Abstract
Motor unit activity in human eye muscles during the vestibulo-ocular reflex (VOR) is not well understood, since the associated head and eye movements normally preclude single unit recordings. Therefore we recorded single motor unit activity following bursts of skull vibration and sound, two vestibular otolith stimuli that elicit only small head and eye movements. Inferior oblique (IO) and inferior rectus (IR) muscle activity was measured in healthy humans with concentric needle electrodes. Vibration elicited highly synchronous, short-latency bursts of motor unit activity in the IO (latency: 10.5 ms) and IR (14.5 ms) muscles. The activation patterns of the two muscles were similar, but reciprocal, with delayed activation of the IR muscle. Sound produced short-latency excitation of the IO muscle (13.3 ms) in the eye contralateral to the stimulus. Simultaneous needle and surface recordings identified the IO as the muscle of origin of the vestibular evoked myogenic potential (oVEMP) thus validating the physiological basis of this recently developed clinical test of otolith function. Single extraocular motor unit recordings provide a window into neural activity in humans that can normally only be examined using animal models and help identify the pathways of the translational VOR from otoliths to individual eye muscles.
Key points
While the eye movements have been well characterized during the vestibulo-ocular reflex, the corresponding motor unit activity in human eye muscles is not well understood.
The present study describes the first single motor unit recordings during the vestibulo-ocular reflex in human eye muscles.
Simultaneous needle and surface recordings identified the inferior oblique as the eye muscle of origin of the ocular vestibular evoked myogenic potential (oVEMP), thus validating the physiological basis of this clinical test of otolith function.
The results demonstrate short-latency vestibulo-ocular projections from the otoliths to individual eye muscles.
Single motor unit activity of eye muscles provides a window into neural activity of the ocular motor nuclei in humans.
Introduction
Little is known about the motor unit activity of individual extraocular muscles during vestibular-evoked eye movements. One reason for this is that the large eye and head movements normally associated with vestibular stimulation preclude holding single motor units for electromyographic (EMG) needle recording. As the activity of ocular motor neurons during the vestibulo-ocular reflex (VOR) often closely reflects that of extraocular motor units, neural activity is typically recorded directly from the brainstem in animals. Only a few studies have recorded extraocular muscle activity following vestibular stimulation in animals (Lorente de Nó, 1934; Szentágothai, 1950; Cohen & Suzuki, 1963). In humans, Magora et al. (1973) recorded vestibular nystagmus using intramuscular electrodes during caloric stimulation. They confirmed the principle of reciprocal innervation of agonist and antagonist eye muscles during both the slow and quick phases of horizontal nystagmus. These studies reported global EMG activity, but did not record from single motor units. In contrast, direct recordings from extraocular motor units provide a window into VOR activation in humans that can otherwise only be studied in animal models.
To allow safe single motor unit recordings in extraocular muscles during the VOR in humans, we used unique forms of vestibular stimulation which leave the head still and evoke only very small eye movements: skull vibration and loud sound (Todd et al. 2007; Welgampola et al. 2009). Sound and vibration have long been known to activate vestibular afferents, and evidence from recent animal studies suggests that in the normal ear they preferentially activate the receptors of irregularly firing otolith afferents (Murofushi & Curthoys, 1997; Curthoys et al. 2006). This is likely to be due to the common evolutionary origin of the otolith and hearing organs in vertebrates, preserving the sensitivity to vibration and sound in mammalian otoliths (Carey & Amin, 2006). Eye movements evoked by these stimuli are therefore a type of otolith-mediated, translational VOR.
Although the eye movements evoked by vibration and sound are very small, the extraocular muscle activity associated with them is robust and can be recorded from surface electrodes placed near the eyes. This muscle activity is independent of the electrical activity generated by the corneoretinal dipole (i.e. the electro-oculogram, EOG; Todd et al. 2007; Welgampola et al. 2009) and the response is called an ocular vestibular evoked myogenic potential (oVEMP) due to its similarity with the cervical VEMP recorded from the neck muscles (Rosengren et al. 2010). The cVEMP is a short-latency, inhibitory potential typically measured from the sternocleidomastoid muscle in response to stimulation of the ipsilateral ear with air-conducted sound (Colebatch et al. 1994; Colebatch & Rothwell, 2004). The oVEMP is also a short-latency reflex, but has a ‘crossed’ projection, i.e. is primarily recorded in the eye contralateral to the stimulated ear (Iwasaki et al. 2007). Recent clinical studies in patients with selective lesions of the superior or inferior divisions of the vestibular nerve have suggested that oVEMPs are mediated by afferents coursing in the superior nerve division (e.g. Iwasaki et al. 2009; Shin et al. 2012), which includes afferents from the utricle and anterior hook of the saccule. oVEMPs are largest when measured from surface electrodes placed beneath the eyes during up-gaze, suggesting that the inferior extraocular muscles are important contributors to the reflex. Under these conditions, the oVEMP consists of a series of peaks of electrical activity, typically beginning with a negative peak at ∼10 ms (n10) (Todd et al. 2007; Chihara et al. 2009; Welgampola et al. 2009). As this n10 peak is abolished in the eye contralateral to a vestibular lesion, the oVEMP can be used to test otolith function in patients with vestibular dysfunction (Iwasaki et al. 2007).
