Abstract
The phase resetting response of the human circadian pacemaker to light depends on the timing of exposure and is described by a phase response curve (PRC). The current study aimed to construct a PRC for a 1 h exposure to bright white light (∼8000 lux) and to compare this PRC to a <3 lux dim background light PRC. These data were also compared to a previously completed 6.7 h bright white light PRC and a <15 lux dim background light PRC constructed under similar conditions. Participants were randomized for exposure to 1 h of either bright white light (n= 18) or <3 lux dim background light (n= 18) scheduled at 1 of 18 circadian phases. Participants completed constant routine (CR) procedures in dim light (<3 lux) before and after the light exposure to assess circadian phase. Phase shifts were calculated as the difference in timing of dim light melatonin onset (DLMO) during pre- and post-stimulus CRs. Exposure to 1 h of bright white light induced a Type 1 PRC with a fitted peak-to-trough amplitude of 2.20 h. No discernible PRC was observed in the <3 lux dim background light PRC. The fitted peak-to-trough amplitude of the 1 h bright light PRC was ∼40% of that for the 6.7 h PRC despite representing only 15% of the light exposure duration, consistent with previous studies showing a non-linear duration–response function for the effects of light on circadian resetting.
Key points
The human circadian pacemaker generates near-24-h rhythms that set the timing of many physiological, metabolic and behavioural body rhythms, and is synchronized to environmental time primarily by the 24 h light–dark cycle.
The magnitude and direction of the resetting response of the pacemaker to light depends on the time of day of exposure, and the change in responses over the day is summarized in a phase response curve (PRC).
A previous PRC showed that a 6.7 h bright white light exposure maximally shifted the circadian pacemaker by over 3 h.
We show that a PRC to a 1 h bright white light pulse maximally shifted the circadian pacemaker by ∼2 h, despite representing only ∼15% of the exposure duration.
This study demonstrates that the circadian pacemaker is sensitive to short-duration light pulses with a non-linear relationship between light duration and the amount of resetting.
Introduction
The human circadian pacemaker requires appropriately timed daily exposure to light–dark cycles to remain entrained to the 24 h solar day. In the absence of light input (Lockley et al. 1997) or when exposed to a light–dark cycle with stimulus strength that is too weak to support entrainment, the circadian pacemaker usually exhibits a near-24-h period (Middleton et al. 1996; Czeisler et al. 1999; Wright et al. 2001). While non-photic time cues can contribute weakly to entrainment in humans (Klerman et al. 1998; Danilenko et al. 2003), exposure to the light–dark cycle is the most powerful environmental synchronizing signal.
The phase-resetting response of the human circadian pacemaker to light depends on the circadian phase at which the light stimulus is given and can be summarized by a phase response curve (PRC). A PRC is a plot of the magnitude and direction of phase shift response versus the circadian phase of the resetting stimulus (Hastings & Sweeney, 1958). In humans, six PRCs have been constructed in response to white light, with varying illuminance levels and durations of exposure (Honma & Honma, 1988; Czeisler et al. 1989; Minors et al. 1991; Jewett et al. 1994; Van Cauter et al. 1994; Khalsa et al. 2003). While direct comparison of these PRCs is difficult due to differences in methodology, they are generally consistent and show that light exposure occurring in the early biological night induces a phase delay shift of the circadian pacemaker, whereas light exposure occurring in the late biological night induces a phase advance shift. All but one of the PRCs in humans is classified as weak Type 1 (Winfree, 1980): the phase transition curve (PTC) plotting final phase versus initial phase yields a slope close to 1, indicating weak phase resetting. Strong Type 0 resetting, in which the stimulus drives the pacemaker to the same final phase regardless of initial phase and results in a PTC with an average slope of 0, is possible in humans following three consecutive days of exposures to 7,000–12,000 lux for 5 h (Czeisler et al., 1989). When light is centred near the critical zone, or point of singularity, as indicated by the core body temperature minimum, suppression of the amplitude of the circadian oscillation occurs (Czeisler et al. 1989; Jewett et al. 1994).
