Abstract
Ischaemia, inflammation, and exercise lead to tissue acidosis, which induces pain and mechanical hyperalgesia. Corresponding to this, enhanced thin-fibre afferent responses to mechanical stimulation have been recorded in vitro at low pH. However, knowledge about how this sensitization by low pH occurs is lacking. In this study, we found that all three types (rapidly adapting (RA), intermediately adapting and slowly adapting) of mechanically activated currents recorded with the whole cell patch-clamp method were sensitized by low pH in rat cultured dorsal root ganglion neurones. This sensitization was mainly observed in neurones positively labelled with isolectin B4 (IB4), which binds to versican, a chondroitin sulfate proteoglycan. Inhibitors of acid-sensitive channels (amiloride and capsazepine) did not block sensitization by low pH except in RA neurones, and extracellular calcium was not involved even in the sensitization of this type of neurone. A broad spectrum kinase inhibitor and a phospholipase C inhibitor (staurosporine and U73122) failed to block pH-induced sensitization in IB4-positive neurones, suggesting that these intracellular signalling pathways are not involved. Notably, both excess chondroitin sulfate in the extracellular solution and pretreatment of the neurone culture with chondroitinase ABC attenuated this low pH-induced sensitization in IB4-positive neurones. These findings suggest that a change in interaction between mechanosensitive channels and/or their auxiliary molecules and the side chain of versican on the cell surface causes this sensitization, at least in IB4-positive neurones. This report proposes a novel mechanism for sensitization that involves extracellular proteoglycans (versican).
Key points
Tissue acidosis is caused in many pathological and physiological conditions (e.g. ischaemia, inflammation and exercise) and induces pain and mechanical hyperalgesia.
An augmented mechanical response of thin-fibre afferents by low pH has been reported, but the sensitizing mechanism has not been determined.
In this study we examined whether mechanically activated (MA) currents recorded from the soma of cultured sensory neurones were augmented by low pH application.
We showed that low pH mainly sensitized MA currents of IB4-positive neurones expressing an extracellular matrix proteoglycan, versican, and this sensitization was attenuated by manipulating the extracellular matrix proteoglycan, but not by blocking intracellular signalling pathways.
These results show us a novel sensitizing mechanism involving extracellular matrix proteoglycans, which is different from currently popular sensitizing mechanisms involving intracellular signalling pathways.
Introduction
Mechanical hyperalgesia is one of the major symptoms of many inflammatory and neuropathic conditions in not only the skin and viscera, but also joint and muscle. Sensitization of the afferent responses to mechanical stimulation by inflammatory mediators has been well studied in many tissues, except in skin where only a few mediators are reported to sensitize nociceptor responses to mechanical stimulation (Steen et al. 1992; Lechner & Lewin, 2009). In the past ten years transducing and sensitizing mechanisms of mechanical responses of afferents have been studied by directly pressing the somas or neurites of the cultured dorsal root ganglion (DRG) neurones. Three kinds of mechanically activated (MA) inward currents have been reported with this method (Drew et al. 2002; Hu & Lewin, 2006), namely rapidly adapting (RA), intermediately adapting (IA), and slowly adapting (SA), based on their inactivation time constants (τ).
Several candidate neuronal mechanotransduction channels have been proposed: amiloride-sensitive acid-sensing ion channels (ASICs) (Jones et al. 2005), transient receptor potential (TRP) vanilloid 4 (TRPV4) (Alessandri-Haber et al. 2003), TRP ankyrin 1 (TRPA1) (Kwan et al. 2006; Vilceanu & Stucky, 2010; Brierley et al. 2011) and other channels (see Tsunozaki & Bautista, 2009 for review). However, the contribution of these proposed channels to mechanotransduction has been a matter of dispute (Drew et al. 2004, and other papers). Meanwhile, Coste et al. (2010) have identified new cation channels in mice, Piezo1 and 2, expressions of which are essential for RA current. Piezo2 is highly expressed in DRG neurones.
Both tissue acidosis, naturally occurring in ischaemia, inflammation and exercise, and injection of acid induce pain and mechanical hyperalgesia in humans (Steen & Reeh, 1993; Frey Law et al. 2008) and long-lasting mechanical hyperalgesia in animals (Sluka et al. 2001). Acidification also enhances thin-fibre afferent responses of the skin (Steen et al. 1992) and the muscle (Hotta et al. 2010) to mechanical stimulation in vitro. Two channels have been implicated in the neuronal responses to low pH: ASICs (Waldmann et al. 1997) and TRPV1 (Tominaga et al. 1998). In addition, some proton-sensing G protein-coupled receptors have also been reported to be involved (Ludwig et al. 2003; Huang et al. 2007). However, the molecular mechanisms of low pH-induced sensitization have not been addressed.
Chondroitin sulfate (CS), which is one component of cartilage and connective tissues in many organs, is often clinically used for the control of pain possibly by facilitating connective tissue regeneration. It has also been reported in a randomized study of humans that CS shows an analgesic effect on knee osteoarthritis (Möller et al. 2010). Moreover, Bogen et al. (2009) showed that mechanical hyperalgesia after monocyte chemoattractant protein 1 (MCP-1) injection to the skin is attenuated by chondroitinase ABC (an enzyme that cleaves CS) in rat in vivo. However, there is no report that CS or chondroitinase ABC suppresses mechanical sensitization by inflammatory mediators in a cell-based assay.
In the present study, we show that MA currents induced by pressing the DRG neurones are potentiated by low pH application within the physiological or pathological range. We have also shown that these neurones are mainly isolectin B4 (IB4) positive and that CS, a component of IB4-binding proteoglycan (versican), contributes to this low pH-induced sensitization of IB4-positive neurones.