The oVEMP provides a rare opportunity to investigate eye muscle activity during the translational VOR in humans. As evidence from oVEMP studies suggests involvement of the inferior extraocular muscles, we targeted the inferior oblique (IO) and inferior rectus (IR) muscles and recorded single motor unit activity using concentric needle electrodes. The technique was based on a method used to determine the basis of the cVEMP recorded from the cervical muscles (Colebatch & Rothwell, 2004). Our main interest was in the initial latency and the time course of muscle activity. We determined the contribution of these muscles to the clinical oVEMP and used the oVEMP as a model to study the VOR in response to impulsive otolith stimulation at the level of individual extraocular muscles.
Methods
Subjects
Three normal volunteers were studied over 11 sessions and each subject was tested at least 3 times (1 female, 2 males; age range 34–48 years; 1 left and 10 right eye recordings). The subjects had no history of conductive hearing loss or vestibular, neurological or ophthalmologic disease (excepting a refractive error). The participants (all co-investigators) gave written informed consent according to the Declaration of Helsinki and the study was approved by the local ethics committee (Kantonale Ethik-Kommission Zurich, 2010–0177/3). We tested only co-investigators due to the high level of cooperation required. This is not likely to have biased the results as the very short reflex latency precludes voluntary control. The subject in the video (online Supplemental Material) gave her consent for publication.
Vestibular stimulation
The vestibular stimuli were unshaped 500 Hz bursts of vibration and sound. These stimuli were chosen as they are used in both clinical end experimental contexts to elicit oVEMPs and assess otolith function. Vibration produces robust oVEMPs with large amplitude and is a common oVEMP stimulus. In contrast, sound stimulation is a more traditional cVEMP stimulus, but evokes smaller oVEMPs. Both stimuli were included as they are likely to test varying, though probably overlapping, populations of otolith afferents with different directional sensitivity. Sound stimulation also activates a single ear, allowing investigation of the laterality of the response.
Vibration was delivered with a hand-held ‘minishaker’ positioned over the hairline near Fz (500 Hz, 4 ms, minishaker model 4810; amplifier model 2706, Brüel & Kjaer P/L, Naerum, Denmark). Measurement of the head acceleration evoked by this stimulus showed that the initial acceleration peak was largest and most consistent in the interaural (y) axis (a ‘bowing out’ of the mastoids of 0.1 g), with smaller peaks in inconsistent directions in the x (0.06 g) and z (0.03 g) axes (n= 10 subjects, accelerometer ADXL330, Analog Devices, Norwood, MA, USA).
The sound stimuli were delivered with calibrated headphones and custom amplifier at 142 dB peak SPL (500 Hz, 4 ms, TDH 39, Telephonics Corp., Farmingdale, NY, USA). Both stimuli were generated with customized software using a laboratory interface (micro1401, Cambridge Electronic Design (CED), Cambridge, UK) and delivered at a rate of 7.5 Hz up to a maximum of 2000 repetitions per trial (typically 1000 per trial).
Single motor unit recordings
Subjects reclined to approximately 20 deg above the horizontal. We used ultra thin (0.3 mm diameter, 30G) disposable concentric needles (Neuroline, Ambu A/S, Ballerup, Denmark) to record single motor units. For IO recordings, topical anaesthetic was applied to the skin beneath the eye (lidocaine gel 2%, Cantonal Pharmacy, Zurich, Switzerland). The needle was inserted through the skin at the medial aspect of the inferior orbital margin into the muscle belly and held in position during the recording period (Breinin, 1962). For IR recordings, topical anaesthetic was applied to the eye (oxybuprocaine drops 0.4%, Théa Pharma S.A., Schaffhausen, Switzerland) and a lid retractor was inserted. The eye was rotated manually using forceps and the needle inserted into the IR under visual guidance. We attempted to record data in all three subjects from both the IO and IR muscles using vibration and sound stimulation. This was achieved for the IO muscle (from all three subjects and both stimulation types). However, measurement from the IR muscle was discontinued due to a minor scleral perforation in one subject, and thus IR data were available only in two subjects with vibration stimulation. In all cases, audio feedback was provided to aid placement of the electrode, to help subjects maintain constant activation of one or more motor units and to confirm that a motor unit originated from the intended muscle. The needle insertion was always performed by the same ophthalmologist (K.L.). In contrast to standard surface oVEMPs recorded in maximal up-gaze, gaze angle varied in needle recordings and was adjusted to allow only a small, distinguishable number of units to be recorded at once; however, it was typically near neutral position. EMG was recorded with the same micro1401 data acquisition interface and custom software as described above. Data were sampled at 50 kHz for 100 ms (from 40 ms before to 60 ms following stimulus onset), amplified and bandpass filtered (5 Hz to 10 kHz).
Surface oVEMP recordings
Surface potentials were recorded simultaneously using an active electrode placed just below the infra-orbital margin and a reference directly below it on the cheek, with the earth placed nearby (Blue sensor N, Ambu A/S, Ballerup, Denmark). For comparison, standard surface oVEMPs were recorded during maximal up-gaze with the active electrode placed closer to the lower lid. Negative potentials at the active electrodes were displayed as upward deflections. Latencies were measured at the response peak.
