Abstract
Acetylcholine (ACh), a candidate neurotransmitter that has been implicated in taste buds, elicits calcium mobilization in Receptor (Type II) taste cells. Using RT-PCR analysis and pharmacological interventions, we demonstrate that the muscarinic acetylcholine receptor M3 mediates these actions. Applying ACh enhanced both taste-evoked Ca2+ responses and taste-evoked afferent neurotransmitter (ATP) secretion from taste Receptor cells. Blocking muscarinic receptors depressed taste-evoked responses in Receptor cells, suggesting that ACh is normally released from taste cells during taste stimulation. ACh biosensors confirmed that, indeed, taste Receptor cells secrete acetylcholine during gustatory stimulation. Genetic deletion of muscarinic receptors resulted in significantly diminished ATP secretion from taste buds. The data demonstrate a new role for acetylcholine as a taste bud transmitter. Our results imply specifically that ACh is an autocrine transmitter secreted by taste Receptor cells during gustatory stimulation, enhancing taste-evoked responses and afferent transmitter secretion.
Key points
Acetylcholine (ACh), a classical neurotransmitter, stimulates M3 muscarinic receptors on Receptor (Type II) taste bud cells
ACh is synthesized by, and released from Receptor (Type II) taste bud cells during gustatory stimulation.
This muscarinic autocrine feedback amplifies taste-evoked Ca2+ signals and enhances afferent neurotransmitter (ATP) release from Receptor (Type II) cells.
Taste Receptor cells in mice lacking M3 muscarinic receptors display depressed sensitivity to gustatory stimulation
The findings highlight a new signalling pathway in taste buds and may explain taste disturbances (i.e. side effects) associated with certain anticholinergic drugs.
Introduction
Mammalian taste buds utilize an array of neurotransmitters to analyse and transmit gustatory signals to the CNS in response to taste stimulation. While the full extent of these interactions is far from elucidated, roles have been revealed for ATP (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007), serotonin (Kaya et al. 2004; Huang et al. 2005), GABA (Cao et al. 2009; Dvoryanchikov et al. 2011; Huang et al. 2011), and noradrenaline (Huang et al. 2008a; Zhang et al. 2010), with many other candidate transmitters implicated. One taste transmitter that has not been extensively characterized to date is acetylcholine (ACh). Decades ago, acetylcholinesterase (AChE) was characterized in taste tissue and between individual taste cells (Macintosh, 1941), suggesting a role for ACh in taste buds. Later studies suggested the presence of muscarinic receptors in canine lingual epithelia (Simon & Baggett, 1992), and showed that ACh elicits calcium mobilization in a subset of taste cells (Ogura, 2002). Moreover, ACh receptors have been localized within the taste bud (Ogura & Lin, 2005; Eguchi et al. 2008; Oliveira-Maia et al. 2009; Rogachevskaja et al. 2010), implying that cholinergic signalling is utilized in taste transduction. There remains, however, no identification of which cells respond to ACh or the probable source(s) of ACh that evoke responses in taste cells. Consequently, there is yet no physiological role suggested for ACh during taste reception.
Taste buds consist of three principal cell types (Chaudhari & Roper, 2011), any one of which may represent a candidate target for ACh. Type I cells are glial-like in nature and ensheath the other two classes with thin lamellar processes. Type II cells express G-protein coupled receptors (GPCRs) for sweet, bitter and umami compounds, and communicate with afferent nerve fibres by secreting the excitatory neurotransmitter ATP through pannexin 1 channels (Huang et al. 2007; Romanov et al. 2007). Because of their role in transducing sweet, bitter and umami, these cells have been termed Receptor cells. Type III cells are the only cells that possess synapses, defined morphologically (Yang et al. 2000). Consequently, these cells have been termed Presynaptic cells. Presynaptic cells respond to sour (acid) taste stimulation (Richter et al. 2003; Huang et al. 2006; Huang et al. 2008b) as well as to ATP released by Receptor cells (Richter et al. 2003; Huang et al. 2006; Huang et al. 2007). Presynaptic cells secrete serotonin which inhibits taste responses in Receptor cells, thereby providing negative feedback during gustatory stimulation (Huang et al. 2009), possibly due to the more rudimentary non-synaptic release mechanism of ATP through pannexin channels, which may require fast desensitization due to their high conductivity. Thus, the taste bud functions as a complex unit, employing several transmitters in the generation of taste signals.
The function of ACh in taste buds is unresolved, though it plays a documented role in other peripheral chemosensory organs (Li & Matsunami, 2011; Krasteva et al. 2012). ACh has been hypothesized to be an efferent transmitter released from parasympathetic nerve fibres within the taste bud (Inoue et al. 1992). Indeed, evidence for cholinergic innervation of taste buds has been presented (Ogura et al. 2007). Alternatively, ACh may be stored and released from within the taste bud during gustatory stimulation. In this report, we employed scanning laser confocal calcium imaging and transmitter biosensors to examine the actions of ACh in mouse taste buds. Our results identify Receptor (Type II) taste cells as the source of ACh release in taste buds and highlight a cholinergic contribution to taste transduction.