Most of these previous studies employed substantial background light that was considered ‘dim’ and thought to be relatively inert at that time. Subsequent studies demonstrated that low levels of light do in fact suppress melatonin (Brainard et al. 1988; Jasser et al. 2006) and phase shift the human circadian pacemaker (Zeitzer et al. 2000; Burgess et al. 2003; Gooley et al. 2011), particularly (but not necessarily) if preceded by dimmer light or darkness (Hebert et al. 2002; Smith et al. 2004; Jasser et al. 2006; Chang et al. 2011). The phase-resetting response for stimuli resulting in delay shifts saturates at ∼1000 lux if preceded by exposure to ∼15 lux during the daytime (Zeitzer et al. 2000, 2005). By comparison, exposure to ∼100 lux of white light, which corresponds to ordinary room light, elicits a half-maximal phase shift response (Zeitzer et al. 2000) and suppresses melatonin by ∼70%, even when not preceded by dimmer light or darkness (Gooley et al. 2011). The phase-resetting response also has a non-linear response to duration of light exposure (Rimmer et al. 2000; Burgess et al. 2003; Gronfier et al. 2004). These results may explain, in part, why the PRCs constructed to date do not show substantial differences in their amplitude despite employing different lighting intensity and duration characteristics (e.g. ∼3–6 h, 2,500–12,000 lux); all the stimuli used previously may be close to saturation of the duration– and/or intensity–response function. To date, no studies have looked at the effect of a single continuous bright light pulse shorter than 3 h across all circadian phases.
The aim of the current study was to construct PRCs for a 1 h pulse of bright light (∼8,000 lux) versus dim light control (<3 lux) and compare them to our previously published PRC for a 6.7 h exposure (Khalsa et al. 2003) and the heretofore unpublished PRC for the ∼15 lux background light used in that protocol.
Methods
Ethical approval
The study was approved by the Human Research Committee at Brigham and Women's Hospital, in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to enrolling in the study and were paid for their participation.
Participants and pre-study conditions
We studied 39 young, healthy participants (18 female, 21 male; mean age ± SD = 21.9 ± 2.9 years; range 18–30 years) in the Intensive Physiology Monitoring (IPM) Unit at Brigham and Women's Hospital. All participants underwent a comprehensive physical and psychological exam prior to study. All were healthy and within normal limits for all tests, including an Ishihara colour blindness test. Before entering the study, participants were instructed to maintain a regular, self-selected sleep schedule (8 h time in bed) and report these times by calling a time- and date-stamped voicemail at bedtime and waketime for 3 weeks. Compliance was confirmed with actigraphy (Actiwatch-L, Minimitter, Inc., NY, USA) for at least 7 days prior to admission. Participants were instructed to refrain from use of any prescription, non-prescription and illicit drugs, including caffeine, nicotine and alcohol. Compliance was confirmed by urine and blood toxicology during screening and upon admission.
Inpatient conditions
Figure 1 shows the sleep–wake and light exposure schedule and ambient lighting conditions for four participants (2 light exposures and 2 controls) for 2 of 18 circadian phases studied. Participants were studied for 9–10 days in an environment free of time cues, without access to windows, clocks, watches, TV, radio, internet, telephones or newspapers, and were supervised by staff trained not to reveal information about the time of day. The timing of all events, including meal timing and posture changes were identical in the bright white light and dim background light control conditions; the only difference was the change in light levels to bright white light for 1 h on the intervention day.
Figure 1. Study schedule.
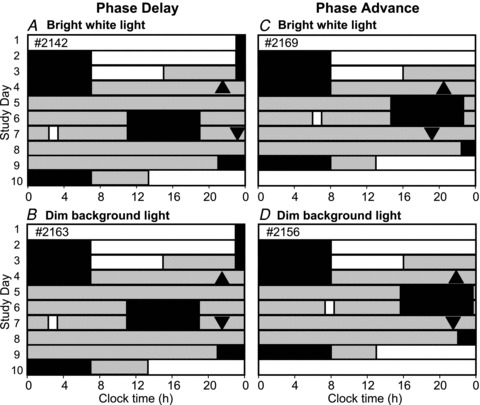
Sleep–wake and light exposure schedules for 2 of the 18 circadian phases used to construct the 1 h bright white light PRC and corresponding dim background light PRC. Study days are plotted on the ordinate and 24 h of clock time on the abscissa. The left panels show the 10-day schedule for the participants randomized to receive the light onset at ∼5 h after dim light melatonin onset (DLMO), a time expected to result in a phase delay, for the light (A) and control (B) conditions. The right panels show the 9-day schedule for the participants randomized to receive the light onset between 9 to 10 h after DLMO, a time expected to result in a phase advance, for the light (C) and control (D) conditions. Phase shifts were calculated from the difference in timing of DLMO between CR1 (▴) and CR2 (▾) over three circadian cycles. The schedules and DLMO times are expressed relative to each participant's habitual sleep–wake schedule. Black bars indicate scheduled sleep episodes. White bars indicate waking light levels of 90 lux and grey bars indicate light levels in <3 lux. Light exposures are indicated on study day 6 or 7 by a white box (∼8000 lux, A and C; control, B and D).