Data analysis
Motor unit spikes were identified using a threshold level and clustered with custom software (MatLab, The MathWorks Inc., Natick, MA, USA) based on an automatic algorithm using wavelets and super-paramagnetic clustering (Quiroga et al. 2004). The recordings typically included multiple units, from which single units were extracted (Fig. 1A–C). Peri-stimulus time histograms consisting of 100 bins of 1 ms width (centred at whole numbers) were constructed for each unit (Fig. 1E and F). The cluster software was set to maximise specificity rather than sensitivity in order to ensure that single unit clusters were not contaminated with extraneous spikes. The accuracy of the software was confirmed for each single unit by visual comparison of the selected spikes with the raw data. For recordings in which spikes from a single unit could not be reliably differentiated, multiple unit histograms were constructed to show the behaviour of several units recorded simultaneously. The number of spikes in each histogram ranged from 305 to 2734 for single unit histograms and 580 to 21,345 for multiple unit histograms. Local maxima and minima in the histogram were detected based on the known properties of the surface oVEMP (Iwasaki et al. 2008). The tallest bin within the first 15 ms after stimulus onset but after the stimulus artefact (5 ms) was selected as the first peak. The peak was accepted if the change in spike count exceeded the mean baseline spike count measured over the 40 ms pre-stimulus period by 2 standard deviations (SD). Using this method, the rate of false positives detected during the pre-stimulus period was under 5%. Subsequent local minima and maxima at expected intervals (∼5 ms) were accepted without applying the SD criterion. Latencies were measured at the local minima and maxima and adjusted to correct for a 0.5 ms delay in the recording system.
Figure 1. Single motor unit recording of the inferior oblique muscle in response to bone-conducted vibration.
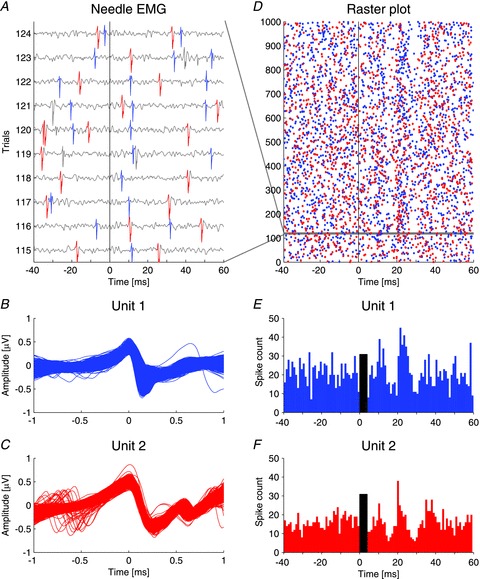
A, needle EMG recording of 10 consecutive trials out of 1000 stimuli with 4 ms bursts of 500 Hz vibration, showing two distinct motor units (blue and red). The black vertical line indicates stimulus onset. B and C, the two distinct single motor units (blue: 2107 spikes, red: 1597 spikes) were aligned to the peaks and identified with a sorting algorithm using wavelets and super-paramagnetic clustering (Quiroga et al. 2004). D, raster plot of the two single motor units over all 1000 stimuli. Spikes from the two units are randomly distributed over the pre-stimulus interval (−40 to 0 ms). After the stimulus (0 to 60 ms) two bands of increased activity emerge at about 11 and 21–22 ms, with bands of decreased activity in between. The horizontal grey band represents the 10 consecutive trials illustrated in A. E and F, peri-stimulus histograms of the firing pattern of the two units derived from the data in the raster plot in D illustrating increased discharge at 11 and 22 ms for the blue unit and 11 and 21 ms for the red unit with periods of decreased discharge in between. The black bars indicate the duration of the stimulus.
Statistical analysis
The values reported are means ± standard deviation. Statistical analysis was performed on data from multiple unit histograms only, due to the small number of single unit histograms. Non-parametric statistics were used (i.e. the Mann–Whitney U and Kruskal–Wallis tests) and the significance level was set at P= 0.05.
Results
The peri-stimulus time histograms showed that the initial motor unit response to brief bursts of vibration and sound was an increase in discharge, for each extraocular muscle tested. The initial increase was followed by a decrease in discharge and then additional increases and decreases, such that the entire response consisted of a series of peaks and troughs of activity with diminishing amplitude over time. The characteristics of the response recorded in the different extraocular muscles (IO and IR) and with the different types of vestibular stimulation (vibration and sound) are described in detail below.
Inferior oblique muscle
Vibration
Following stimulation of the forehead with 4 ms pulses of vibration, we recorded an initial increase in single motor unit discharge in the IO muscle at a mean latency of 10.5 ± 1.1 ms (range 9.5–12.5 ms, n= 6 single units). The discharge at this latency was up to ∼10 times the baseline level seen during the 40 ms pre-stimulus period (mean 3.9 ± 3.2, range 1.8–9.9 times baseline). The duration was very short: for four of six single units duration was only 1 ms (Fig. 2A). The initial peak was followed by a decrease in activity at 17.0 ± 1.6 ms, a second peak in discharge at 22.0 ± 1.4 ms and a subsequent decrease at 27.5 ± 3.0 ms, and the mean interval between the first two peaks was 11.1 ± 2.3 ms. The single units sometimes showed a complete absence of firing after the initial peak (Fig. 2A). We found similar modulation in multiple unit histograms (Table 1). Figure 2B shows an example of a typical multiple unit histogram, which had broad peaks and at least three cycles of increasing and decreasing activity. The addition of several units with slightly different temporal characteristics during the same recording gave the multiple unit histograms a smoother and broader form than the single unit ones. This effect was further enhanced when all units from the same experimental condition recorded in a single subject over several sessions were combined (Fig. 2C).
