design of the study. Jona Walk, Geertje Westerlaken, Nathalie van Uden and Mirjam Belderbos participated in data collection and analysis. All authors declare that they have reviewed the manuscript and that they participated sufficiently to take public responsibility for it.
Disclosure
None of the authors have any affiliations with, or financial involvement in, any organization with a direct financial interest in the subject of the research discussed in this paper.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Inhibitory receptor expression on monocyte subtypes. Surface expression of inhibitory receptors was compared between total monocytes and CD14+CD16+ proinflammatory monocytes in 21 healthy adults. P-values shown are the outcomes of paired-sample t-tests, with *P < 0·05;**P < 0·01 and ***P < 0·001.
Table S1. Expression of seven inhibitory receptors and one membrane-bound ligand in neonates and adults. Summary of median mean fluorescence intensities (MFIs) (a) and percentage of positive expression (b) found for every inhibitory receptor per cell type and sample type. CB, cord blood; NVB, neonatal venous blood; AVB, adult venous blood. Where no value is shown, a receptor was not expressed on that cell type (mean MFI less than twice that of isotype control).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Rennie JM, Roberton NRC. Roberton's textbook of neonatology. London: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- 2.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 3.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–43. [PubMed] [Google Scholar]
- 4.Chelvarajan RL, Collins SM, Doubinskaia IE, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75:982–94. doi: 10.1189/jlb.0403179. [DOI] [PubMed] [Google Scholar]
- 5.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–44. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–9. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 7.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 8.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belderbos ME, van Bleek GM, Levy O, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wit D, Olislagers V, Goriely S, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–2. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 11.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29:315–48. doi: 10.3109/08830181003792803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchini G, Nelson A, Edner J, Lonne-Rahm S, Stavreus-Evers A, Hultenby K. Erythema toxicum neonatorum is an innate immune response to commensal microbes penetrated into the skin of the newborn infant. Pediatr Res. 2005;58:613–6. doi: 10.1203/01.pdr.0000176836.27156.32. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA. 2008;105:7528–33. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steevels TA, Meyaard L. Immune inhibitory receptors: essential regulators of phagocyte function. Eur J Immunol. 2011;41:575–87. doi: 10.1002/eji.201041179. [DOI] [PubMed] [Google Scholar]
- 15.Meyaard L, Hurenkamp J, Clevers H, Lanier LL, Phillips JH. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol. 1999;162:5800–4. [PubMed] [Google Scholar]
- 16.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas ML. Of ITAMs and ITIMs: turning on and off the B cell antigen receptor. J Exp Med. 1995;181:1953–6. doi: 10.1084/jem.181.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–91. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 19.Lebbink RJ, de Ruiter T, Adelmeijer J, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–25. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poggi A, Tomasello E, Ferrero E, Zocchi MR, Moretta L. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte–monocyte colony-stimulating factor. Eur J Immunol. 1998;28:2086–91. doi: 10.1002/(SICI)1521-4141(199807)28:07<2086::AID-IMMU2086>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Maasho K, Masilamani M, Valas R, Basu S, Coligan JE, Borrego F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42:1521–30. doi: 10.1016/j.molimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Steevels TA, Lebbink RJ, Westerlaken GH, Coffer PJ, Meyaard L. Signal inhibitory receptor on leukocytes-1 is a novel functional inhibitory immune receptor expressed on human phagocytes. J Immunol. 2010;184:4741–8. doi: 10.4049/jimmunol.0902039. [DOI] [PubMed] [Google Scholar]
- 23.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu DQ, Li LM, Guo YL, et al. Signal regulatory protein alpha negatively regulates beta2 integrin-mediated monocyte adhesion, transendothelial migration and phagocytosis. PLoS ONE. 2008;3:e3291. doi: 10.1371/journal.pone.0003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Buhring HJ, Zen K, et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–36. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 26.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rygiel TP, Rijkers ES, de Ruiter T, et al. Lack of CD200 enhances pathological T cell responses during influenza infection. J Immunol. 2009;183:1990–6. doi: 10.4049/jimmunol.0900252. [DOI] [PubMed] [Google Scholar]
- 28.Snelgrove RJ, Goulding J, Didierlaurent AM, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–83. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 29.Xi H, Katschke KJ, Jr, Helmy KY, et al. Negative regulation of autoimmune demyelination by the inhibitory receptor CLM-1. J Exp Med. 2010;207:7–16. doi: 10.1084/jem.20091508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SM, Kim EJ, Suk K, Lee WH. Synthetic peptides containing ITIM-like sequences of IREM-1 inhibit BAFF-mediated regulation of interleukin-8 expression and phagocytosis through SHP-1 and/or PI3K. Immunology. 2011;134:224–33. doi: 10.1111/j.1365-2567.2011.03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houben ML, Nikkels PG, van Bleek GM, et al. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PLoS ONE. 2009;4:e6572. doi: 10.1371/journal.pone.0006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 34.Bloemers BL, van Bleek GM, Kimpen JL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr. 2010;156:804–9. doi: 10.1016/j.jpeds.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Sohlberg E, Saghafian-Hedengren S, Bremme K, Sverremark-Ekstrom E. Cord blood monocyte subsets are similar to adult and show potent peptidoglycan-stimulated cytokine responses. Immunology. 2011;133:41–50. doi: 10.1111/j.1365-2567.2011.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koning N, van Eijk M, Pouwels W, et al. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J Innate Immun. 2010;2:195–200. doi: 10.1159/000252803. [DOI] [PubMed] [Google Scholar]
- 37.Rashmi R, Bode BP, Panesar N, et al. Siglec-9 and SHP-1 are differentially expressed in neonatal and adult neutrophils. Pediatr Res. 2009;66:266–71. doi: 10.1203/PDR.0b013e3181b1bc19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 39.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 40.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83:799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez IB, Pasquinelli V, Jurado JO, et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202:524–32. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 42.Ma CJ, Ni L, Zhang Y, et al. PD-1 negatively regulates interleukin-12 expression by limiting STAT-1 phosphorylation in monocytes/macrophages during chronic hepatitis C virus infection. Immunology. 2011;132:421–31. doi: 10.1111/j.1365-2567.2010.03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC) Early-onset and late-onset neonatal group B streptococcal disease – United States, 1996–2004. MMWR. 2005;54:1205–8. [PubMed] [Google Scholar]
- 44.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 45.Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79:828–36. doi: 10.1189/jlb.0705370. [DOI] [PubMed] [Google Scholar]
- 46.Jansen CA, Cruijsen CW, de Ruiter T, et al. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur J Immunol. 2007;37:914–24. doi: 10.1002/eji.200636678. [DOI] [PubMed] [Google Scholar]
- 47.Juutistenaho S, Eskola M, Sainio S, Aranko K, Kekomaki R. Association of stress-related perinatal factors and cord blood unit hematopoietic progenitors is dependent on delivery mode. Transfusion. 2010;50:663–71. doi: 10.1111/j.1537-2995.2009.02467.x. [DOI] [PubMed] [Google Scholar]
- 48.Lebbink RJ, Meyaard L. Non-MHC ligands for inhibitory immune receptors: novel insights and implications for immune regulation. Mol Immunol. 2007;44:2153–64. doi: 10.1016/j.molimm.2006.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