Methods
Ethical approval
All experimental procedures were approved by the University of Miami Animal Care and Use Committee. Animals were killed via exposure to CO2 followed by cervical dislocation.
Animals
Mice used in these experiments included C57BL/6 mice (wild-type); transgenic mice expressing enhanced green fluorescent protein (GFP) under control of the PLCβ2 promoter (PLCβ2-GFP mice) (Kim et al. 2006); transgenic mice expressing GFP under the GAD67 promoter (GAD-GFP mice) (Chattopadhyaya et al. 2004); and genetic knockout mice missing the muscarinic receptors M1 and M3, along with wild-type animals from the same mixed genetic background (a gift from Dr J. Wess, NIDDK, Bethesda, MD, USA). Receptor (Type II) taste cells selectively express GFP in PLCβ2-GFP mice, thereby facilitating their identification in living tissues (Kim et al. 2006). Similarly, the majority, but not all Presynaptic (Type III) cells fluoresce green in GAD-GFP mice (Tomchik et al. 2007).
Taste cell isolation
Lingual epithelium was peeled from the circumvallate papillae after injection with 2.5 mg ml−1 dispase II (Roche), 1 mg ml−1 trypsin inhibitor (Sigma) and 1 mg ml−1 collagenase A (Roche). The epithelium was subsequently bathed in Ca2+-free Tyrode for 20 min, pinned, and whole taste buds collected with a fire polished micropipette. Taste buds were then transferred to a shallow recording chamber, secured with Cell-Tak (BD Biosciences, San Jose, CA, USA), and superfused with normal Tyrode solution.
Cellular biosensor technique
Acetylcholine biosensors consisted of CHO-K1 cells stably expressing M3 receptors (CRL-1981, ATCC, Manassas, VA, USA) and loaded with 5 μm Fura-2-AM (Rodriguez-Diaz et al. 2011). These cells are sensitive to ACh in the micromolar range (EC50= 1.4 μm). Endogenous purinoceptors on the biosensors were desensitized by incubating the cells in 500 μm ATP for 60 min prior to the experiments (Huang et al. 2007). Biosensors did not respond to any of the taste stimuli utilized in this study, or to 5-HT (3 nm), noradrenaline (10 nm) or GABA (10 um), or to depolarization with 50 mm KCl. For ATP biosensors, we used Fura-2-loaded CHO cells expressing P2X2 and P2X3 receptors (Huang et al. 2007).
Experiments were performed on whole taste buds and on isolated taste cells. If the latter, taste cells were also loaded with Fura-2 and imaged along with biosensors. Biosensors were drawn onto a fire-polished micropipette with gentle suction and manipulated into contact with taste buds. Fura-2 images (F340, F380) were acquired ratiometrically using an Olympus (Tokyo, Japan) IX71 inverted fluorescence microscope with a 20× water immersion objective. Image analysis was carried out using Imaging Workbench 6.0 (Indec Biosystems, Santa Clara, CA, USA), with traces plotted as intracellular calcium concentration using a Ca2+ imaging calibration kit (Invitrogen, USA) (Grynkiewicz et al. 1985).
Lingual slice preparation
Lingual slices of 100 μm containing circumvallate papillae taste buds loaded with either Calcium Green Dextran (10 kDa), or Calcium Orange (both 2 mm in 50% DMSO, Invitrogen, Carlsbad, CA, USA) when GFP cellular markers was used were cut from blocks of lingual tissue using a vibrating blade microtome (Leica VT1000 S) (Caicedo et al. 2000). Slices were placed in a shallow recording chamber and attached to the coverslip base using Cell-Tak. Lingual slices were perfused at room temperature with Tyrode buffer containing elevated Ca2+ (8 mm; see below). Elevating Ca2+ improved the stability of the recordings and improved signal-to-noise ratio. We perfused the recording chamber at a rate of 2 ml min−1 and focally applied taste stimuli to the apical tips of taste buds (see below), to ensure that taste solutions only contacted the apical pores of the taste buds, and not the parenchyma. Focal taste application and buffer perfusion at 2 ml min−1 assured that tastant was entirely cleared from the bath in 15–30 s.