Figure 2. Peri-stimulus histograms of single, multiple and summated motor units in response to vibration and sound.
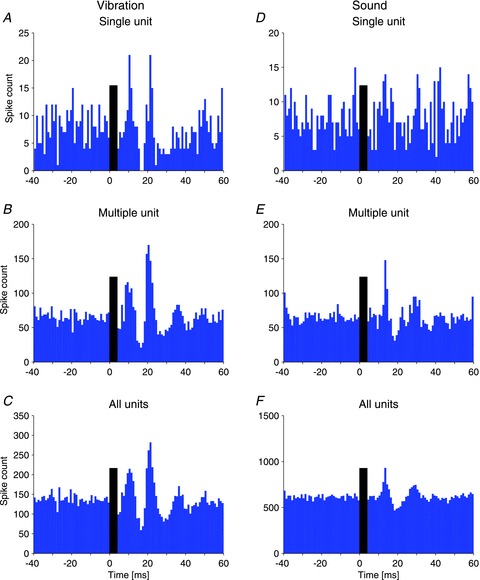
A, the single unit histogram shows the response of one distinct unit to 4 ms bursts of 500 Hz vibration. A sharp peak in discharge is seen at 11 and 22 ms with complete suppression in between. B, the multiple unit histogram shows the behaviour of several indistinguishable units recorded simultaneously. The same pattern of increased and decreased discharge as above is seen, but with broader peaks. C, summation of the spikes from all units recorded in the same subject confirms the pattern. D, the single unit recording in response to 4 ms tone bursts of 500 Hz sound in the contralateral ear shows a similar pattern of increased and decreased discharge with slightly longer latencies. E, the pattern is replicated in multiple unit histograms. F, the summated histogram of spikes from all recorded units. The black bars indicate the duration of the stimulus.
Table 1.
Latencies and amplitudes of the first three peaks of extraocular motor unit activity from multiple unit histograms
| Excitation 1 | Inhibition 1 | Excitation 2 | |||||
|---|---|---|---|---|---|---|---|
| Latencya | Amplitudeb | Latency | Amplitude | Latency | Amplitude | ||
| Inferior oblique muscle | |||||||
| Vibration | Mean | 10.2 | 2.8 | 15.5 | 0.5 | 21.3 | 1.5 |
| SD | 1.4 | 1.3 | 2.3 | 0.2 | 1.9 | 0.4 | |
| n | 13 | 13 | 13 | 13 | 13 | 13 | |
| Sound | Mean | 13.1 | 1.5 | 19.2 | 0.7 | 27.4 | 1.3 |
| SD | 0.5 | 0.4 | 1.8 | 0.1 | 2.4 | 0.1 | |
| n | 11 | 11 | 11 | 11 | 11 | 11 | |
| Inferior rectus muscle | |||||||
| Vibration | Mean | 14.5 | 3.5 | 20.5 | 0.4 | 27.8 | 1.4 |
| SD | 0.0 | 1.3 | 3.2 | 0.3 | 1.0 | 0.2 | |
| n | 4 | 4 | 4 | 4 | 4 | 4 | |
Latency units are milliseconds.
Amplitude units are a ratio of the spike count at the response peak compared to the mean spike count over the baseline period.
Sound
The responses evoked by sound versus vibration are compared in Fig. 2. Following stimulation with 4 ms bursts of air-conducted sound, there was an initial increase in discharge of the IO muscle contralateral to the stimulus at a mean latency of 13.3 ± 1.3 ms (range 11.5–14.5 ms, amplitude 2.0 ± 0.2 times baseline, duration 1 ms, n= 4 single units), with subsequent troughs and peaks of activity at latencies of 17.3, 29.3 and 34.3 ms (Fig. 2). The effect was similar in the multiple unit histograms (Table 1). The initial peak evoked by sound occurred significantly later than that evoked by vibration (13.1 vs. 10.2 ms, P < 0.001) and the interval between peaks of increased discharge was longer for sound stimulation (14.3 vs. 11.1 ms, P= 0.003), possibly due to the sound stimuli being less intense relative to vibration.
In contrast to the contralateral ear, stimulation of the ear ipsilateral to the IO muscle produced no consistent effect across the three subjects. For two of the subjects, insufficient vestibular activation by sound may have contributed to this lack of effect. The stimulus intensity was at the upper limit of safe acoustic stimulation levels, but was still close to the threshold to sound in the ipsilateral ears of these subjects. However, in one subject no response was seen even with stimulation at 10 dB above the oVEMP threshold for that ear.
Inferior rectus muscle
In recordings from the IR muscle using vibration (Fig. 3), the initial increase in discharge occurred at 14.5 ± 0 ms (3.5 ± 1.3 times baseline level, n= 4 multiple unit recordings in two subjects), significantly delayed compared to that recorded from the IO muscle following identical vestibular stimulation (14.5 vs. 10.2 ms, P= 0.002). The subsequent peaks showed a similar delay, such that the entire IR response was offset from the IO response by approximately 4–5 ms (Table 1). This delay by half a cycle caused the IR to be active while the IO was not (and vice versa). Therefore the activity of the IO and IR muscles was reciprocal.
Figure 3. Comparison of inferior rectus and inferior oblique eye muscle response to vibration.