Circadian phase assessments
Phase assessments were made by determining the melatonin secretory profile during two constant routine (CR) procedures (Duffy & Dijk, 2002), before and after the experimental light exposure or control (Fig. 1). During the CR, participants were asked to remain awake while supervised in an environment free of time cues in constant dim light (<3 lux), while maintaining a constant semi-recumbent posture and fed hourly snacks, with nutritional intake (150 mEq Na+, 100 mEq K+ (±20%)) controlled on an isocaloric (Basal Energy Expenditure ×1.3) diet, and provided 2000 ml fluids per 24 h. The duration of the CRs was varied systematically (CR1, 29.50–52.17 h; CR2, 32.50–55.17 h) to ensure that all 18 light exposure and control phases occurred at the same time relative to the sleep–wake cycle, i.e. in the middle of a 16 h wake episode preceded and followed by an 8 h sleep episode in darkness (Fig. 1).
Melatonin assessment
From day 2, plasma was drawn every 30–60 min through an indwelling venous cannula and kept patent with a heparinised saline infusion (5 IU heparin ml−1 in 0.45% NaCl). Blood samples were transferred to EDTA tubes and kept in ice before centrifugation (1500g for 10 minutes), and stored at −20°C. Five participants (n= 2 light exposures and n= 3 control) did not have sufficient plasma samples during at least one of the CR procedures for phase analysis; in these participants, salivary melatonin was used to assess circadian phase. Melatonin was assayed using direct radioimmunoassay (Pharmasan Labs, Osceola, WI, USA). For plasma, the limit of detection was 0.7 pg ml−1, the intraassay coefficient of variation (CV) ranged from 5.7 to 12.1% and the interassay CV ranged from 8.4 to 13.2%. For saliva, the intraassay CV ranged from 2.7 to 8.1% and the interassay CV ranged from 12.6 to 18.7%.
Lighting conditions
Lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/HO/EW, 95 W; F32T8/ADV841/A, 32 W; F25T8/TL841, 25 W; Philips Lighting, The Netherlands) with digital ballasts (Lutron Electronics Co., Inc., PA, USA) transmitted through a UV-stable filter (Lexan 9030 polycarbonate with prismatic lens, GE Plastics, MA, USA). Ambient room lighting during scheduled wake episodes on baseline days was approximately 90 lux at the cornea (approximately 0.23 W m−2 (∼89 lux) at 137 cm from the floor in the vertical plane, and had a maximum of 0.48 W m−2 (∼150 lux) at 187 cm from the floor in the horizontal plane anywhere in the room) (Fig. 1, white bars). From midway through the third baseline day, ambient lighting was reduced to ∼0.5 lux (0.001 W m−2) at 137 cm from the floor in the vertical plane with a maximum <3 lux (0.01 W m−2) at 187 cm from the floor in the horizontal plane anywhere in the room (Fig. 1, grey bars). Participants were in darkness (<0.02 lux, <0.00006 W m−2) during scheduled sleep. During the 1 h bright light exposure, all ceiling lamps were turned on at full brightness. Participants wore Uvex glasses (Uvex Winter Optical, Smithfield, RI, USA) to ensure exclusion of ultraviolet light and were instructed to look at a fixed location on the upper wall for 6 min at a time, alternating with 6 min of free gaze episodes during which time participants could look elsewhere in the room (5 cycles of fixed–free episodes during the 1 h exposure). During both fixed and free gaze episodes, participants were instructed to avoid photophobic behaviour. A technician remained with the participant for the entire exposure to ensure compliance. During the <3 lux dim background light exposure, participants followed an identical supervised gaze behaviour protocol. Participants were seated 1.5 h prior to, and for 3 h following, the exposure to both light conditions to control for any postural influences on the melatonin profile.
Participants were randomized to the light condition (bright white light, <3 lux dim background light) and circadian phase of exposure (1–18) prior to CR1. Light exposure was timed relative to each participant's baseline wake time with the midpoint of the 18 pre-determined light exposure/control phases occurring every 80 min (∼20 degree intervals across the circadian cycle.). Due to inter-individual differences in phase angle of entrainment, the final distribution of light exposure/control phases varied somewhat from the scheduled 80 min intervals.