Methods
Primary cultures of DRG neurones from Sprague–Dawley rats (SLC Inc., Japan) 2–13 days after birth were used for all experiments. Rat pups were kept with their mothers until the experiments under a 12 h light/dark cycle (light between 8.00 and 20.00 h) in an air-conditioned room (22–24°C). All experiments were conducted according to the Regulations for Animal Experiments in Nagoya University, and the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions in Japan.
DRG culture preparation
DRG neurones were prepared as previously reported (Lechner & Lewin, 2009). Briefly, animals were killed using pure CO2 gas and 25–30 DRGs including those from all lumbar segments were removed. The DRGs were digested with collagenase IV (0.5–1.0 mg ml−1, Sigma, St Louis, MO, USA) and trypsin (0.025–0.05%, Invitrogen, Carlsbad, CA, USA) for 30 min and 20 min, respectively, at 37°C. Trypsin inhibitor (Wako, Osaka, Japan) was then used to stop the digestion. They were washed with a growth medium (Dulbecco's Modified Eagle's Medium/Ham's F-12 (Wako)) supplemented with Glutamax–1 (Invitrogen), penicillin–streptomycin, 0.8% glucose, 5% fetal bovine serum and 100 ng ml−1 nerve growth factor (7S-NGF, Sigma), and triturated using fire-polished Pasteur pipettes. DRG neurones were plated on glass coverslips precoated with poly-l-lysine (0.1 mg ml−1, Sigma) and laminin (20 μg ml−1, Invitrogen). Cultures were used for patch-clamp experiments within the next 2 days.
Patch-clamp experiments and mechanical stimulation
Whole cell patch-clamp recordings were made at room temperature. Patch pipettes were pulled from borosilicate glass capillaries (Narishige, Tokyo, Japan) and filled with a solution consisting of (mm): KCl 130, NaCl 10, MgCl2 1, EGTA 1, and Hepes 10, adjusted to pH 7.3 with 1 n KOH. ATP (2 mm) and GTP (2 mm) were also added. The extracellular solution contained (mm): NaCl 140, KCl 5, CaCl2 2, MgCl2 2, glucose 10, and Hepes 10, adjusted to pH 7.4 with 1 n NaOH. Ca2+-free extracellular solution was made by replacing CaCl2 with 5 mm EGTA. All recordings were made using an Axopatch 200B amplifier (Axon Instruments, Inc., Union City, CA, USA) in combination with Clampex software (Axon Instruments, Inc.). Inactivation time constants (τ) were calculated by fitting the current traces with single exponential functions and total charge transfer was also calculated as an area under the curve of current trace by Clampfit software (Axon Instruments, Inc.). Pipette resistances were 3–6 MΩ.
Mechanical stimulation was performed according to previous reports (Hu & Lewin, 2006; Lechner & Lewin, 2009). Briefly, MA currents were recorded in the voltage clamp mode (−60 mV) and mechanical stimuli to the cell surface were applied using a heat-polished glass pipette (tip diameter: 3–5 μm) as a stimulation probe, which was positioned at an angle of 45–50 deg to the surface of the dish. The probe was driven by a piezo-controlled micromanipulator (Nanomotor MM3A, Kleindiek Nanotechnik, Reutlingen, Germany) with a velocity of 1.8 μm ms−1. For the single mechanical stimulus, the probe was moved forward to press the cell and kept in this position for 500 ms, then moved backward. The amount of probe displacement was controlled by setting the step number of the micromanipulator. In this study, we configured the manipulator to 0.525 μm step−1. The step sizes used to stimulate the neurones were usually started from four steps (2.1 μm) and gradually increased in an increment of one or two steps until the induced MA current reached 100 pA. After a current over 100 pA was observed with a certain number of steps, the repetitive stimulation was applied with the same number of steps. The number of steps for repetitive stimulation was determined for every DRG neurone. The range of evoked MA current by repetitive stimulation was 106–1071 pA (median: 245 pA), while the number of stimulation steps was 3–10 (median: 6 steps). The interval of repetitive stimulations was 30 s. To see the effect of low pH on the MA currents, each low pH solution (pH 6.2, 6.6, 7.0) was applied for 30 s and then washed out with extracellular solution (pH 7.4) for 30 s. Mechanical stimulation was given shortly before changing the solution. MA currents were fitted with single exponential functions and classified into three types of current (rapidly adapting (RA), intermediately adapting (IA), slowly adapting (SA)) based on their inactivation time constants (τ). In the present study, we defined the currents as RA, τ < 3 ms; IA, 3 ≤τ≤ 30 ms; and SA, τ > 30 ms. In some RA neurones, action potentials (APs) were evoked in current clamp mode by repetitive 80 ms current injections, increasing from 40 to 800 pA with increments of 40 pA, to determine whether their APs have a hump.
Isolectin B4 labelling
The DRG neurones were labelled with isolectin B4 (IB4) of Griffonia simplicifolia after patch-clamp recording. Five microlitres of IB4 conjugated to Alexa-568 (1 mg ml−1, Invitrogen) was added to the chamber and diffused for 20 min, then washed out for 10 min with extracellular solution. Neurones were visualized by fluorescence microscopy with appropriate filters and those having a bright red cell surface were defined as IB4-positive neurones.
Blockade of intracellular signalling
Two kinds of inhibitors were added to the pipette solution to inhibit the intracellular signalling pathway. We used 100 or 500 nm staurosporine as a broad-spectrum kinase inhibitor (Karaman et al. 2008) and 1 μm U73122 as a phospholipase C (PLC) inhibitor (Jin et al. 1994).
Degradation of chondroitin sulfate proteoglycan on the neuronal surface by chondroitinase ABC
One portion of the DRG neurones was incubated with 2 U ml−1 chondroitinase ABC (Seikagaku Co., Tokyo, Japan) in 100 mm Tris-HCl supplemented with 15 mm sodium acetate and 5 mm CaCl2, pH 7.4 for 30 min at 37°C (Kinsella et al. 2003). Thereafter, they were washed out twice with the extracellular solution and used for patch-clamp experiments.