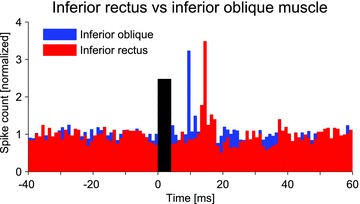
Multiple unit histograms from the two muscles in the same subject show similar discharge patterns in response to the identical stimulus (black bar), but with a delay of half a cycle (∼5 ms) in the IR muscle. The spike count was normalized for comparison of the two units.
Relationship to surface recordings
The surface response is compared to the motor unit response in Fig. 4. An initial negativity was recorded in the simultaneous surface oVEMPs in the majority of instances, i.e. the n10 potential of the oVEMP. The surface recordings tended to resemble one another and had similar n10 latencies in all conditions, probably due to the fact that the surface response represents a combination of activity from both the IO and IR muscles during neutral gaze (n10 from IO vibration histograms 11.8 ± 1.9 ms, IO sound 11.5 ± 0.7 ms, IR vibration 12.0 ± 2.0 ms, P= 0.393). The pattern of repeated increases in discharge at intervals of ∼10 ms was also reflected in these surface recordings.
Figure 4. Relationship of needle EMG recordings to surface oVEMP recordings.
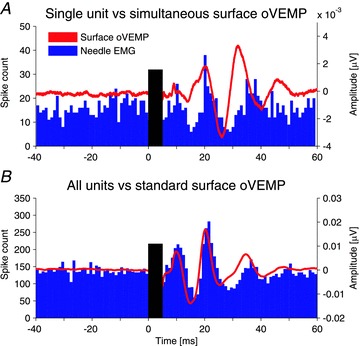
A, single unit histogram in response to vibration (blue bars) overlaid with simultaneous surface oVEMP recording (red trace). Both data sets show peaks of increased activity at about 10–11 and 21 ms with periods of suppression in between. B, the sum of spikes from all units recorded in the same subject (same histogram as in Fig. 2C) matches closely the surface oVEMP recorded under standard conditions in up-gaze.
The simultaneously recorded surface responses were small (Fig. 4A) and there was a small positive peak preceding the n10 in a small minority (4 of 28) of multiple unit recordings. In contrast, large, well-formed oVEMPs were recorded in all subjects under the conditions typically used for this test (during up-gaze and with electrodes close to the eyes) (Fig. 4B). This difference was due to the compromise necessary to record from needle and surface electrodes simultaneously: namely restricted vertical gaze to reduce the number of active motor units, and surface electrodes placed further away from the eye to allow space for needle insertion. Despite this, the simultaneous surface recordings resembled the single unit peri-stimulus histograms (Fig. 4A). This relationship was, however, much clearer on comparison of the oVEMP recorded under optimal conditions with the sum of all motor units of the respective subject (Fig. 4B), though the surface response tended to precede the motor unit response.
Discussion
We successfully recorded single unit extraocular muscle activity in response to vestibular stimulation in alert human subjects. Stimulation of the vestibular otolith organs with 4 ms bursts of vibration produced a series of excitability changes in the IO and IR muscles. There was a highly reproducible initial increase in activity of the IO at 10.5 ms and of the IR at 14.5 ms. The latency of this initial peak was consistent within each stimulus condition, and the duration was very short, often just 1 ms, confirming that the extraocular muscles exercise great precision in the control of vestibular-evoked eye movements.
Evidence of origin of the ocular vestibular evoked myogenic potential (oVEMP)
Our results provide strong evidence that the surface potential recorded in the clinical oVEMP test is indeed produced by an excitation of the IO muscle. Although the nature of our data does not allow us to distinguish between excitation and disinhibition, inhibition and disfacilitation, for simplicity and consistency with other vestibular studies, we refer to the peaks of activity as excitation and the troughs as inhibition. Our findings represent the missing link in the chain of evidence for this test of otolith function. The striking burst of IO motor unit firing at 10.5 ms following the vibration stimulus was reflected in the simultaneous surface recordings that showed an initial negative peak, similar to the n10 peak typically recorded in the clinical oVEMP. In addition, oVEMPs recorded under optimal conditions in up-gaze closely matched the summated peri-stimulus histograms from the IO (Fig. 4). Previous studies of surface oVEMPs in several stimulation and gaze conditions had suggested that the IO played an important role (Rosengren et al. 2005; Todd et al. 2008a; Welgampola et al. 2009), but definitive evidence of the reflex polarity and muscle of origin could only be provided by direct recordings from within the muscle. We also recorded excitation in the IR muscle, but this consistently occurred some 4–5 ms later, indicating that the IR muscle does not contribute to the initial, clinically relevant n10 oVEMP peak. Although we did not record from the horizontal rectus muscles, we expect that they would have also been activated by our otolith stimulus, due to the involvement of the otoliths (in particular the utricle) in responding to laterally directed translational acceleration (Fernandez & Goldberg, 1976). Animal studies have shown strong projections from the utricle to these muscles (Suzuki et al. 1969; Uchino et al. 1996). Experimental oVEMP studies have shown that distinct responses can be recorded from surface electrodes placed over the lateral rectus muscle when the muscle is activated by abduction (Todd et al. 2008a; Govender et al. 2011). However, although we cannot rule out a contribution from these muscles, we propose that they probably do not contribute significantly to the clinical oVEMP recorded from beneath the eye as they are not activated with vertical gaze and their bellies lie further away from the electrodes.