Illuminance and irradiance measurements were taken frequently using an IL1400 radiometer/powermeter with SEL-033/Y/W and SEL-033/F/W detectors, respectively. The mean ± SD illuminance was 8334 ± 1048 lux and 0.52 ± 0.10 lux for the bright white light exposure and <3 lux dim background light, respectively, as measured at the participants’ forehead in the direction of gaze. When measured at a height of ∼137 cm (54 in) at 0 degrees in the vertical plane, the light levels were 8020 ± 899 and 0.53 ± 0.05 lux, respectively.
Statistical analysis
Phase shifts (mean ± SD) were calculated as the difference between initial phase and final phase of the melatonin rhythm over three circadian cycles measured during the first and second CR, respectively (Fig. 2). Melatonin phase was defined as the dim light melatonin onset (DLMO) calculated from 25% of the fitted three-harmonic peak-to-trough amplitude of the melatonin rhythm during the first constant routine (Fig. 2). The bright white light and <3 lux dim background PRC and control data were each fitted with a two-harmonic function of the form
Figure 2. Individual plasma melatonin profiles before and after bright or dim background light exposure.
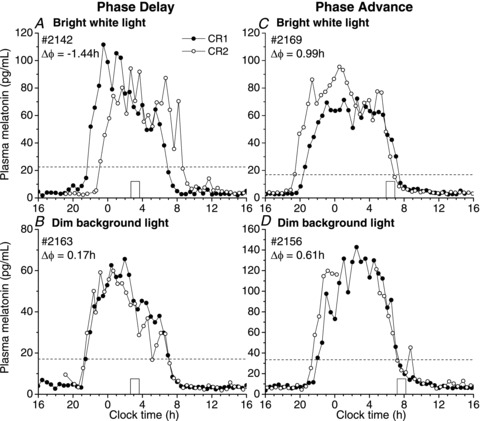
Individual plasma melatonin profiles are shown for four participants corresponding to Fig. 1 during constant routine (CR) 1 (•) and CR2 (◦) following exposure to a 1 h bright white light pulse (top panels) or the corresponding <3 lux dim background light condition (bottom panels). The timing of the bright white or <3 lux dim background light exposure on the day before CR2 is marked by an open box. In panel A, the 1 h bright white light exposure caused a −1.44 h phase delay shift (Δϕ) of the circadian pacemaker when timed to occur in the late subjective night compared to an advance of +0.17 h in the <3 lux dim background light condition (panel B). In panel C, bright white light exposure in the early subjective morning caused a +0.99 h phase advance compared to +0.61 h in the <3 lux dim background light (panel D). Dim light melatonin onset (DLMO) level is indicated for each participant by a dotted line.
| (1) |
where x is circadian phase and y is phase shift. Parameters μ, A, ϕA, B and ϕB represent the mean phase shift and the amplitude and phase of the first and second harmonics, respectively. These parameters and 95% confidence intervals were estimated using a Levenberg–Marquardt algorithm implemented in OriginPro 8.5 (Northampton, MA, USA). The adjusted R2 (Adj R2) value was computed as a goodness of fit measure of the two-harmonic function to data.
PRC for 6.7 h bright white light pulse and corresponding dim background light
We have previously published a PRC for a 6.7 h exposure to bright white light (between ∼5000 and ∼9000 lux in the angle of gaze) that used near-identical methods to those employed here (Khalsa et al. 2003). In addition to the duration of the experimental light exposure, the major differences in the protocol were the timing and intensity of the background light. Dim light started at the beginning of CR1 (as opposed to midway through baseline day 3 as described above) and was slightly higher, with a maximum of 15 lux (0.048 W m−2) at 187 cm from the floor in the horizontal plane anywhere in the room and typically ∼3.3 lux (0.009 W m−2) at 137 cm from the floor in the vertical plane. Participants’ ages ranged from 19 to 44 years (mean age ± SD = 27.2 ± 7.5 years).