Drugs and enzyme application
Low pH solutions (pH 6.2, 6.6 and 7.0) were made from the extracellular solution by adjusting the pH with 1 n NaOH. Amiloride (AMI) (Sigma) and capsazepine (CPZ) (Sigma) were dissolved into DMSO, and then diluted with the extracellular solution to the appropriate concentration. Staurosporine (Wako) and U73122 (Sigma) were also dissolved in DMSO and then diluted with the intracellular solution. The final DMSO concentration was less than 0.1%. Low pH, AMI and CPZ solutions were locally applied through a Y-shaped plastic tube close to the recorded neurones (Murase et al. 1989). Sodium chondroitin sulfate (CS) from swine cartilage was kindly donated by Zeria Pharmaceutical Co., Ltd. CS was directly dissolved into the extracellular solution, and 200 μl of 1 or 3% CS was added to the extracellular solution in the chamber (volume of the chamber: 2 ml) to a final concentration of 0.1 or 0.3% and left for 2 min. After this treatment, low pH solution was locally applied through the Y-tube.
Statistical analysis
The percentages of neurones were compared using the χ2 test. Other results are expressed as mean ± SEM. The peak current amplitude was analysed first by repeated measures ANOVA, followed by Dunnett's multiple comparison test. Increases in the total charge transfer by MA current within IB4-positive or -negative neurones, and the effects of acid-sensitive channel blockers and CS on low pH-induced sensitization were analysed with Student's paired t test. Differences of increase in the total charge transfer by MA current at pH 6.2 between IB4-positive and -negative neurones, and differences in the percentage increase of peak current amplitude between chondroitinase ABC-treated and -untreated groups were analysed with Student's t test. P < 0.05 was considered to be a significant change.
Results
MA current of small DRG neurones was potentiated by low pH
We used small diameter DRG neurones that were presumed to be mostly nociceptive. The median diameter of these neurones was 23 μm (range: 17.5–29 μm, n= 130). Furthermore, these neurones showed distinctive AP shapes with a hump (Fig. 1A, inset) that indicate the expression of nociceptive specific sodium channels (Stucky & Lewin, 1999). In our preliminary data, recording of APs in 235 small neurones (range: 16–30 μm, median: 23.5 μm) produced by current injections showed a hump with only two exceptions. The MA current type of these exceptional neurones was RA (see below), as previously reported (Hu & Lewin, 2006). Almost all DRG neurones responded to the mechanical stimulation. We observed three types of MA currents distinguished by inactivation time constants (τ): RA, τ < 3 ms; IA, 3 ≤τ≤ 30 ms; and SA, τ > 30 ms, in the present study (RA, Fig. 1A and B; IA, Fig. 1C; SA, Fig. 1A and D).
Figure 1. Low pH application potentiates all types of MA currents in nociceptive DRG neurones (sample recordings).
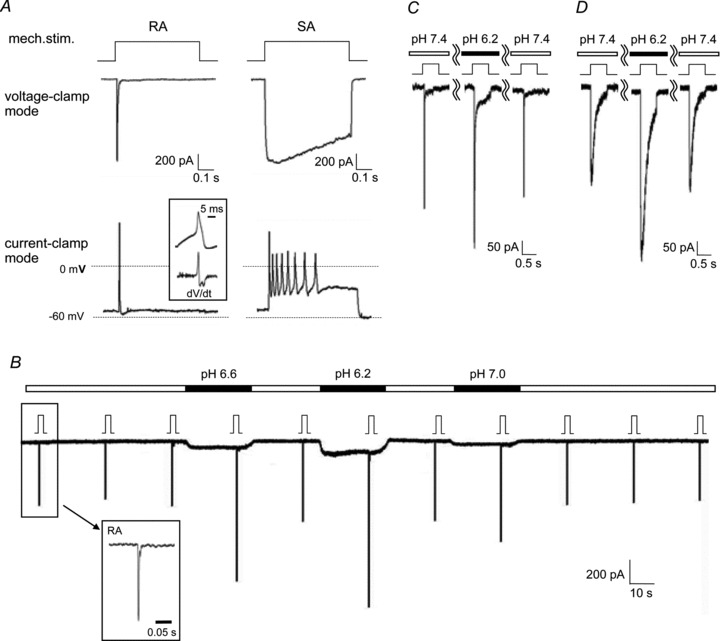
A, mechanical stimulation evoked currents and action potentials (APs). From top to bottom: representation of mechanical stimulation (stimulation steps were 5 for RA and 6 for SA), current recording in voltage clamp mode and voltage recording in current clamp mode. Many APs were observed in an SA neurone in current clamp mode (right bottom), while only one AP was evoked in an RA neurone (bottom left) with almost the same peak current amplitude as SA. Inset: an AP with a hump (two minima in first dV/dt) evoked with the current injection method in the same RA neurone. B, continuous sample recording of MA currents and effects of low pH application. Each low pH solution (pH 6.2, 6.6 and 7.0) was applied for 30 s and washed out with extracellular solution (pH 7.4) for 30 s. The application order of the low pH solution was randomized. Mechanical stimulation was applied every 30 s at the end of low pH application (about 25 s from application start) and was held for 500 ms. The low pH application period is marked with black bars and the markers of the mechanical stimuli are shown just above the trace. Stimulation steps were 4. The current type of this neurone was RA (see recording in inset with an expanded time scale, τ: 1.8 ms). IA (C) and SA (D) types of MA currents were also potentiated by low pH (6.2). Stimulation steps were 4 for IA and 6 for SA.
We also recorded APs evoked by mechanical stimulations to clear the relation between MA current type and AP frequency. SA current generated more frequent APs than RA current. For example, eight APs were observed in an SA neurone in current clamp mode, while only one AP was evoked in an RA neurone with almost the same peak current amplitude as SA (Fig. 1A).