Scanning laser confocal Ca2+ imaging
In the lingual slice preparation, we imaged cells confocally to record Ca2+ transients in cells while maintaining sufficient z-resolution to ensure sampling from a single cell. All stimuli were dissolved in Tyrode solution. KCl and pharmacological agents were bath-applied. We applied taste stimuli to the taste pore via a 2 μm ‘puffer’ micropipette, using an MSC PLI-100 pressure source (Medical Systems, Greenvale, NY, USA). Taste stimuli were ejected by applying a pressure of 3 p.s.i. for 2 s. Responses from cells were recorded using an Olympus BX50WI laser scanning confocal microscope (Olympus Optical, Thornwood, NY, USA), scanning with argon and krypton lasers, and using the Fluoview image analysis package. Images were captured at 2 s intervals. Responses are presented as changes in relative fluorescence, ΔF/F0: (F–F0)/F0. Photobleaching of the sample was corrected by plotting the gradual decline of signal over time (typically with an exponentially decreasing baseline) and subtracting this baseline from the response (Caicedo et al. 2000). Only cells that maintained consistent responses for the duration of experimentation were included in our analysis (see Data analysis, below). We identified taste cell types either physiologically or through GFP expression in PLCβ2-GFP or GAD-GFP mice.
RT-PCR analysis
RNA was extracted from isolated taste buds and from surrounding non-taste epithelium (DeFazio et al. 2006). Suitable positive control tissues were obtained and validated for genes of interest, with water acting as a negative control. Genomic contamination was removed from samples using DNase 1, and RNA was reverse transcribed with Superscript III (Invitrogen, Carlsbad, CA, USA). A sample the equivalent of 2 taste buds was used for each analysis, with all results run in triplicate. Amplification was carried out on an iCycler with reagents from Bio-Rad (Hercules, CA), for a total of 37 cycles for each gene of interest, with β-actin at 30 cycles and PLC-β2 at 35 cycles acting as controls.
Solutions and taste stimuli
Unless otherwise indicated, chemicals were purchased from Sigma (St Louis, MO, USA). Normal Tyrode solution contained the following (in mm): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, 10 sodium pyruvate, 5 NaHCO3 pH 7.4, 310–320 mosmol l−1. To prepare buffer with elevated calcium (for confocal calcium imaging) or with nominally zero calcium, we substituted equimolar sodium with calcium. Similarly, high-potassium Tyrode solution for depolarizing taste cells was prepared by substituting equimolar KCl (50 mm) for NaCl.
Taste stimuli used in these experiments consisted of a mixture of the following: an artificial sweetener, 100 μm SC45647 (2-[[[4-(aminomethyl)anilino]-[[(1R)-1 -phenylethyl]amino]methyl]amino]ethane-1,1-diol) (a gift from Dr G. Dubois, Coca Cola, Atlanta, GA, USA); bitter compounds, 30 μm cycloheximide and 200 μm denatonium; and umami compounds 200 mm monopotassium l-glutamate (MPG) with 1 mm inosine 5-monophosphate (IMP). Fluorescein (2 μm) was added to the tastant mixture when applied focally in the lingual slice preparation to track stimulation and to ensure consistent application of tastants. 1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) was purchased from Tocris Bioscience (Ellisville, MO, USA) and MT7 from Peptides International (Louisville, KY, USA).
Data analysis
Baseline fluorescence was established for a minimum of 100 s before a stimulus was presented. We scored positive responses only if (1) the peak ΔF/F was at least two times the baseline fluctuation for that cell, (2) responses could be elicited at least twice in the same cell by the same stimulus and appeared approximately of the same amplitude, and (3) responses were cell-specific (i.e. were observed in specific cells, not in all dye-loaded cells in the field of view; and not recorded in background regions) (Caicedo et al. 2000). This assured that signals in the lingual slice preparation were not generated by tissue movement or a stimulus artifact. Data analyses were performed in Microsoft Excel, and Prism v.5 (Graphpad Software, Inc, La Jolla, CA, USA), with data displayed as in a manner appropriate for respective sample size (Drummond & Vowler, 2011).
Results
Acetylcholine elicits Ca2+ responses in taste receptor cells
Previous researchers have shown acetylcholine (ACh) or carbachol (CCh) elicits Ca2+ mobilization in mouse taste receptor cells (Ogura, 2002; Eguchi et al. 2008). We used a different approach – intact taste buds in a lingual slice – to examine this phenomenon in greater detail, and to localize the actions of ACh to apical (chemosensory) versus basolateral (synaptic) regions of taste buds.
Taste buds in lingual slices showed robust and repeatable Ca2+ responses to ACh (Fig. 1A), that were blocked with atropine (dashed line, 5 μm). Ca2+ responses were evoked by bath-applied ACh (which stimulates apical and basolateral regions of taste cells), but applying ACh focally onto the apical tips of taste cells was ineffective (Fig. S1). This finding suggests that ACh receptors are located on the basolateral membranes of taste cells, implicating a synaptic role in taste.