In addition to the 6.7 h bright white light PRC data (Khalsa et al. 2003), a PRC for the <15 lux background light together with the displacement in the timing of the sleep–wake cycle was also derived using the same methodology. Twelve additional participants (2 female, 10 male; mean age ± SD = 24.7 ± 4.8 years; range 19–34 years) were studied and received continuous background light (<15 lux) during the light exposure day; the timing of all other events was identical to the bright white light condition. The midpoint of the <15 lux dim background light exposure was timed every 120 minutes (∼30 deg intervals across the circadian cycle). Plasma melatonin data from these participants were analysed as described above.
Statistical comparisons of the 1 h bright white light and <3 lux dim background light PRCs to the 6.7 h bright white light and <15 lux background light PRCs were based on the 95% confidence intervals of the two-harmonic fits of each PRC. Non-overlapping confidence intervals indicated significant differences between the PRCs.
Results
One hour bright white and <3 lux dim background light PRCs
Of the 39 participants who started the study, two (2176, 2178) were disempanelled prior to randomization of light exposure due to an inability to tolerate CR conditions. Two participants (21B6 and 2141, control condition) did not have sufficient plasma or saliva samples during at least one of the CR procedures and were excluded from further analysis. One participant (22L6) had very low plasma melatonin levels throughout the study (<4 pg ml−1) and was subsequently excluded from analysis. A total of 34 participants (15 female, 19 male; mean age ± SD = 21.8 ± 2.7 years; range 18–30 years) were therefore included in the 1 h bright white light PRC (n= 18) and <3 lux background light (n= 16) analysis.
Exposure to a 1 h bright white light pulse reset the circadian pacemaker according to a conventional Type 1 PRC, with maximal phasedelay and advance shifts of −2.02 h to 1.20 h, respectively (Fig. 3A). When data were fitted with a two-harmonic function (Adj R2= 0.55), we observed maximal phase delay and advance shifts of −1.75 h and 0.45 h, respectively (Fig. 3B). The phase-resetting response of the circadian pacemaker changed from phase advances to phase delays ∼3 h before DLMO and switched from delays to advances at ∼9 h after DLMO (phase at which the fit curve crosses 0, represented by a continuous horizontal line in Fig. 3). The crossing at 0 indicates no net phase shift at these circadian phases. This relationship, however, depends on the definition of what is considered ‘no net phase shift’. Without the influence of a light–dark cycle, prior experiments (Czeisler et al. 1999; Duffy et al. 2011) show that circadian phase will drift to an earlier or later clock time each day, depending on each individual's intrinsic circadian period. In this study, phase shifts were computed over three 24 h cycles in dim background light (<3 lux). When adjusted for intrinsic circadian period based on published averages (24.18 h, Czeisler et al. 1999), −0.54 h becomes the line of no net phase shift (dashed horizontal line, Fig. 3). According to 95% confidence intervals generated from the fitted two-harmonic function (Fig. 3C), phase delay shifts were significantly different from −0.54 h in a region spanning light exposure onset from ∼2 h before DLMO to ∼6 h after DLMO. Phase advances were significantly different from the −0.54 h no net phase shift line outside this region; there was no evidence of an extended ‘dead zone’, in which no phase shifts were generated.
Figure 3. Phase response curve to 1 h of bright white light or <3 lux dim background light.
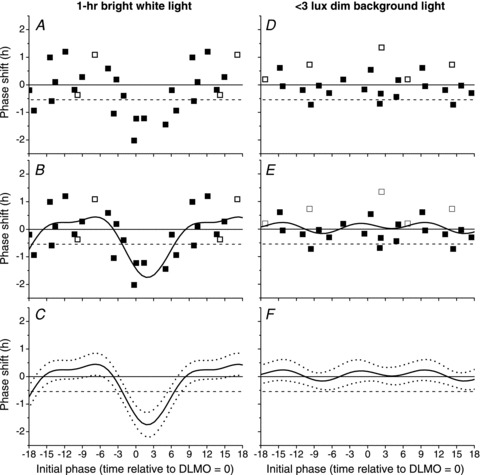
Exposure to a 1 h pulse of bright white light (left panels) or <3 lux dim background light (right panels). Raw phase shifts were computed using either plasma (▪) or salivary (□) melatonin (panels A, B, D and E) and fitted with a two-harmonic function (continuous line, panels B, C, E and F). Significant phase delays and advances were determined by comparing the overlap of 95% confidence intervals (dashed lines, panels C and F) to the zero phase shift line (continuous horizontal line, all panels) and the −0.54 h no net phase shift line based on drift due to intrinsic circadian period (dashed horizontal line, all panels). Initial phase was defined as the DLMO on CR1 relative to the onset of light exposure; negative values on the abscissa indicate onset of light exposure occurring prior to DLMO on CR1 and positive values indicate onset of light exposure occurring after DLMO on CR1.