We examined whether these MA currents are potentiated or not in acidic conditions. During the periodical recording of MA currents from a single neurone, three levels of low pH solutions (pH 7.0, 6.6, 6.2) were applied in random order (Fig. 1B). Mechanical stimulation was given every 30 s at the end of perfusion of each solution. Acid sensitive currents (e.g. Fig. 1B) evoked by low pH were observed in almost all neurones. Only one neurone out of 130 neurones recorded failed to show any kind of acid sensitive currents. This exceptional neurone responded to mechanical stimuli, but was not sensitized by low pH.
The net peak current amplitude evoked by mechanical stimuli was evaluated by subtracting the acid sensitive current amplitude. Before low pH application, we performed three sequential mechanical stimulations at pH 7.4 to check the stability of MA current (a sample recording in Fig. 1B). This stability was confirmed if the ratio of the peak current amplitude of the second response to the mean current amplitude of the first and the third responses was around 1 (mean: 1.00, range: 0.82–1.19, standard deviation = 0.075, n= 130). Based on this analysis, we defined potentiation as an increase of > 20% over the mean of the MA current at pH 7.4 before and 30 s after the low pH application. A sample recording of sensitization by low pH in an RA neurone was shown in Fig. 1B. IA and SA types were also potentiated (sample by pH 6.2 in Fig. 1C and D). MA currents were potentiated in 17 neurones out of 45 with application of pH 6.2 (38%, Fig. 2A). Both the percentage of neurones potentiated (Fig. 2A) and the magnitude of augmentation of the peak current amplitude in potentiated neurones (judged on the effect of pH 6.2) were increased in a pH-dependent manner (n= 17, P < 0.01, Fig. 2B). This sensitization was reversible and the MA current amplitude returned in 30 s to almost the same level as before low pH application (Fig. 1B–D). MA currents were attenuated (decrease of < 20%) in 6 neurones and another 22 neurones were not affected by low pH application.
Figure 2. Summary of MA current potentiation by low pH.
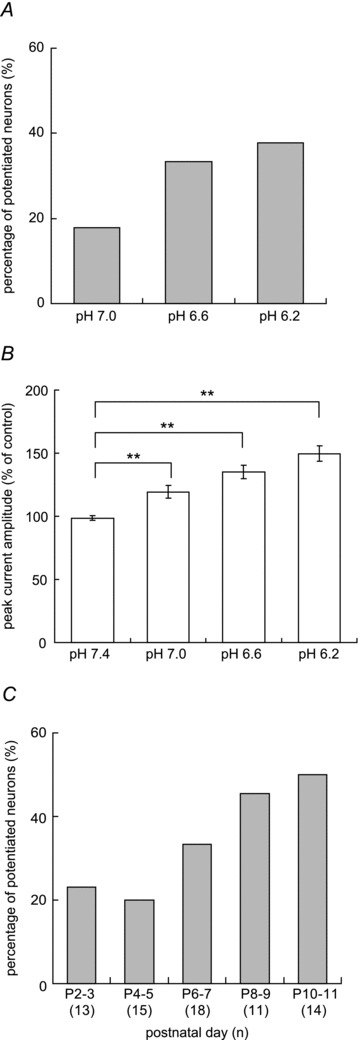
A, percentage of neurones potentiated by different pH applications. The criterion for potentiation was > 20% increase of the peak MA current amplitude during low pH application compared with the mean peak MA current amplitude at pH 7.4 before and 30 s after the low pH application. Each neurone was challenged by all levels of low pH in random order. n= 45. B, peak MA current amplitude (% of control) of potentiated neurones by pH 6.2 increased in a pH-dependent manner. Error bars represent SEM. **P < 0.01 (repeated measures ANOVA followed by Dunnett's multiple comparison test). n= 17. C, difference in the percentage of neurones potentiated by pH 6.6 at different postnatal days. The number of examined neurones is in parentheses under each bar. Note: the percentage of potentiated neurones at postnatal day 13 (though by pH 6.2) was almost the same (namely 47%) as that by pH 6.6 at postnatal day 10–11 (not shown in the figure).
Interestingly, potentiation of MA currents by low pH was observed in only about 20% of DRG neurones obtained from rats at early postnatal days (P2–P5). In contrast, around 40% or more of DRG neurones were sensitized after postnatal day 6 (Fig. 2C). This change during the postnatal period was similar to the postnatal change in percentage of neurones positively stained with IB4 (Bennett et al. 1996). Therefore, we classified the neurones by IB4 staining and compared pH-induced sensitization of MA currents between IB4-positive and -negative groups.
IB4-positive neurones were more frequently potentiated by low pH
We performed IB4 labelling on all neurones after repetitive mechanical stimulation and low pH application. The percentage of sensitized neurones was significantly higher in IB4-positive neurones than in IB4-negative ones in every MA current type (P < 0.05, χ2 test, Fig. 3A). There was no significant difference in the proportion of current types between IB4-positive and -negative neurones (Fig. 3B).
Figure 3. IB4-positive DRG neurones are more frequently potentiated by low pH application.