Figure 1. Acetylcholine (ACh) stimulates M3 muscarinic receptors on mouse taste bud cells.
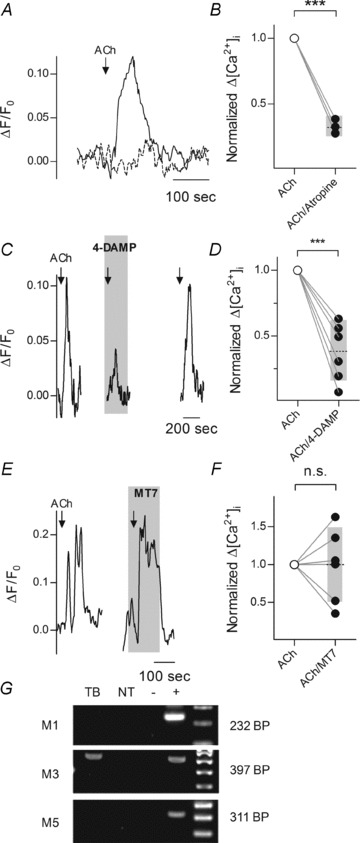
Lingual slices of mouse circumvallate papillae containing intact taste buds were prepared as described (Caicedo et al. 2002; Dando & Roper, 2009) and were stimulated with bath-applied ACh. A, 1 μm ACh (arrow) mobilized Ca2+ in Receptor (Type II) taste cells (continuous line). In the presence of atropine (5 μm, dashed line), responses were much attenuated). Cells were loaded with Calcium Orange and identified as Receptor cells using GFP under the control of the PLCβ2 promoter. B, ACh-evoked responses (open circles) were blocked by atropine (5 μm) (filled circles), consistent with muscarinic receptor activation. P < 0.0001, Student's two-tailed paired t test. Grey bar and dotted line denote mean and 95% CI. C, blocking M3 receptors with 4-DAMP (5 μm, shaded area) inhibited ACh-evoked responses in taste Receptor cells. D, summary of experiments testing 4-DAMP (5 μm). P < 0.05, Student's two-tailed paired t test. E, MT7 (50 nm), an M1 antagonist, did not significantly affect ACh-evoked responses in Receptor cells. F, summary of data showing lack of effect of MT7 (50 nm) on ACh responses, P= 0.95, Student's two-tailed paired t test. A, C and E, ordinate = relative change in fluorescence (see Methods), B, D and F, ordinate =Δ[Ca2+]i normalized to the level before antagonist. G, RT-PCR showed expression of M1, M3, and M5 ACh receptors in CV taste buds (TB), non-taste epithelium (NT), water (−, a negative control), and positive controls (+; M1, brain; M3, kidney; M5, brain). Only M3 was detected in taste buds at this sensitivity.
Consistent with previous reports (Ogura, 2002; Eguchi et al. 2008), we found ACh responses were blocked by atropine (5 μm) (Fig. 1B), indicating that ACh responses were generated by muscarinic ACh receptors. To date, there has been disagreement concerning which muscarinic receptors are responsible for the ACh sensitivity of taste buds (Ogura, 2002; Eguchi et al. 2008). Thus, we tested 4-DAMP (5 μm), an M3-selective receptor antagonist. 4-DAMP significantly reduced ACh-evoked calcium mobilization in taste cells (Fig. 1C and D). In contrast, the M1 antagonist MT7 (at 50 nm) had little to no effect (Fig. 1E and F). Conclusively, RT-PCR analysis of mRNA from isolated taste buds showed M3 but not M1 or M5 receptors are expressed in taste cells (Fig. 1G), consistent with the pharmacological testing. Collectively, the data show that ACh evokes Ca2+ mobilization in taste buds via M3 muscarinic receptors.
To localize the specific target(s) of ACh in taste buds, we isolated taste cells from transgenic mice expressing GFP in Receptor (Type II) taste cells (Kim et al. 2006). ACh-evoked Ca2+ mobilization was only recorded from GFP-expressing (Receptor) cells (8 of 19 cells). No ACh responses were recorded from cells lacking GFP (0 of 15 cells; P= 0.0045, Fisher's exact test).
ACh potentiates taste-evoked signals in Receptor (Type II) cells
We next investigated what role ACh might play during gustatory stimulation. Using the lingual slice preparation to study taste buds in a relatively intact environment (as opposed to taste buds or taste cells isolated in vitro), we recorded Ca2+ responses evoked by focal application of sweet, bitter, or umami taste stimuli to the taste pore. Such focal application of tastants reliably evokes Ca2+ mobilization in Receptor (Type II) taste cells (Tomchik et al. 2007; Dando & Roper, 2009). Importantly, taste-evoked responses to focal application were significantly enhanced by superfusing the preparation with ACh (1 μm) during taste stimulation (Fig. 2A and B).