Raw data from the <3 lux background light PRC are also plotted in Fig. 3D. Data were fitted with a two-harmonic function (Adj R2=−0.03, Fig. 3E), indicating that there was no discernable PRC in the <3 lux background light group. According to 95% confidence intervals (Fig. 3F), all phase shifts were significantly different from 0.
Comparison of 1 h and 6.7 h bright white light and corresponding dim background light PRCs
Figure 4 shows the 6.7 h bright white light PRC (left panels, previously published in Khalsa et al. 2003) and the <15 lux background light PRCs (right panels). Exposure to a 6.7 h pulse between ∼5000 and ∼9000 lux reset the circadian pacemaker according to a conventional Type 1 PRC with maximal phase delays and advances of −3.80 h and 2.09 h, respectively (Fig. 4A). When data were fitted with a two-harmonic function (Adj R2= 0.89), maximal phase delays and advances of −3.44 h and 2.02 h, respectively, were observed (Fig. 4B) and phase shifts were significantly different from −0.54 h across all circadian phases (Fig. 4C). The <15 lux background light PRC was generated by exposing subjects to <15 lux of ambient white light across the circadian cycle. Circadian phase shifts were significantly different from −0.54 h throughout most of the phase delay and advance regions (Fig. 4D and F), suggesting that even dim light elicits circadian phase-dependent resetting of the melatonin rhythm (Adj R2= 0.53). Maximal phase delays and advances (−1.81 h and 0.22 h, respectively) were significantly smaller in the <15 lux background light PRC, however, compared to the 6.7 h bright white light PRC.
Figure 4. Phase response curve to 6.7 h of bright white light or <15 lux dim background light.
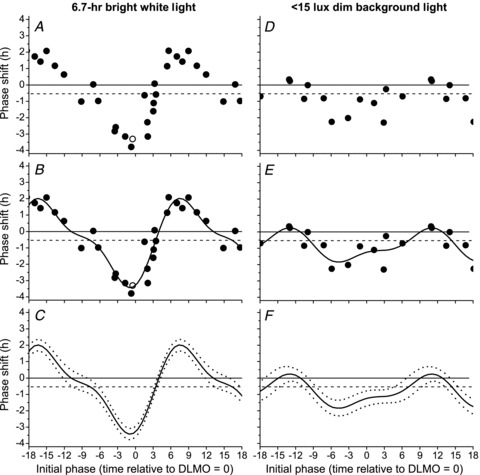
Exposure to a 6.7 h pulse of bright white light (left panels) or <15 lux dim background light (right panels). Raw phase shifts were computed using either plasma (•) or salivary (□) melatonin. All data were plotted using the same conventions as described in Fig. 3.
Figure 5A shows the raw data for both the 1 h and 6.7 h bright white light PRCs plotted on the same scale. Note that the abscissa for circadian phase has been adjusted from previous figures, which used circadian phase as DLMO relative to light onset, to circadian phase as DLMO relative to the midpoint of the light exposure to align the PRCs. The fitted peak-to-trough amplitude of the 1 h PRC in the maximal delay region was ∼50% of that of the 6.7 h PRC (−1.75 h versus−3.44 h, respectively) despite representing only ∼15% of the duration; the fitted peak-to-trough amplitude of the 1 h bright light PRC in the maximal advance region was ∼22% of that of the 6.7 h bright light PRC (0.45 h versus 2.02 h, respectively). When adjusted for intrinsic circadian period based on published averages (24.18 h, Czeisler et al. 1999), the two PRCs cross the −0.54 h line at approximately the same phase. According to 95% confidence intervals for both the 1 h and 6.7 h bright white light PRCs (Fig. 5B), the two PRCs were not statistically different (i.e. confidence intervals overlap) except for a small window around the maximal delay and advance region of the 6.7 h PRC. The <3 lux background light PRC was plotted with the <15 lux background light PRC (Fig. 5, right panels). There were significant differences between these two dim light PRCs at most circadian phases according to 95% confidence intervals (Fig. 5D) due to the significant phase delays and advances (relative to the −0.54 h no net phase shift line) observed in the <15 lux background light group.
Figure 5. Comparison of 1 h and 6.7 h bright white light and dim background light PRCs.