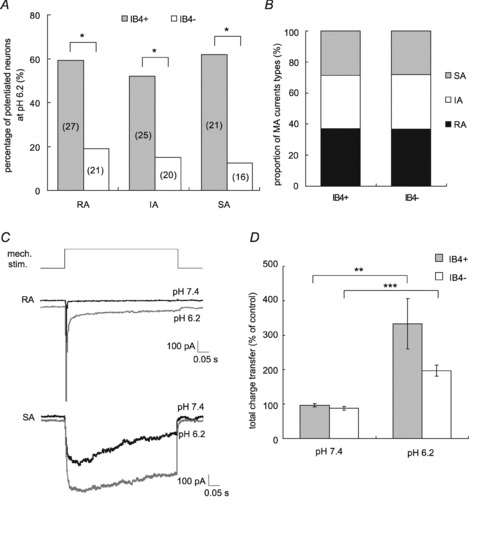
A, percentage of neurones potentiated by pH 6.2 application. Potentiation percentage was significantly greater in IB4-positive neurones than in -negative ones in every MA current type. *P < 0.05 (χ2 test). The number of examined neurones is in parentheses on each bar. B, proportion of current types in IB4-positive and -negative neurones. There was no significant difference between groups (IB4-positive: n= 73, IB4-negative: n= 57). C, sample recordings showing total charge transfer change. Upper panel shows an RA-type response (grey trace with pH 6.2 application, black trace with pH 7.4) and lower panel shows an SA-type response. The total charge transfer increased as a result of inactivation time constant prolongation. D, increase in the total charge transfer of MA current in neurones potentiated by low pH application. IB4-positive: n= 14, IB4-negative: n= 9. Error bars represent SEM; **P < 0.01, ***P < 0.001 (Student's paired t test).
Low pH application also prolonged the inactivation time constant (τ) of MA current (Fig. 3C), and increased the total charge transfer. On average, the total charge transfer of MA current at pH 6.2 was 3.3-fold greater than at pH 7.4 in the potentiated IB4-positive group (n= 14, P < 0.01, Student's paired t test, Fig. 3D), while there was a 2.0-fold increase in the IB4-negative group (n= 9, P < 0.001). The magnitude of facilitation tended to be larger in the IB4-positive group, but the difference between groups was not significant (P > 0.05, Student's t test).
Contribution of acid sensitive channels
It is well known that DRG neurones have two kinds of acid sensitive channels: acid-sensing ion channels (ASICs) and transient receptor potential vanilloid (TRPV) 1. Capsazepine (CPZ), a selective TRPV1 antagonist, and amiloride (AMI), an ASICs antagonist, were used to evaluate the involvement of these channels. In a preliminary study, we used the mixture of 10 μm CPZ and 200 μm AMI in 18 neurones (IB4-positive, 10 neurones; IB4-negative, 2; not identified, 6). Low pH reapplication was started after application of this antagonist mixture for 1 min. Currents directly evoked by low pH were suppressed 40–100% by this mixture in all types of MA neurones. In contrast to this, the sensitized mechanical response was completely inhibited only in RA neurones (sample in Fig. 4A, summary in Fig. 4B), but not in other neurones. Next, we used each antagonist separately to identify which antagonist was effective on sensitization of RA current. Ten micromolar CPZ was effective, while 200 μm AMI did not attenuate the sensitization (P < 0.05, Student's paired t test, Fig. 4C). CPZ at a ten times lower concentration was also effective on this sensitization (peak current amplitude (% of control) without 1 μm CPZ, 145 ± 9%; with 1 μm CPZ, 114 ± 2%; P < 0.05, Student's paired t test, n= 6).
Figure 4. Effects of acid sensitive channel blockers on mechanical sensitization by low pH.
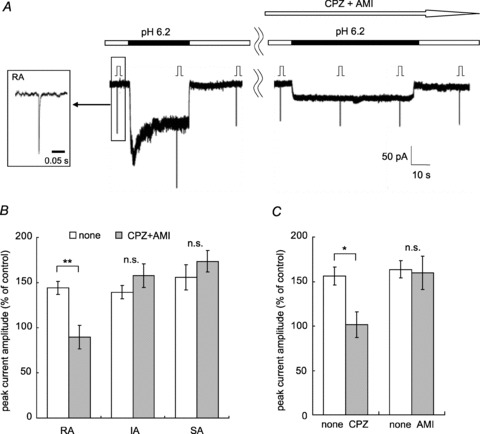
A, sample recordings of the RA current. The mixture of 10 μm capsazepine (CPZ) and 200 μm amiloride (AMI) was applied during the time period marked with an open arrow. This RA current was potentiated with pH 6.2 (left) and the blocker mixture attenuated the potentiation (right). Stimulation steps were 4. Inset shows MA current form with an expanded time scale (τ: 1.7 ms). B, comparison of the peak current amplitude (% of control) with and without blocker mixture (10 μm CPZ + 200 μm AMI) in each type of MA current. White bars, without blockers; grey bars, with blockers. Only RA current potentiation was blocked by the mixture. RA, n= 4; IA, n= 7; SA, n= 7. C, the effect of each blocker on sensitization of RA current. White bars, without blocker; grey bars, with CPZ or AMI. Ten micromolar CPZ was effective on RA current potentiation with pH 6.2 (n= 6), while 200 μm AMI had no effect (n= 6). Error bars represent SEM **P < 0.01, *P < 0.05, n.s., no significant difference (Student's paired t test).
To determine whether the increase in intracellular Ca2+ caused by TRPV1 is involved in sensitization by low pH, we performed experiments under Ca2+-free conditions (n= 8). We found that the RA current was potentiated even in the Ca2+-free conditions (Fig. 5).
Figure 5. No contribution of extracellular Ca2+ to RA current potentiation by low pH.
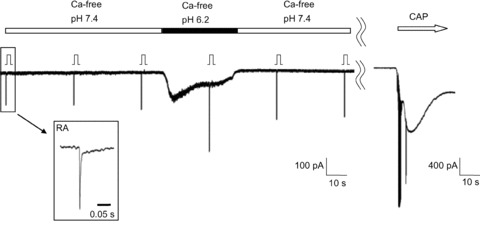
Sample recording showing the effect of Ca2+-free extracellular solution. RA current potentiation was also observed under Ca2+-free conditions. Stimulation steps were 6. Inset: MA current form with an expanded time scale (τ: 2.9 ms). This neurone responded to 1 μm capsaicin (CAP), a TRPV1 agonist (white arrow).