Our data provide new evidence for a short-latency, crossed projection to the IO muscle following otolith stimulation in humans. Most of our recordings were made with vibration, as it is a more powerful vestibular stimulus; however, it is a bilateral stimulus and cannot be used to stimulate one ear in isolation. The recordings with sound were therefore important to examine the laterality of the projections, although sound and vibration may not activate identical populations of otolith afferents. We found that the motor unit response to sound stimulation was similar to that following vibration, but was present only when the ear contralateral to the muscle was stimulated. This confirms that the otolith projection underlying the IO muscle response is crossed, and supports the data from surface recordings, which showed a contralateral oVEMP projection (Chihara et al. 2007; Iwasaki et al. 2007). The small surface responses seen with ipsilateral sound stimulation may therefore have an alternative source, possibly the IR muscle. It is currently not clear which otolith afferents are responsible for this crossed projection to the IO muscle. Recent evidence from human studies has suggested that oVEMPs are mediated by fibres coursing in the superior nerve division of the vestibular nerve (e.g. Iwasaki et al. 2009; Shin et al. 2012), which contains all utricular and some saccular afferents. Saccular projections to the eyes are not well understood and may be weaker than utriculo-ocular projections (Isu et al. 2000). Our data are consistent with those of Suzuki et al. (1969), who demonstrated a contralateral excitatory projection from the utricle to the IO muscle in cats; however, contradictory data from Uchino et al. (1996) suggest that this projection is predominantly ipsilateral. Our results are consistent with studies of translational head movements in humans, which have recorded similar VOR latencies around 12–15 ms (Bronstein & Gresty, 1988; Crane et al. 2003), but human studies have also provided conflicting data of otolith-ocular projections (Diamond & Markham, 1983; Lempert et al. 1998). Further studies will be needed to elucidate the detailed projections from different vestibular organs to individual eye muscles.
The latency of the surface response recorded during up-gaze appeared to slightly precede the IO motor unit response, increasingly so for subsequent peaks. A similar ‘acceleration’ can also be seen in the later waves of surface responses recorded with different vertical gaze angles (Govender et al. 2009). The mechanism underlying this effect is likely to involve the normal pattern of muscle recruitment, whereby an increase in muscle tension leads to recruitment of progressively larger muscle fibres with higher conduction velocity (Henneman et al. 1965; Grantyn et al. 1977; Toft et al. 1989).
Implications for the vestibulo-ocular reflex
Our motor unit recordings demonstrate how individual eye muscles respond to an impulsive vestibular stimulus. The method is analogous to animal studies that record from neurons in the ocular motor nuclei, providing a window into the same neural circuit in humans. In addition, it allows differentiation between the innervation patterns of individual eye muscles, although we could not distinguish different types of muscle fibres (i.e. twitch and non-twitch fibres) (Wasicky et al. 2004). We found reciprocal activity in the IO and IR muscles, which are both controlled by the oculomotor nucleus and nerve (Fig. 3), consistent with Sherrington's law of reciprocal innervation of antagonist muscles (Sherrington, 1906). Although the IO and IR muscles are not classic antagonists, they produce opposing vertical movements and can therefore be expected to show reciprocal activation during vertical eye movements. Based on this law, one might have predicted an initial inhibition of the IR, coinciding with the excitation of the IO, but we recorded only a delayed excitation of the IR, coinciding with the first inhibition of the IO. As single motor unit responses recorded from the neck muscles under similar experimental conditions begin with a clear inhibition of muscle activity (Colebatch & Rothwell, 2004), our technique would have allowed us to see an early inhibition if it were present. The basis of this 4–5 ms reflex delay in the IR compared to the IO muscle is not known, but it illustrates the complexity of the otolith-ocular reflex. The delay is greater than expected for a single additional synapse, suggesting that there is refined processing within the translational VOR pathway to produce the appropriate combination of muscle activity. In fact, these latencies are probably not absolute and may change with different initial directions of skull vibration and consequent activation of different populations of otolith afferents, even when the afferents originate from the same otolith organ. As extraocular muscle activity and eye movements are intrinsically linked, any change in evoked eye movement will result in a different pattern and timing of eye muscle activity. Latency shifts have been reported in oVEMP surface recordings following a simple reversal in the initial direction of skull vibration, with stimulus frequencies up to 500 Hz (Todd et al. 2008a; Cai et al. 2011; Jombik et al. 2011). This suggests that the translational VOR is capable of encoding the net direction of such stimulation and produces meaningful responses in the appropriate extraocular muscles, even though vibration and sound are short-duration, high frequency stimuli.