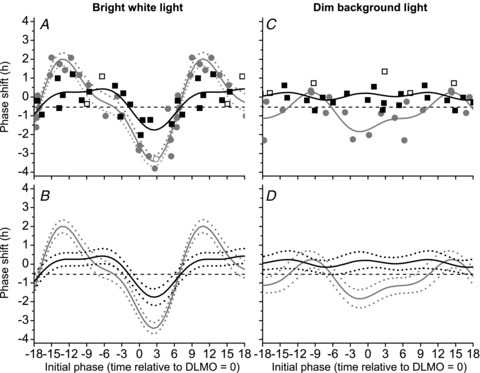
The raw data (symbols), two-harmonic fits (continuous lines) and 95% confidence intervals (dashed lines) for both the 1 h (black) and 6.7 h PRCs (grey) to bright white light (left panels) and dim background light (right panels) were plotted by circadian phase defined as the DLMO relative to the midpoint of light exposure, with negative values indicating midpoint of light exposure occurring before DLMO and positive values indicating midpoint of light exposure occurring after DLMO.
Discussion
A 1 h bright white light exposure induced Type 1 resetting of the circadian rhythm of melatonin with maximal phase delay and advance shifts of −2.02 and 1.20 h, respectively. A two-harmonic function fitted to the raw data resulted in a PRC with a fitted peak-to-trough amplitude of 2.20 h. In comparison, a previously published 6.7 h bright white light PRC (Khalsa et al. 2003) had a fitted peak-to-trough amplitude of 5.46 h. The 1 h exposure, which represents only 15% of the duration of the 6.7 h exposure, was therefore able to generate 40% of the response generated in the 6.7 h bright white light PRC, adding to evidence from previous results in human experiments (Rimmer et al. 2000; Gronfier et al. 2004) that suggest a non-linear duration–response function for circadian phase resetting in humans. When the fitted 1 h bright white light PRC was compared relative to the line of intrinsic circadian drift (−0.54 h over 3 days), we did not observe a dead zone in the PRC, consistent with the previous study with 6.7 h bright white light. The present study therefore suggests that the human circadian pacemaker is sensitive to light at all circadian phases, even for shorter durations of light exposure, and that there is no dead zone when circadian drift is taken into account.
This present study includes the first published reports of dim background light PRCs, in which participants were exposed to continuous background dim light using the same procedures that were used to construct PRCs in response to bright white light. The maximal phase delay and advance shifts imposed by the <15 lux background light PRC may have been due to a significant phase-resetting effect of the ∼15 lux background (Zeitzer et al. 2000) and/or to non-photic effects due to a shift in the timing of meals, activity and the sleep–wake cycle (Duffy et al. 1996; Klerman et al. 1998; Barger et al. 2004; Gronfier et al. 2004; St Hilaire et al. 2007). In the study by Duffy et al. (1996), exposure to a 5 h dark pulse (<0.03 lux) against a ∼15 lux background over multiple cycles caused a phase advance shift of 1.74 h over 10 cycles, and phase delay shift of 3.24 h, suggesting that alteration of the <15 lux light–dark cycle was sufficient to affect the circadian pacemaker.
Prior to this study, the shortest duration of light used to construct a PRC was a single 3 h exposure to 5000–9000 lux light (Minors et al. 1991), although not all delay phases were studied. Phase advances and delays of ∼2 h in the core body temperature (CBT) rhythm were observed in response to a 3 h exposure. In addition, one participant was exposed to 9000 lux for only 1 h centred 0.5 h before temperature minimum and exhibited a phase delay shift of ∼1 h. This is comparable to results from our present study in which we found a phase delay shift of 0.94 h for a 1 h light exposure onset occurring ∼7 h after DLMO, a time that corresponds approximately with core body temperature minimum.
The methodology used to construct the PRCs reported in this study involved constant routine procedures which required prolonged wakefulness. Each subject underwent CRs of different durations to ensure that the experimental light exposure was centred in the middle of the waking day. The variable CR lengths are necessary to achieve a symmetrical sequence of light–dark exposures before and after the experimental pulse, given the potentially large confounding effects of asymmetric light exposures (Czeisler et al. 1989). This methodology, however, introduces a significant amount of sleep deprivation, and the resulting sleep debt may not be completely dissipated by the 8 h sleep opportunity given immediately prior to the light exposure day. Burgess (2010) recently reported that partial sleep deprivation can reduce the magnitude of phase advances in response to bright light, which suggests that the circadian system may be even more responsive to a 1 h pulse of bright light administered in the phase advance region than we report here. It is not known whether partial sleep loss similarly reduces the magnitude of phase delays.