Figure 2. ACh potentiates taste responses in taste buds.

Ca2+ mobilization in taste cells in the lingual slice preparation was assayed with laser scanning confocal Ca2+ imaging. Neurotransmitter secretion from isolated taste buds was measured with ATP biosensors. A, in the lingual slice preparation, taste-evoked Ca2+ mobilization was enhanced by perfusing the tissue with ACh (1 μm, present throughout shaded area). The traces show mean responses ± SEM from 15 cells. Arrows indicate focal application of taste stimulus. Note, perfusing ACh itself (shaded area) generated a small response, consistent with data in Fig. 1. This ACh response was taken into account by aligning the traces such that the maximum response to ACh was set to ΔF/F0= 0, i.e. the baseline for taste-evoked responses was re-set to zero. Focally applied tastant responses were measured from that new baseline. Ordinate shows ΔF/F0 from dye-loaded taste cells. B, summary of effects of ACh (1 μm) on taste-evoked responses. n= 15, P < 0.001, Student's two-tailed paired t test. Ordinate, Ca2+ responses normalized to the control stimulus. C, ATP biosensors were positioned near taste buds to detect taste-evoked neurotransmitter (ATP) release in the absence and in presence (shaded area) of 1 μm ACh. Traces show mean response ± SEM from 12 biosensors. Ordinate, [Ca2+]i in ATP biosensors. D, summary of effects of ACh (1 μm) on taste-evoked ATP secretion, n= 15, P < 0.01, Student's two-tailed paired t test. Ordinate, biosensor responses normalized to control stimulus.
We then isolated taste buds from vallate papillae and tested whether ACh affects taste-evoked secretion of the taste neurotransmitter ATP (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007) using neurotransmitter biosensors (see Methods). Stimulating taste buds with a bitter/sweet taste solution elicited ATP release (Fig. 2C), and most importantly, this taste-evoked ATP release was significantly elevated by the presence of ACh (1 μm) (Fig. 2D).
ACh is synthesized in, and released from, Receptor (Type II) taste cells
We investigated potential source(s) of ACh to determine whether this transmitter might be intrinsic to taste buds. RT-PCR on mRNA isolated from taste buds showed that the biosynthetic enzyme choline acetyltransferase (ChAT) was expressed only in taste tissue and not in surrounding non-taste epithelium (Fig. 3A), indicating that ACh can be synthesized within the taste bud and may be stored there for release during taste reception.
Figure 3. Receptor (Type II) cells release ACh in response to taste stimulation.
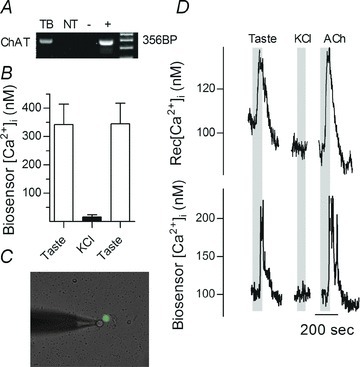
A, RT-PCR showed mRNA for choline acetyltransferase (ChAT), the biosynthetic enzyme for ACh, is expressed at high levels in taste buds (TB), but not in the surrounding non-taste epithelium (NT). Controls, brain (+) and water (−). B, summary of data from 12 biosensor cells, testing ACh secretion from isolated taste buds in response to taste and KCl stimulation. Mean ± SEM shown. Bitter/sweet taste solution, but not KCl depolarization (50 mm) evoked ACh release. C, an ACh biosensor was positioned near an isolated Receptor (Type II) cell, identified using GFP-PLCβ2 mice (see Methods). D, as shown in C, a Receptor cell was apposed to an ACh biosensor cell. The traces show transmitter secretion was evoked by taste stimulation but not by depolarization with 50 mm KCl (bars). Responses in the isolated Receptor cell and biosensor were recorded concurrently (upper and lower traces, respectively). Ordinates indicate [Ca2+]i in Receptor cell (upper trace) and in ATP biosensor (lower trace).
Acetylcholine biosensors are sensitive and specific detectors
To identify whether taste cells might indeed secrete ACh, we obtained CHO cells stably expressing highly sensitive muscarinic receptors and used these cells as ACh biosensors. ACh biosensors responded well to 1 μm ACh but were unaffected by taste stimulation, eliminating a potential source of artifacts (Supplemental Material, Fig. S2A). Furthermore, ACh-evoked responses in biosensors were not altered by the presence of taste stimuli (Fig. S2B). Because taste buds secrete several neurotransmitters, including ATP, noradrenaline, serotonin and GABA, we tested whether any of these transmitters stimulated ACh biosensors. None of these transmitters evoked a false positive (Fig. S2C and D). Parenthetically, purinoceptors endogenous to these biosensors had been desensitized, as described in Methods. Lastly, biosensor cells did not respond with Δ[Ca2+]i to KCl depolarization (Figure S2D), in agreement with other biosensor cells constructed from genetically engineered CHO cells (Huang et al. 2007).