Absence of contribution of intracellular kinase and phospholipase C signalling pathways
It has been reported that intracellular signalling pathways that include some forms of protein kinase and phospholipase Cγ contribute to the sensitization to mechanical stimulation in IB4-positive (or glial cell line-derived neurotrophic factor (GDNF) sensitive) neurones (Hucho et al. 2005; Bogen et al. 2008). To clarify any involvement of these intracellular signalling mechanisms in the low pH-induced sensitization, we examined the effects of a strong and broad-spectrum kinase inhibitor, staurosporine, and a phospholipase C inhibitor, U73122. Addition of 100 nm staurosporine and 1 μm U73122 did not affect the percentage of IB4-positive neurones sensitized by low pH (Fig. 6). Staurosporine at 100 nm may not be sufficient to block kinases, therefore, we additionally examined the effect of staurosporine alone at a higher concentration (500 nm) and found it did not significantly decrease the percentage of the sensitized neurones. The percentage increases of the peak current amplitude of sensitized neurones were 153 ± 4% with no treatment, 209 ± 20% with 100 nm staurosporine + 1 μm U73122 treatment, and 159 ± 18% with 500 nm staurosporine treatment.
Figure 6. Effects of intracellular signalling enzyme inhibitors on mechanical sensitization by low pH in IB4-positive neurones.
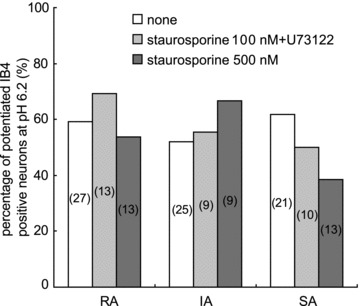
Percentages of potentiated IB4-positive neurones were compared in the presence and absence of inhibitors. White bars, percentage of potentiated neurones without inhibitors (same as IB4-positive neurones in Fig. 3A); pale grey bars, with a mixture of 100 nm staurosporine + 1 μm U73122; and dark grey bars, 500 nm staurosporine in patch pipette solution. The number of examined neurones is in parentheses on each bar. Percentage of sensitization in every MA current type was not significantly different with or without inhibitors (χ2 test). Note: among SA neurones, the percentage of neurones sensitized in the 500 nm staurosporine group tended to be lower than that in the non-treated group. Theoretically, however, the difference would not be significant even if the number of staurosporine-treated neurones was increased to 21 (the same number as the non-treated group) and none of them showed sensitization.
Contribution of chondroitin sulfate (CS)
Versican is a type of chondroitin sulfate proteoglycan and has been identified as an IB4-binding protein on DRG neurones (Bogen et al. 2005; Wu et al. 2005). Versican has many side chains of CS, and such polysaccharide moieties are often necessary for extracellular matrix functions. The above-mentioned result that IB4-positive neurones were more frequently augmented by low pH than IB4-negative ones would suggest some contribution of versican to this sensitization. Thus, we examined the involvement of versican, especially focused on CS side chains, in pH-induced sensitization in two ways: by adding excess CS to the external solution that may replace versican and by pretreating the DRG neurones with chondroitinase ABC, an enzyme that destroys the integrity of versican.
The effects of low pH on RA and IA mechanical responses of the IB4-positive group were attenuated by the presence of 0.1% CS in the extracellular solution (RA, n= 6; IA, n= 7; P < 0.05, Student's paired t test, Fig. 7A and B), but SA current potentiation needed a higher concentration (0.3%) to be blocked (n= 6, P < 0.05, Student's paired t test, Fig. 7B). In contrast, in IB4-negative neurones the mechanical responses facilitated by low pH were changed neither by 0.1% CS (peak current amplitude (% of control) with 0.1% CS; RA, 133 ± 11% (n= 6); IA, 162 ± 8% (n= 5)) nor by 0.3% CS (SA, 147 ± 6% (n= 3)). The grand average of the peak current amplitude in the IB4-negative group was not significantly different (144 ± 7% without CS vs. 146 ± 6% with CS), while in the IB4-positive group it was significantly different (144 ± 7% without CS vs. 97 ± 7% with CS; P < 0.0001, Student's paired t test). Thus, the suppressive effect of CS appears to be specific for IB4-positive neurones.
Figure 7. Chondroitin sulfate contribution to low pH-induced sensitization of IB4-positive neurones.
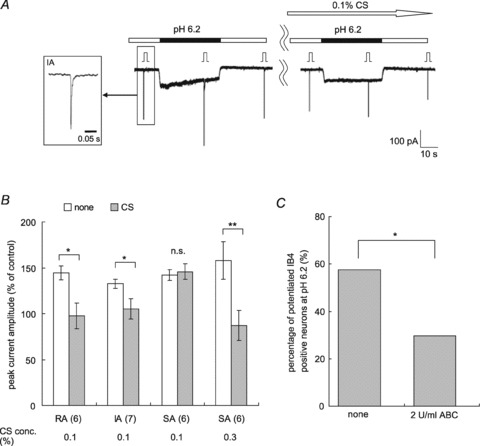
A, sample recordings from an IB4-positive neurone. This IA current was potentiated with pH 6.2 (left) and 0.1% CS attenuated this potentiation (right). The white arrow shows CS application and the black bars show pH 6.2 application. Inset shows the MA current form of this neurone with an expanded time scale (τ: 5.6 ms). Stimulation steps were 4. B, summary of the effect of CS on the IB4-positive neurones. White bars, the peak current amplitude before CS application; grey bars, after CS. The number of examined neurones is in parentheses under the graph. Error bars represent SEM. **P < 0.01, *P < 0.05, n.s., no significant difference (Student's paired t test). Note: CS application reversed the sensitization of MA current of IB4-positive neurones with pH 6.2, but it was not effective in IB4-negative neurones sensitized by low pH (RA, n= 6; IA, n= 5; SA, n= 3; see the text). C, effect of chondroitinase ABC treatment on the sensitization with pH 6.2. After pretreatment with 2 U ml−1 chondroitinase ABC (37°C, 30 min), the percentage of sensitized neurones was significantly decreased. *P < 0.05 (χ2 test). Non-treated group, n= 73; chondroitinase ABC-treated group, n= 54.