The extraocular motor unit response to impulsive otolith stimulation consisted of multiple peaks of activity. In both the IO and IR muscles, alternating peaks and troughs of activity followed the initial excitation, at intervals of ∼5 ms (i.e. a ∼10 ms cycle). The resonance in the IR recordings was identical to that in the IO, but was offset by half a cycle (∼5 ms), suggesting that the frequency is a general feature of the ocular motor system following this type of stimulation. The same resonance of ∼100 Hz has been noted in surface oVEMP recordings and is independent of the frequency of the stimulus (Todd et al. 2008b, 2009). The origin of this effect is unclear. Todd et al. (2009) suggested it was due to a positive feedback loop involving afferents from the extraocular muscles. We wondered whether these secondary peaks and troughs just reflected simple synchronisation of the motor units (Türker & Powers, 2005). Yet this would presuppose a regular baseline firing rate of 100 Hz, higher than the firing rate of most recorded units (e.g. Fig. 1). Moreover, surface recordings suggest that the resonance persists at this frequency with different angles of gaze and is independent of the background motor unit firing rate (Govender et al. 2009). Data from oVEMP studies in patients with unilateral vestibular loss suggest that the later peaks may be recorded in the absence of the first peak and trough (Iwasaki et al. 2007). Evidence from patients with bilateral vestibular loss indicates that the entire surface response is vestibular dependent, as all peaks disappear with complete vestibular loss (Iwasaki et al. 2008), while the later peaks may receive bilateral input, as unilateral loss of vestibular function often abolishes only the first surface peak in response to vibration (Iwasaki et al. 2007). Studies using similar sound and vibration stimuli (delivered with smaller vibrators that can be operated in magnetic fields for eye movement measurement with search coils) have reported transient eye movements which appear to show some resonance as well, albeit at slightly lower frequency (Todd et al. 2007; Welgampola et al. 2009; Aw et al. 2011). Pulses of galvanic vestibular stimulation also produce a VOR response with very small, 100 Hz resonance of position (Aw et al. 2006). This suggests that, regardless of the mechanism, the pattern of increasing and decreasing muscle activity has a measurable effect on the evoked eye movements.
Conclusion
By recording EMG activity directly from the IO and IR muscles during vestibular stimulation with vibration and sound, we were able to determine the myogenic basis of the oVEMP, a new measure of otolith function, and identify the IO as the source for the clinically relevant n10 potential. However, our use of oVEMP stimulation and recording techniques also provided a unique opportunity to record single unit motor activity during vestibular activation, as the extraocular muscles are significantly activated but the evoked eye movements are very small. This allowed stable measurement of VOR-related extraocular muscle activity in alert human subjects. Using this technique, we were able to demonstrate the presence of short-latency, excitatory projections from the otoliths to the IO and IR muscles in humans. Unilateral stimulation with sound confirmed that the projection to the IO muscle is crossed. Our data therefore provide evidence for very short-latency VOR projections from the otolith organs, similar to those evoked by the semicircular canals during the rotational VOR. The data also showed that the IO and IR muscles, which are antagonists for vertical eye movements, had reciprocal activation in response to our stimulus and that both muscles responded with repeated bursts of activity. As the contribution of individual extraocular muscles to eye movements cannot reliably be deduced from eye movement recordings, studies of the single motor unit activity of extraocular muscles are important as they provide a window into the underlying neural commands controlling eye movements in humans.
Acknowledgments
We would like to thank J. G. Colebatch for his help in developing the single unit recording technique and for kindly providing some of the recording software and equipment. Additional equipment was funded by the Neuro-Otology Society of Australia. We are grateful to R. Quian Quiroga for providing open access to the code of his spike detection and sorting algorithm. We would like to thank B. J. Hess and C. J. Bockisch for providing helpful comments on the manuscript. The study was supported by the Swiss National Science Foundation, the Betty and David Koetser Foundation for Brain Research and the Zurich Centre for Integrative Human Physiology (University of Zurich). S.M.R. was supported by the National Health and Medical Research Council of Australia. The authors declare no conflict of interest.
Glossary
- cVEMP
cervical vestibular evoked myogenic potential
- EMG
electromyography
- IO
inferior oblique eye muscle
- IR
inferior rectus eye muscle
- oVEMP
ocular vestibular evoked myogenic potential
- VOR
vestibulo-ocular reflex
Author contributions
K.P.W. and S.M.R. both equally designed the study, analysed the data and wrote the manuscript. R.M. and V.S. contributed to the recordings and review of the manuscript. D.S. contributed to the study conception, recordings and the manuscript. K.L. performed the needle recordings and contributed to the manuscript. All authors approved the final version of the manuscript. The research was conducted at the University Hospital Zurich, Switzerland.
Supplementary material
Supplemental Material video
References
- Aw ST, Aw GE, Todd MJ, Bradshaw AP, Halmagyi GM. Three-dimensional vibration-induced vestibulo-ocular reflex identifies vertical semicircular canal dehiscence. J Assoc Res Otolaryngol. 2011;12:549–558. doi: 10.1007/s10162-011-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw ST, Todd MJ, Halmagyi GM. Latency and initiation of the human vestibuloocular reflex to pulsed galvanic stimulation. J Neurophysiol. 2006;96:925–930. doi: 10.1152/jn.01250.2005. [DOI] [PubMed] [Google Scholar]
- Breinin GM. The Electrophysiology of Extraocular Muscle with Special Reference to Electromyography. Toronto: University of Toronto Press; 1962. [Google Scholar]
- Bronstein AM, Gresty MA. Short latency compensatory eye movement responses to transient linear head acceleration: a specific function of the otolith-ocular reflex. Exp Brain Res. 1988;71:406–410. doi: 10.1007/BF00247500. [DOI] [PubMed] [Google Scholar]