Stimulating taste buds with tastants reliably evoked biosensor responses, indicating ACh release. ACh biosensor responses could only be obtained when the biosensor was closely apposed to a taste bud; moving the biosensor even micrometres away from the taste bud eliminated taste-evoked biosensor responses. ACh release was observed when taste buds were stimulated with a taste mixture (sweet and bitter stimuli), but not when taste buds were depolarized with KCl (Fig. 3B). The taste mixture selectively stimulates Receptor (Type II) cells, whereas KCl depolarization is an effective stimulus only for Presynaptic (Type III cells) (DeFazio et al. 2006). These findings implicate Type II cells in the taste bud were the origin of taste-evoked ACh release.
To identify unambiguously which taste cells secrete ACh, we next isolated individual Receptor and Presynaptic cells from transgenic mice expressing GFP markers for these cell types and monitored taste-evoked ACh secretion with biosensors (Fig. 3C). Eight out of nine identified Receptor cells released ACh upon taste stimulation (Fig. 3D). In contrast, only one Presynaptic cell out of five elicited a biosensor response when the Presynaptic cell was stimulated (50 mm KCl) and even at that, the biosensor readout was weak, suggesting a false positive. The difference in incidence of ACh release from Receptor versus Presynaptic cells was significant at the P= 0.02 level (Fisher's exact test). These findings are consistent with the predominant source of ACh released during taste bud stimulation being mainly, if not exclusively Receptor (Type II) cells.
Cholinergic mechanisms during taste transduction
If ACh enhances ATP secretion (Fig. 2) and also if taste stimulation triggers Receptor cells to secrete ACh (Fig. 3), then one might expect that blocking cholinergic receptors during gustatory stimulation would lead to a reduced secretion of ATP. We tested this by measuring taste-evoked ATP secretion from taste buds before and after adding atropine to block muscarinic receptors. Indeed, on its own, atropine (5 μm) significantly and reversibly reduced taste-evoked ATP secretion (Fig. 4A and B). As a further test of the role of muscarinic enhancement of taste transmitter release and to verify that atropine was not directly interfering with transmitter secretion, we measured taste-evoked ATP secretion with and without atropine in taste buds isolated from genetically mutant littermates lacking M1 and M3 receptors (double knock out, DKO, mice) (Bymaster et al. 2003). Biosensor measurements showed that taste-evoked ATP secretion was on average 80% of that observed in wild-type animals in taste buds from double knockout mice. Importantly, atropine had no effect on taste-evoked ATP release in taste buds from M1/M3 DKO mice, in marked contrast to taste buds from wild-type mice (Fig. 4C–E).
Figure 4. ACh from Receptor cells enhances neurotransmitter (ATP) secretion during taste stimulation.
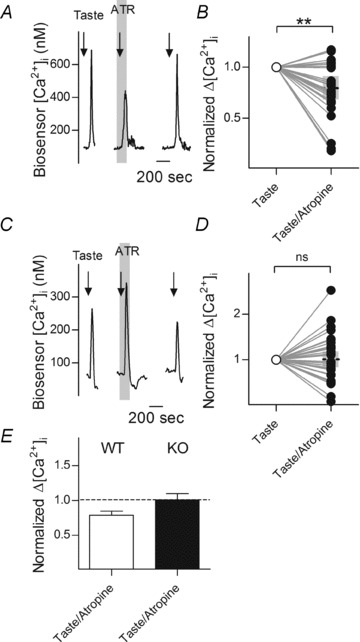
ATP biosensors were positioned near taste buds of wild-type (WT) and mutant mice lacking both M1 and M3 muscarinic receptors (KO) to monitor taste-evoked transmitter secretion, as in Fig. 2. A, in a WT mouse, taste-evoked ATP release was reduced by atropine (5 μm). Arrows indicate taste stimulation. Shaded area shows addition of atropine. (Parenthetically, atropine has no effect on ATP biosensors.) B, summary of effects of atropine in WT mice, from experiments as in A. n= 25, P= 0.02, Student's two-tailed paired t test. C, in an M1/M3 double knockout mouse, atropine (5 μm) did not affect taste-evoked ATP secretion. D, summary of experiments from M1/M3 double knockout mice, as in E. n= 32, P= 0.94, Student's two-tailed paired t test. E, comparison of actions of atropine on taste-evoked ATP release in wild-type (open bar) and double knockout (filled bar) mice; dashed line indicates response of same cell to taste without atropine. Mean+SEM shown by bar. Ordinates in A and C show [Ca2+]i in biosensor cells, ordinates in B, D and E indicate biosensor responses normalized to control stimulus.