Chondroitinase ABC pretreatment also effectively inhibited MA current potentiation by low pH. We treated the DRG cultures with 2 U ml−1 chondroitinase ABC to digest CS just before the patch-clamp experiment. The percentage of sensitized IB4-positive neurones in the treated group was 30% (16/54 neurones), which was significantly less than 58% (42/73 neurones) in the group without enzymatic treatment (P < 0.05, Fig. 7C). The percentage increase of peak current amplitude of the chondroitinase ABC treated group was 109 ± 5% (n= 54) and that of the untreated group was 130 ± 4% (n= 73); there was a significant difference (P < 0.01, Student's t test). These results clearly show that the integrity of versican is needed for low pH-induced sensitization.
Discussion
We demonstrated in this study that low pH sensitized (increased) all three types (RA, IA and SA) of MA currents that were previously reported (Hu & Lewin, 2006; Lechner & Lewin, 2009) in rat nociceptive cultured DRG neurones. This observation is in accordance with previous findings that the mechanical response of thin-fibre muscle (Hotta et al. 2010) and cutaneous (Steen et al. 1992) afferents can be sensitized by low pH. The present results suggest that this afferent sensitization in the tissue can be directly (meaning not through activation of other cells) induced at neuronal terminals. In addition, we also showed that this sensitization was less frequently observed in the early postnatal days (P2–P5) and the percentage of sensitized neurones increased at later postnatal age (P6–P11). This change with postnatal age may be one reason why a previous report failed to detect any facilitation of MA currents with pH 6.4 (Drew et al. 2002).
We have also shown that the percentage of neurones having MA currents sensitized by low pH was significantly higher in IB4-positive neurones than in IB4-negative ones. There are some reports that IB4-positive neurones contribute to mechanical sensitization and the mechanical response itself. Bogen et al. (2009) showed that mechanical hyperalgesia after MCP–1 injection to the skin is attenuated by chondroitinase ABC or IB4-saporin in behavioural tests. They speculated that mechanical sensitization is caused by the interaction of MCP–1 with the chondroitin sulfate side chain of versican on IB4-positive neurones. Moreover, it has been reported that Mrgprd, a sensory neurone-specific G protein-coupled receptor, which is expressed specifically in IB4-positive neurones, is clearly related to mechanical sensitivity by behavioural tests under genetic ablation of this type of neurone (Cavanaugh et al. 2009). The authors of that study concluded that IB4-positive neurones having Mrgprd might have a preferred sensitivity to mechanical stimuli, at least in mice. Our data in this report also support the importance of IB4-positive neurones to mechanical sensitization and the involvement of versican.
In the present study, CPZ was effective in suppressing RA current potentiation by low pH, indicating that TRPV1 contributes to this sensitization of RA neurones. However, our findings do not support the possibility that calcium influx through TRPV1 channels contributes to this sensitization as a second messenger, because RA current was also potentiated even under the extracellular calcium-free condition. This result raises the possibility that TRPV1 is positioned near RA channels, possibly Piezo2 (Coste et al. 2010), and that conformational change of TRPV1 through channel opening may influence the mechanical sensitivity of these channels. Contrary to RA, ASICs and TRPV1 are not involved in low pH sensitization of IA and SA currents. In addition, the involvement of the intracellular kinase pathway and the PLC pathway were not confirmed in this sensitization. These observations together suggest that other mechanisms are involved.
There remains a possibility that MCP–1, which exists in vesicles in rat DRG neurones (Dansereau et al. 2008) and is known to induce mechanical hyperalgesia in rats (Bogen et al. 2009), is released by depolarization during low pH application and sensitizes MA currents. However, in this case too, versican is involved (Bogen et al. 2009).
The most important observation in this study is that addition of chondroitin sulfate (CS) to the extracellular solution attenuated the mechanical sensitization by low pH in IB4-positive neurones. CS at 0.1% attenuated sensitization in RA and IA, and a higher concentration of CS was able to inhibit sensitization of SA. We have no clear answer yet as to why there are different sensitivities to CS among the MA current types. We also found that the pretreatment of DRG culture with chondroitinase ABC attenuated the percentage of neurones sensitized by low pH. These results suggest involvement of CS in this sensitization. In contrast to the IB4-positive neurones, neither addition of CS nor treatment with chondroitinase ABC influenced the mechanical sensitization of IB4-negative neurones. Therefore, we speculate that the CS side chain of versican itself is involved in this mechanical sensitization.
How versican interacts with MA channels is then an interesting point to consider. Protons might influence the dissociation state of amino acids or other acidic side chains of versican, and change the shape of this molecule, thereby changing the interaction between versican and MA channels and/or their auxiliary molecule(s). This might consequently change the propagation of mechanical force to MA channels so as to increase the probability that the channels will open, or the number of open MA channels. Based on the above, the effect of CS and chondroitinase ABC could be explained as follows: we suppose that versican interacts with MA channels and/or their auxiliary molecule(s) through their CS side chain, and excess CS added might replace this interaction and so reduce versican's ability to interact with MA channels/auxiliary molecules. Chondroitinase ABC removes CS from versican; this will also make versican unable to interact with MA channels/auxiliary molecules. The full elucidation of the mechanism of interaction between versican and MA channels/auxiliary molecules will require further experiments.