The results reported herein have important implications for the development of the most effective and energy-efficient methods for phase-shifting the human circadian pacemaker. Phase resetting by light can treat circadian misalignment as experienced after transmeridian travel, during shift-work schedules, in circadian rhythm disorders including advanced- and delayed-sleep phase disorders, and can be used to adapt to extreme photoperiods or exposure to altered spectral environments (e.g. extreme latitudes, space exploration). Exposure to shorter duration light exposures may reduce the undesirable side-effects associated with therapeutic use of light exposure such as glare, visual discomfort, ‘jitteriness’, headaches and nausea (e.g. Terman & Terman, 1999) and thus may lead to better compliance.
Acknowledgments
The authors thank Claude Gronfier, Ph.D. for discussions on the data analysis and interpretation; Kurt A. Smith, Ph.D. for assistance in conducting the studies; Melissa Hines, KC Malvey, Conor O’Brien and Ralph Todesco for recruitment and screening; Joseph M. Ronda and colleagues for technical, computer, and lighting support; Elizabeth B. Klerman, M.D., Ph.D. for medical supervision; the technical, dietary and laboratory staff, nurses and physicians at the Centre for Clinical Investigation in partnership with the Harvard Catalyst and Division of Sleep Medicine, Brigham and Women's Hospital; and Pharmasan Labs (Osceola, WI, USA) for melatonin assays. This work was supported by a grant from the National Institute of Mental Health (R01 MH45130) and was performed in a General Clinical Research Center supported by M01 RR02635. Collection of data by S.B.S.K. was partially supported by NHLBI F33 HL009588. S.W.L. was supported by a fellowship from The Wellcome Trust, UK (060018/B/99/Z), J.J.G. and M.A.St.H. were supported by a National Heart, Lung and Blood Institute fellowship in the program of training in Sleep, Circadian and Respiratory Neurobiology at Brigham and Women's Hospital (T32 HL079010). C.A.C. was supported in part by the Air Force Office of Scientific Research and S.W.L. and C.A.C. were supported in part by the National Space Biomedical Research Institute through the National Aeronautics and Space Administration (NCC 9-58). M.A.St.H., J.J.G., S.B.S.K. and R.E.K. have no conflicts of interest to disclose. S.W.L. and C.A.C. have no conflicts of interest directly related to the submitted work, but a fuller list of their activities can be found in Supplemental material.
Glossary
- CBT
core body temperature
- CR
constant routine
- CV
coefficient of variation
- DLMO
dim light melatonin onset
- PRC
phase response curve
- PTC
phase transition curve
Author contributions
Conception and design of the experiments (S.B.S.K., R.E.K., C.A.C. and S.W.L.), collection, analysis and interpretation of data (M.A.St.H., J.J.G., S.B.S.K., R.E.K., C.A.C. and S.W.L.), preparing the manuscript (M.A.St.H. and S.W.L.), revisions to the manuscript for important intellectual content (all authors). All authors approved the final version to be published.
Supplemental material
References
- Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1077–R1084. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D. Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Research. 1988;454:212–218. doi: 10.1016/0006-8993(88)90820-7. [DOI] [PubMed] [Google Scholar]
- Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms. 2010;25:460–468. doi: 10.1177/0748730410385544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? JBiol Rhythms. 2003;18:170–178. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk D, Wright KP, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: Influence of sleep timing, social contact and light exposure. J Physiol. 1996;495:289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van RE, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Sweeney BM. A persistent diurnal rhythm of luminescence in Gonyaulax polyedra. Biological Bulletin. 1958;115:440–458. [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: A further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, III, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol Regul Integr Comp Physiol. 1998;274:R991–R996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Klerman EB, Khalsa SBS, Wright KP, Jr, Czeisler CA, Kronauer RE. Addition of a non-photic component to a light-based mathematical model of the human circadian pacemaker. J Theoretical Biol. 2007;247:583–599. doi: 10.1016/j.jtbi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999;60:799–808. [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Winfree AT. The Geometry of Biological Time. New York: Springer-Verlag; 1980. [Google Scholar]
- Wright KP, Jr, Hughes R, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci U S A. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Khalsa SB, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, Czeisler CA. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol Regul Integr Comp Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