Discussion
Our findings show that ACh potentiates taste-evoked signals in, and augments taste-evoked transmitter (ATP) secretion from, Receptor (Type II) cells. Simon & Baggett (1992) first identified muscarinic receptors in lingual epithelium, with Ogura (2002) subsequently describing muscarinic Ca2+ mobilization in rat taste cells. However, distinctions between Receptor and Presynaptic cells had not been defined at that time and thus earlier studies were not able to more precisely identify the targets for ACh in taste buds. Further, muscarinic receptors in taste buds have alternately been reported as the subtype M1 (Ogura, 2002) or M3 (Eguchi et al. 2008) by immunocytochemistry and RT-PCR, respectively. The present findings, using pharmacology and RT-PCR, establish that taste Receptor cells respond to ACh via M3 receptors. Rogachevskaja and colleagues (2010) also recently reported that muscarinic receptors, most likely either M3 or M5, induced Ca2+ transients in mouse taste cells with an EC50 of 2.5 μm. Their observations are in good agreement with our own, save for the fact that they electrophysiologically identified the cells as Type I, and not Type II taste cells.
The mechanism whereby activating muscarinic M3 receptors augments taste responses is not known. There are reports that muscarinic receptors interact with olfactory receptors to enhance odour-evoked responses (Firestein & Shepherd, 1992; Li & Matsunami, 2011). For example, Li & Matsunami (2011) co-applied odour stimuli with M3 ligands and showed that agonists increased, and antagonists attenuated odour-evoked responses in HEK 293T cells that were co-expressing ORs and M3, similar to our present findings using gustatory stimuli and native taste cells. Li & Matsunami reported that M3 formed stable functional complexes with the odorant receptor OR-S6, but further details of the mechanism of interaction remain to be reported.
Nicotinic receptors have been implicated in taste buds but have been studied mainly with reference to activation of bitter taste by nicotine (Dahl et al. 1997; Oliveira-Maia et al. 2009). We found no evidence that ACh elicited responses when applied to the apical (i.e. chemosensory) tips of taste cells. This could be attributed to the differing experimental conditions in which these studies were carried out, the taste field studied, or simply be due to the fact that as nicotine is a small, lipophilic molecule, it could conceivably reach additional receptors on the basolateral membranes of taste cells. Further, all the actions of ACh on taste cells were characterized by muscarinic agents and RT-PCR, with the presumed targets being basolaterally located synaptic receptors. The actions of nicotine as a bitter alkaloid taste may involve apical nicotinic receptors, and nAChR subunits have been reported in lingual tissues containing taste buds (Oliveira-Maia et al. 2009). Although we found no evidence for nicotinic synaptic receptors, we cannot completely rule out their presence. ACh has one of the shortest half-lives of neurotransmitters due to the exceptional efficiency of acetylcholinesterase. Choline, however, has no such restriction. As the M3 receptor subtype is sensitive to both ACh and to a lesser degree to choline (Kurzen et al. 2007), we cannot exclude the possibility of endocrine control of the taste bud through the action of choline. However, the vesicular acetylcholine transporter (VAChT) has been observed in TRPM5- and PLCβ2-positive cells (Eguchi et al. 2008). This would suggest that ACh is released via exocytosis from taste Receptor cells, in agreement with our results.
Exocytosis of ACh would likely be triggered by Ca2+ release from internal stores, as Receptor cells are known to lack voltage gated calcium channels in the mouse (Clapp et al. 2006). We cannot, of course, rule out cholinergic innervation of the taste bud from extrinsic sources such as autonomic input. Any such innervation would be eliminated during isolation of taste buds and thus not open to testing in our experiments. In support of that possibility, Ogura (2002) showed nerve fibres innervating taste buds were immunopositive for the vesicular acetylcholine transporter VAChT.
Our findings may help explain some of the alterations in taste perception associated with drugs used for treating asthma (Drewnowski, 1989). Unpleasant taste is a well-established side effect of atropine derivatives (e.g. ipratropium bromide) taken as inhaled bronchodilators (Newnham, 2001). If topically applied ipratropium bromide penetrates the lingual epithelium and into taste buds (which remains to be determined), it would block the endogenous action of ACh, reducing the sensitivity of Receptor (Type II) cells, and may be the source of such taste disturbances. Perversion of taste due to cholinergic dysfunctions or caused by use of cholinergic drugs might merit further study of peripheral gustatory sensory involvement.
Acknowledgments
Many thanks to Dr J. Wess (NIDDK, Bethesda, MD, USA) for providing genetic mutant animals, Dr Y. J. Huang for maintaining biosensor cells, and to Dr G. Dvoryanchikov for assistance with RT-PCR. This research was supported by NIH grants 2R01DC007630 and 5R01DC000374 to S.D.R.