Involvement of intracellular signals in sensitization of mechanical response has been reported (Koda & Mizumura, 2002; Di Castro et al. 2006). It was also reported in an in vivo experiment that hyperalgesia by GDNF involved versican (Bogen et al. 2008). The latter authors speculated that glycosaminoglycan side chains of versican might interact with GDNF or soluble GFRα1 secreted by surrounding tissue, causing intracellular signal transduction via PLCγ and kinases mentioned above. However, the hypothesis of involvement of intracellular signals does not seem to match our present findings because the mixture of a broad and robust kinase inhibitor, staurosporine, and PLC inhibitor, U73122, failed to decrease the percentage of IB4-positive neurones sensitized by low pH. The magnitude of sensitization (percentage increase of the peak current amplitude) became larger in the group treated with 100 nm staurosporine + 1 μm U73122, but not in the group treated with 500 nm staurosporine. We have no clear explanation for this difference.
These findings also suggest that in pH induced sensitization the possibility of involvement of proton-sensing G protein-coupled receptors is low. Our present results together indicate that the extracellular interaction between protons and versican causes mechanical sensitization.
This is the first report to show that sensitization of primary afferents can be induced by interaction with cell surface proteoglycans, without activating an intracellular signal transmission cascade. CS is one kind of glucosaminoglycans that exists in cartilage and connective tissues in many organs. It has been reported that hyaluronans, another kind of glucosaminoglycans that are used in joint pain treatment, reduce stretch-activated ion channel activity in vitro (de la Peña et al. 2002). CS also attenuated knee osteoarthritis pain in a randomized study of humans (Möller et al. 2010). In addition, recent reports show important roles of glucosaminoglycans in network formation in the central nervous system. Chondroitinase ABC was found to promote primary afferent sprouting in spinal cord injury (Bradbury et al. 2002; Barritt et al. 2006), and more recently, the role of CS in the central nervous system has been widely investigated (Kwok et al. 2011). Chondroitinase ABC also attenuated acute pain induced by MCP–1 injection to the skin (Bogen et al. 2009). Our results show that CS is important not only in chronic but also in acute pain, and they may provide a different perspective for pain treatment with CS and chondroitinase ABC.
We also reported that low pH-induced sensitization occurs in about 20% of IB4-negative neurones. RA current may be sensitized through TRPV1 activation; even so it is still unclear how IA and SA currents of IB4-negative neurones are sensitized.
In summary, the present study showed that low pH directly sensitized sensory neurones, especially IB4-positive neurones. Sensitization of IA and SA currents involved neither acid sensitive channels (ASICs and TRPV1) nor an intracellular kinase/PLC signal transmission cascade, but was suppressed by addition of CS or chondroitinase ABC. From these results, we propose a novel mechanism of sensitization in which change induced by protons of the extracellular matrix (the sulfated glycosaminoglycan side chain of versican) may alter the transmission of force to MA channels and/or their auxiliary molecule(s).
Acknowledgments
The authors thank Dr H. Ishibashi for teaching us the patch-clamp technique and Dr G. R. Lewin and his colleagues, especially Dr S. G. Lechner, for advice on the mechanical stimulation technique. We also thank Dr B. Lynn for kindly reading our manuscript, giving valuable comments on it and correcting the English. Sodium chondroitin sulfate was kindly donated by Zeria Pharmaceutical Co., Ltd. This work was supported in part by grants from Japan Society for the Promotion of Science (No. 23390154) and from the Ministry of Education, Culture, Sports, Science and Technology (No. 21026015) in Japan, and Chiyoda Mutual Life Foundation.
Glossary
- AMI
amiloride
- AP
action potential
- ASICs
acid-sensing ion channels
- CPZ
capsazepine
- CS
chondroitin sulfate
- DRG
dorsal root ganglion
- GDNF
glial cell line-derived neurotrophic factor
- IA
intermediately adapting
- IB4
isolectin B4
- MA
mechanically activated
- MCP-1
monocyte chemoattractant protein 1
- PLC
phospholipase C
- RA
rapidly adapting
- SA
slowly adapting
- TRP
transient receptor potential
Author contributions
A.K. performed all experimental work, analysed and interpreted the data. K.M. conceived the study. A.K., K.K. and K.M. designed experiments, drafted, critically revised and approved the final version of the article.
References
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dreger M, Gillen C, Schröder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J. 2005;272:1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCγ, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol. 2011;589:3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106:757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- de la Peña E, Sala S, Rovira JC, Schmidt RF, Belmonte C. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro. Pain. 2002;99:501–508. doi: 10.1016/S0304-3959(02)00260-9. [DOI] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Taguchi T, Mizumura K. Low pH enhances response of thin muscle afferents to mechanical stimuli. Adv Exp Med Biol. 2010;669:315–318. doi: 10.1007/978-1-4419-5692-7_64. [DOI] [PubMed] [Google Scholar]
- Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Tzeng JN, Chen YJ, Tsai WF, Chen CC, Sun WH. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci. 2007;36:195–210. doi: 10.1016/j.mcn.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Lo TM, Loh HH, Thayer SA. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Tran PK, Weiser-Evans MC, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury: role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler Thromb Vasc Biol. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- Koda H, Mizumura K. Sensitization to mechanical stimulation by inflammatory mediators and by mild burn in canine visceral nociceptors in vitro. J Neurophysiol. 2002;87:2043–2051. doi: 10.1152/jn.00593.2001. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Lechner SG, Lewin GR. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol. 2009;587:3493–3503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Möller I, Perez M, Monfort J, Benito P, Cuevas J, Perna C, Domenech G, Herrero M, Montell E, Verges J. Effectiveness of chondroitin sulphate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2010;18(Suppl. 1):S32–S40. doi: 10.1016/j.joca.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154:113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tsunozaki M, Bautista DM. Mammalian somatosensory mechanotransduction. Curr Opin Neurobiol. 2009;19:362–369. doi: 10.1016/j.conb.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]