- Cai KY, Rosengren SM, Colebatch JG. Cervical and ocular vestibular evoked myogenic potentials are sensitive to stimulus phase. Audiol Neurootol. 2011;16:277–288. doi: 10.1159/000321988. [DOI] [PubMed] [Google Scholar]
- Carey J, Amin N. Evolutionary changes in the cochlea and labyrinth: Solving the problem of sound transmission to the balance organs of the inner ear. Anat Rec. 2006;288A:482–489. doi: 10.1002/ar.a.20306. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Iwasaki S, Ushio M, Fujimoto C, Kashio A, Kondo K, Ito K, Asakage T, Yamasoba T, Kaga K, Murofushi T. Ocular vestibular-evoked myogenic potentials (oVEMPs) require extraocular muscles but not facial or cochlear nerve activity. Clin Neurophysiol. 2009;120:581–587. doi: 10.1016/j.clinph.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Iwasaki S, Ushio M, Murofushi T. Vestibular-evoked extraocular potentials by air-conducted sound: another clinical test for vestibular function. Clin Neurophysiol. 2007;118:2745–2751. doi: 10.1016/j.clinph.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki J-I. Eye movements induced by ampullary nerve stimulation. Am J Physiol. 1963;204:347–351. doi: 10.1152/ajplegacy.1963.204.2.347. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol. 2004;115:2567–2573. doi: 10.1016/j.clinph.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Crane BT, Tian J, Wiest G, Demer JL. Initiation of the human heave linear vestibulo-ocular reflex. Exp Brain Res. 2003;148:247–255. doi: 10.1007/s00221-002-1301-8. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Diamond SG, Markham CH. Ocular counterrolling as an indicator of vestibular otolith function. Neurology. 1983;33:1460–1469. doi: 10.1212/wnl.33.11.1460. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Govender S, Rosengren SM, Colebatch JG. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin Neurophysiol. 2009;120:1386–1391. doi: 10.1016/j.clinph.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Govender S, Rosengren SM, Todd NP, Colebatch JG. Ocular vestibular evoked myogenic potentials produced by impulsive lateral acceleration in unilateral vestibular dysfunction. Clin Neurophysiol. 2011;122:2498–2504. doi: 10.1016/j.clinph.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Grantyn R, Grantyn A, Schaaf P. Conduction velocity, input resistance and size of cat ocular motoneurons stained with Procion yellow. Brain Res. 1977;135:167–173. doi: 10.1016/0006-8993(77)91062-9. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Isu N, Graf W, Sato H, Kushiro K, Zakir M, Imagawa M, Uchino Y. Sacculo-ocular reflex connectivity in cats. Exp Brain Res. 2000;131:262–268. doi: 10.1007/s002219900292. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Chihara Y, Smulders YE, Burgess AM, Halmagyi GM, Curthoys IS, Murofushi T. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colebatch JG, Curthoys IS. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68:1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, MacDougall HG, Halmagyi GM, Curthoys IS. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119:2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Jombik P, Spodniak P, Bahyl V. Direction-dependent excitatory and inhibitory ocular vestibular-evoked myogenic potentials (oVEMPs) produced by oppositely directed accelerations along the midsagittal axis of the head. Exp Brain Res. 2011;211:251–263. doi: 10.1007/s00221-011-2681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert T, Gianna C, Brookes G, Bronstein A, Gresty M. Horizontal otolith-ocular responses in humans after unilateral vestibular deafferentation. Exp Brain Res. 1998;118:533–540. doi: 10.1007/s002210050309. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Observations on nystagmus. Acta Otolaryngol. 1934;21:416–437. [Google Scholar]
- Magora A, Gonen B, Zelig S, Sachs U, Zauberma H. Electromyographic investigation of extraocular muscles in vestibular nystagmus evoked by caloric stimulation. Ophthalmic Res. 1973;4:91–98. [Google Scholar]
- Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol. 1997;117:66–72. doi: 10.3109/00016489709117994. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116:1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:636–651. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, USA: Yale University Press; 1906. [Google Scholar]
- Shin BS, Oh SY, Kim JS, Kim TW, Seo MW, Lee H, Park YA. Cervical and ocular vestibular-evoked myogenic potentials in acute vestibular neuritis. Clin Neurophysiol. 2012;123:369–375. doi: 10.1016/j.clinph.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969;68:350–362. doi: 10.3109/00016486909121573. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950;13:395–407. doi: 10.1152/jn.1950.13.6.395. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118:381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by impulsive transmastoid accelerations. Clin Neurophysiol. 2008a;119:1638–1651. doi: 10.1016/j.clinph.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Colebatch JG. Tuning and sensitivity of the human vestibular system to low-frequency vibration. Neurosci Lett. 2008b;444:36–41. doi: 10.1016/j.neulet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Govender S, Colebatch JG. Low-frequency tuning in the human vestibular-ocular projection is determined by both peripheral and central mechanisms. Neurosci Lett. 2009;458:43–47. doi: 10.1016/j.neulet.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Toft E, Sinkjaer T, Andreassen S. Mechanical and electromyographic responses to stretch of the human anterior tibial muscle at different levels of contraction. Exp Brain Res. 1989;74:213–219. doi: 10.1007/BF00248294. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Black box revisited: a technique for estimating postsynaptic potentials in neurons. Trends Neurosci. 2005;28:379–386. doi: 10.1016/j.tins.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Sasaki M, Sato H, Imagawa M, Suwa H, Isu N. Utriculoocular reflex arc of the cat. J Neurophysiol. 1996;76:1896–1903. doi: 10.1152/jn.1996.76.3.1896. [DOI] [PubMed] [Google Scholar]
- Wasicky R, Horn AK, Buttner-Ennever JA. Twitch and nontwitch motoneuron subgroups in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Migliaccio AA, Myrie OA, Minor LB, Carey JP. The human sound-evoked vestibulo-ocular reflex and its electromyographic correlate. Clin Neurophysiol. 2009;120:158–166. doi: 10.1016/j.clinph.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