Glossary
- AChE
acetylcholine esterase
- CCh
carbachol
- ChAT
choline acetyltransferase
- DKO
double knockout
- GAD67
glutamate decarboxylase 67
- GPCR
G-protein coupled receptor
- M1/3/5
muscarinic receptor 1/3/5
- MT7
muscarinic toxin 7
- nAChR
nicotinic acetylcholine receptor
- PLCβ2
phospholipase C, β2
- TRPM5
transient receptor potential melastatin
- VAChT
vesicular acetylcholine transporter
Author contributions
R.D. and S.R. conceived and designed these experiments, R.D. collected, and with S.R. analysed and interpreted, results, and R.D. and S.R. drafted the article. Both authors approved the final version of the manuscript.
Supplementary material
Supplementary Figure S1
Supplementary Figure S2
References
- Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28:437–442. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci. 2000;20:7978–7985. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim KN, Roper SD. Individual mouse taste cells respond to multiple chemical stimuli. J Physiol. 2002;544:501–509. doi: 10.1113/jphysiol.2002.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proc Natl Acad Sci U S A. 2009;106:4006–4011. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2011;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in taste buds through ATP signaling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A. Taste responsiveness in eating disorders. Ann N Y Acad Sci. 1989;575:399–408. doi: 10.1111/j.1749-6632.1989.tb53260.x. discussion 408–399. [DOI] [PubMed] [Google Scholar]
- Drummond GB, Vowler SL. Show the data, don't conceal them. J Physiol. 2011;589:1861–1863. doi: 10.1113/jphysiol.2011.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci. 2011;31:5782–5791. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K, Ohtubo Y, Yoshii K. Functional expression of M3, a muscarinic acetylcholine receptor subtype, in taste bud cells of mouse fungiform papillae. Chem Senses. 2008;33:47–55. doi: 10.1093/chemse/bjm065. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Firestein S, Shepherd GM. Neurotransmitter antagonists block some odor responses in olfactory receptor neurons. Neuroreport. 1992;3:661–664. doi: 10.1097/00001756-199208000-00001. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008a;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008b;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One. 2011;6:e25 471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Yamaai T, Kitada Y. Parasympathetic postganglionic nerve fibers in the fungiform papillae of the bullfrog, Rana catesbeiana. Brain Res. 1992;596:299–304. doi: 10.1016/0006-8993(92)91561-r. [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCβ2 promoter in a functional class of taste receptor cells. Chem Senses. 2006;31:213–219. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schutz B, Langheinrich AC, Chubanov V, Gudermann T, Ibanez-Tallon I, Kummer W. Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol. 2012;137:483–497. doi: 10.1007/s00418-012-0911-x. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- Li YR, Matsunami H. Activation state of the M3 muscarinic acetylcholine receptor modulates mammalian odorant receptor signaling. Sci Signal. 2011;4:ra1. doi: 10.1126/scisignal.2001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh FC. The distribution of acetylcholine in the peripheral and the central nervous system. J Physiol. 1941;99:436–442. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham DM. Asthma medications and their potential adverse effects in the elderly: recommendations for prescribing. Drug Saf. 2001;24:1065–1080. doi: 10.2165/00002018-200124140-00005. [DOI] [PubMed] [Google Scholar]
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. doi: 10.1152/jn.2002.87.6.2643. [DOI] [PubMed] [Google Scholar]
- Ogura T, Lin W. Acetylcholine and acetylcholine receptors in taste receptor cells. Chem Senses. 2005;30(Suppl 1):i41. doi: 10.1093/chemse/bjh103. [DOI] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Tallini YN, Shui B, Kotlikoff MI, Lin W. Immuno-localization of vesicular acetylcholine transporter in mouse taste cells and adjacent nerve fibers: indication of acetylcholine release. Cell Tissue Res. 2007;330:17–28. doi: 10.1007/s00441-007-0470-y. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, Desimone JA, Nicolelis MA, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci U S A. 2009;106:1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547:475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogachevskaja OA, Romanov RA, Yatzenko YE, Kolesnikov SS. Muscarinic acetylcholine receptors operative in mouse taste cells. Biochemistry (Moscow) 2010;4:97–103. [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, Baggett HC. Identification of muscarinic acetylcholine receptors in isolated canine lingual epithelia via voltage clamp measurements. Arch Oral Biol. 1992;37:685–690. doi: 10.1016/0003-9969(92)90072-g. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kolli T, Hivley R, Jaber L, Zhao FI, Yan J, Herness S. Characterization of the expression pattern of adrenergic receptors in rat taste buds. Neuroscience. 2010;169:1421–1437. doi: 10.1016/j.neuroscience.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


